Abstract
Women with myocardial ischemia without obstructive coronary artery disease (CAD) often have microvascular coronary dysfunction (MCD). Traditional cardiac risk factors (RFs) contribute modestly to MCD detected by invasive coronary reactivity testing (CRT). Cardiac magnetic resonance imaging (CMRI) is an emerging noninvasive technique used to evaluate MCD. We evaluated RFs related to CMRI myocardial perfusion in women with MCD. 53 women with CRT confirmed MCD underwent adenosine stress and rest CMRI (1.5 Tesla). The myocardial perfusion reserve index (MPRI) was calculated (Pie Medical) with lower MPRI meaning less perfusion reserve. Relationships between RFs and MPRI were examined.
The mean age was 54±10 years with a mean body mass index (BMI) of 26.2±4.2. The mean MPRI was 1.63±0.39. Hypertension, dyslipidemia, elevated BMI, and post-menopausal status were inversely related to MPRI, while ever smoking, age, family history of CAD, history of irregular menses, and history of menopausal hormone therapy (MHT) or oral contraceptive (OC) use were not. Dyslipidemia and BMI remained significant independent predictors of MPRI. Regression modeling demonstrated that the RFs of dyslipidemia, obesity, hypertension, family history of CAD, and history of irregular menses explained 27% of the observed MCD variance.
Conclusions
In conclusion, impaired MPRI measured by CMRI is related to dyslipidemia and elevated BMI in women with MCD. These results suggest traditional RFs contribute modestly to MCD; a larger cohort of women with MCD should be examined to confirm and extend these observations. The impact of traditional CAD RF modification strategies, including optimal medical therapy, should be explored as MCD treatment targets.
Key Words: Microvascular coronary dysfunction (MCD), myocardial perfusion reserve, cardiac risk factors (RFs)
Introduction
Women present with signs and symptoms of myocardial ischemia more frequently than men and also are more frequently found to have no obstructive coronary artery disease (CAD) on angiography (1-3). We have previously shown that up to half of these women with persistent signs and symptoms of chest pain and no obstructive CAD have evidence of ischemia on exercise-stress nuclear magnetic resonance spectroscopy (4). These women have relatively high rates of angina hospitalizations, repeated coronary angiography, treatment costs, and adverse cardiovascular events compared to asymptomatic women (5,6). We have further demonstrated that these subjects often have microvascular coronary dysfunction (MCD) (7). While the importance of traditional cardiac risk factors (RFs) including hypertension, hyperlipidemia, diabetes, age, smoking history and family history of premature CAD as an etiology of obstructive CAD is well established, the relations between RFs and MCD is less explored (8-10).
Invasive coronary reactivity testing (CRT) is the gold standard test used to diagnose MCD. CRT uses intracoronary doses of adenosine, acetylcholine, and nitroglycerin to identify abnormal endothelial dependent and independent pathways that result in abnormal coronary flow reserve and ischemia. Cardiac magnetic resonance imaging (CMRI) is an emerging non-invasive technique that can detect non-segmental, sub-endocardial abnormalities suggestive of myocardial ischemia in subjects with no obstructive CAD (11). We have previously shown that women with MCD have lower myocardial perfusion reserve on CMRI compared to asymptomatic controls (12,13).
Myocardial perfusion reserve index (MPRI) is a semi-quantitative marker used to evaluate the ratio of myocardial blood flow during maximal hyperemia compared to rest derived from CMR images. Previous work in the Multi-Ethnic Study of Atherosclerosis (MESA) study has shown cardiac RFs predict myocardial perfusion on CMRI in asymptomatic adults (14). We evaluated relations between cardiac RFs and CMRI MPRI in symptomatic women with MCD.
Methods
Study subjects
Fifty-three women with signs and symptoms of myocardial ischemia with no obstructive CAD on coronary angiogram and MCD underwent adenosine stress CMRI (1.5 Tesla) and completed questionnaires regarding medical history with supervision. Presence of MCD was determined by previously published standardized methods using intracoronary adenosine, acetylcholine, and nitroglycerin (15-17). All women were recruited from the Barbra Streisand Women’s Heart Center at the Cedars-Sinai Heart Institute in Los Angeles, California between 2006 and 2008. This study was approved by the Cedars-Sinai internal review board and written informed consent was obtained for all subjects.
CMR image acquisition
CMRI was performed in the supine position on a 1.5 Tesla CMR scanner (Sonata, Siemens, Erlangen, Germany) with electrocardiogram (ECG)-gating and a phased-array surface coil (CP Body Array Flex, Siemens, Erlangen, Germany). A previously published highly standardized protocol was used and included assessment of cardiac function and viability in addition to pharmacologic stress and rest perfusion imaging with a total dose of gadolinium contrast of 0.15 mmol/kg (Gadodiamide, GE Healthcare, Milwaukee, WI, USA) (13). Blood pressure and pulse oxygenation were monitored (In vivo, Philadelphia, PA, USA) and recorded before, during, and after adenosine infusion. A 12-lead ECG was recorded prior to and following CMRI. Subjects had caffeine withdrawn for 24 hours prior to the CMRI exam.
The left ventricular short axis was determined by scout imaging, and first-pass perfusion images were obtained in basal, mid and distal short-axis image planes. Care was taken to ensure the short axis slices were not positioned too proximally or distally in the LV. A GRE-EPI hybrid pulse sequence was used for all subjects: field of view =350 × 350 mm2 or minimized dependent upon patient size, slice thickness =8 mm, TR/TE maximum =6.5/1.3 ms, bandwidth/pixel =1,420 Hz, GRAPPA acceleration factor =2, imaging every heartbeat. For pharmacologic stress, adenosine (Adenoscan, Astellas Pharma US, Inc., Northbrook, IL) was injected at a dose of 140 mcg/kg/min intravenously (IV) over a total duration of 4 minutes. 0.05 mmol/kg of gadolinium was administered at mid-stress at 4 mL/s via a second IV catheter, followed by 30 mL saline infusion at 4 mL/s. This was followed 10 minutes later by rest perfusion imaging with the same contrast settings.
CMR image analysis
Quantitative analysis of the first pass perfusion images for MPRI was performed using CAAS MRV 3.3 software (Pie Medical Imaging B.V., Netherlands) by skilled users (LT, DB). Epicardial and endocardial LV myocardial contours (basal, midventricular, and apical slices were manually traced in order to acquire intensity over time curves at rest and stress for 16-segments (segment 17, the LV apex, was not imaged). The LV cavity region of interest was adjusted to include adequate blood pool signal. The calculated relative upslope (RU, maximum upslope of the selected curve, divided by the maximum upslope of the left ventricular cavity curve, with LV cavity input calculated for each slice) was used for calculation of the segmental MPRI, defined as the ratio of RU (stress)/RU (rest) and segmental values were averaged as previously described (18).
Measurement of RFs
Values for body mass index (BMI) were measured and subjects completed a study questionnaire during a baseline visit. Data were obtained for age, presence of hypertension, dyslipidemia, ‘ever smoked’ history, family history of CAD, history of irregular menses, current menopausal status, and historical or current use of oral contraceptives (OCP) or menopause hormone therapy (MHT). Hypertension was defined as high blood pressure requiring treatment, based on JNC VI guidelines (19). Dyslipidemia was defined as abnormal cholesterol requiring treatment based on Adult Treatment Panel (ATP) III guidelines (20). If treatment was given and lipid levels were normal at the time of testing, the patient was still identified as having dyslipidemia. Family history of CAD was defined as a family member with a heart attack. Ever smoked history was defined as self-report of prior smoking. Reproductive history on historical or current use of OCP or MHT was defined as currently or previously using either medication.
Statistical analysis
Summary values for demographic characteristics are expressed as means and standard deviation (mean ± SD) for continuous variables, and frequencies (%) for categorical variables. Relationships between RFs and MPRI were examined by bivariate correlation, uni- and multi-variable linear regression analyses with MPRI as the dependent variable. In particular, point bi-serial correlation was calculated for binary RFs and Pearson correlation coefficient was used for continuous RFs. Each given RF was first modeled separately and RFs were then chosen based on R squared values from linear regression. The RF selection procedure started with a full model including all the RFs, and then sequentially left out the variables with minimal contributions until there was a substantial drop in R squared values. No co-linearity was identified among the variables using variance inflation factors from multivariable regression. Analysis was performed using SAS software (SAS Institute, Cary, North Carolina).
Results
All 53 women completed the CMRI protocol and questionnaire. The subjects were 54±10 years old and BMI was 26.2±4.2. Patient demographics regarding distribution of RFs are listed in Table 1. CRT was performed with at least one abnormality consistent with MCD as previously described (15-17) in all subjects who underwent CMRI.
Table 1. Patient demographics.
| Risk factors | % of subjects |
|---|---|
| Hypertension | 35% |
| Dyslipidemia | 53% |
| Ever smoked | 42% |
| Diabetes | 0% |
| Family history of premature coronary artery disease | 70% |
| Post-menopausal | 55% |
| History of irregular menses | 12% |
| History of menopause hormone therapy | 44% |
| History of oral contraceptive use | 84% |
| Age (mean ± standard deviation) | 54.0±10 years |
| BMI (mean ± standard deviation) | 26.2±4.2 |
BMI, body mass index
There were no complications during CMRI. The resting and peak heart rates were 70.4±12.5 and 106.0±13.4 beats per minute, respectively. The groups mean MPRI was 1.63±0.39, with a lower value indicating less perfusion reserve.
Bivariate correlation analysis between each cardiac RF and MPRI identified associations between post-menopausal status (r=–0.30, P=0.04), hypertension (r=–0.32, P=0.02), dyslipidemia (r=–0.32, P=0.02), and BMI (r=–0.28, P=0.04) (Table 2) (Figure 1). Post-menopausal status no longer predicted MPRI after adjusting for age. Hypertension no longer predicted MPRI after adjusting for dyslipidemia and BMI. However, dyslipidemia and BMI remained significant independent predictors of MPRI in a multivariable model (Table 3).
Table 2. Bivariate correlation analysis showing the correlation between risk factors and MPRI.
| Risk factors | (r, P-value) |
|---|---|
| Hypertension | –0.32, P=0.02 |
| Dyslipidemia | –0.32, P=0.02 |
| Ever smoked | –0.12, P=0.38 |
| Family history of premature coronary artery disease | 0.07, P=0.61 |
| Age, years | –0.24, P=0.09 |
| BMI | –0.28, P=0.04 |
| Post-menopausal status | –0.30, P=0.04 |
| History of irregular menses | –0.18, P=0.21 |
| History of menopause hormone therapy | –0.16, P=0.25 |
| History of oral contraceptive use | 0.18, P=0.19 |
BMI, body mass index
Figure 1.
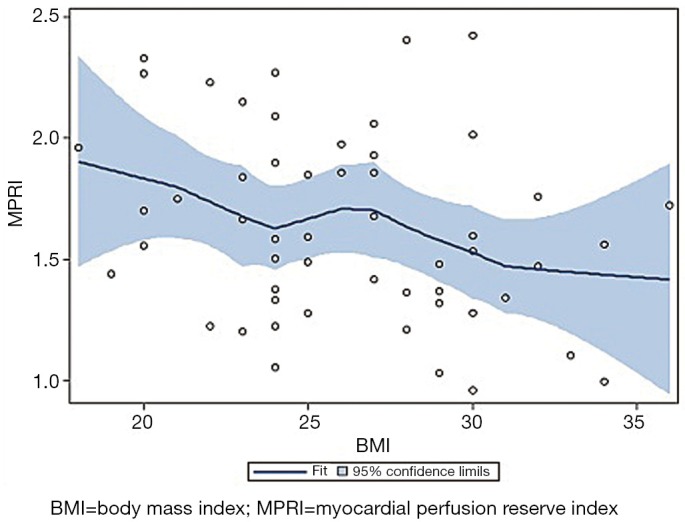
Locally weighted least squares scatterplot smoothing (LOESS) demonstrating the relationship between myocardial perfusion reserve (MPRI, X axis) and body mass index (BMI, Y axis)
Table 3. Multivariable linear regression analysis showing the relationship between risk factors and mean MPRI.
| Risk factors | (β, P-value) |
|---|---|
| BMI adjusted for dyslipidemia (β, P-value) | –0.02, P=0.04 |
| Dyslipidemia adjusted for BMI (β, P-value) | –0.25, P=0.02 |
| Hypertension adjusted for BMI and dyslipidemia (β, P-value) | –0.10, P=0.42 |
| Post-menopausal status adjusted for age (β, P-value) | –0.19, P=0.15 |
| Post-menopausal status adjusted for BMI, dyslipidemia (β, P-value) | –0.09, P=0.42 |
BMI, body mass index
The best multivariable model (Table 4) predicting MPRI using a combination of independent predictors of MPRI (dyslipidemia and BMI) only yielded a R2 of 0.17. When hypertension, family history of CAD, and history of irregular menses were added to the model, the result was an R2 of 0.27.
Table 4. Multivariable linear regression model for prediction of MPRI.
| Risk Factors that predict MPRI | R2 | P-value |
|---|---|---|
| Age, HTN, BMI, dyslipidemia, ever smoked history, family history of CAD, Post-menopausal status, history of MHT or OCP use, history of irregular menses | 0.32 | 0.18 |
| HTN, BMI, dyslipidemia, family history of CAD, post-menopausal status | 0.22 | 0.06 |
| HTN, BMI, dyslipidemia, family history of CAD, history of irregular menses | 0.27 | 0.02 |
| BMI and dyslipidemia | 0.17 | 0.01 |
BMI, body mass index; CAD, coronary artery disease; HTN, hypertension
Discussion
Our study is the first to demonstrate that traditional cardiac RFs are associated with impaired perfusion reserve measured by CMRI MPRI in subjects with MCD. Specifically, obesity and dyslipidemia modestly predict CMRI MPRI in women with MCD. Notably, traditional cardiac RFs account for a relative minority of the observed variability in MPRI. Overall, obesity, dyslipidemia, hypertension, family history of CAD, and history of irregular menses combined explained 27% of the observed variance in MPRI within this population with MCD defined by CRT.
The study addresses a pilot group of subjects testing an overarching concept of whether CMRI can be used as a noninvasive to diagnose MCD. This specific manuscript queries whether CMRI MPRI is related to traditional RFs. Initial analysis of post-menopausal status and hypertension revealed relations to MPRI; however, after adjusting for age, BMI and dyslipidemia, these RFs no longer remained related. This is consistent with prior Women’s Ischemia Syndrome Evaluation (WISE) work which found that systolic blood pressure and post-menopausal status interact (21). In older women, while there are metabolic differences that are exacerbated by hormonal shift after menopause that lead to the clustering of obesity, hypertension, and dyslipidemia (22), our current study findings and our prior work demonstrate that the menopausal status is not distinguishable from aging (21). In addition, we show a history of irregular menses contributes to the predictive model of CMRI MPRI which is also consistent with prior WISE research that demonstrated a polycystic ovary syndrome phenotype is associated with adverse cardiac outcomes (23). The prevalence of prior oral contraceptive use in this study is consistent with population use since the 1960s (24). The observed relationship between BMI and MPRI is not thought to be consequent to imaging artifact from imperfect signal detection with phased array coils using accelerated imaging techniques in larger subjects. A comparison of symptomatic and asymptomatic overweight women is the subject of ongoing study by our group. Our finding that cardiac RFs predict approximately one-quarter of the variance in MCD assessed by CMRI MPRI parallels earlier findings in WISE (8). WISE evaluated women between 1999 and 2005 with ischemic heart disease (IHD). In this cohort, 80% had no obstructive CAD; defined as stenosis greater than 50% in at least one major epicardial coronary artery (8). Among the women who underwent invasive coronary flow reserve measurement using intracoronary adenosine, traditional cardiac RFs (age, diabetes, hypertension, dyslipidemia, family history of premature CAD, post-menopausal status and severity of angiographic CAD) predicted 16% of the variance in coronary flow reserve (8). In this current report we have exclusively studied women with no obstructive CAD who have a diagnosis of MCD and have used different software for evaluation of MPRI (PIE Medical Imaging, vs. Matlab, Mathworks).
Our findings are also consistent with previous work in the MESA study which demonstrated the ability of CMRI to detect subtle changes in myocardial blood flow even in asymptomatic individuals with cardiac RFs (14). The MESA study showed that individuals with greater coronary RF burden have worse myocardial perfusion on CMRI. This further validates the utility of CMRI to detect perfusion abnormalities in subjects documented to have no obstructive CAD.
Determining the relation of traditional RFs to MPRI is important because a previous study has demonstrated that CMRI MPRI predicts event free survival in women with signs and symptoms of ischemia and no obstructive CAD (12). Specifically, Doyle et al demonstrated that an increased burden of RFs, including obesity and smoking history, had annualized event rates as high as 12%. In our MCD cohort, BMI and dyslipidemia best related to myocardial perfusion on CMRI. These results suggest that research evaluating optimal medical therapy aimed at traditional RF modification should be explored as treatment targets in the MCD population. Furthermore, since only 27% of the CMRI MPRI variance is explained by traditional cardiovascular risk, novel RFs, such as psychosocial RFs and cardiac autonomic dysfunction, should be explored as RFs for MCD.
Of note, the current studies relatively small sample size may have contributed to the absence of diabetics in this study cohort, and this may have reduced the predictive value of traditional RFs for MPRI. Because diabetes is a CHD equivalent, we may have failed to recruit diabetic subjects that were already aggressively managed and not referred for specialty care in our clinic, from which we recruited the study cohort. Additionally, diabetic subjects are more likely to have silent ischemia (25), which could contribute to lower rates of referral for angina and evaluation for MCD.
Limitations
Because our study was designed as a pilot study, it is limited by a small sample size. Small sample size likely explains why certain RFs such as age (P=0.09) trended toward significance and other RFs such as hypertension and post-menopausal status related to MPRI in univariate analysis but lost significance in multivariate analysis. The interaction between hypertension and post-menopausal status may also be explained by inter-correlation such that these RFs become non-significant once the major ones (BMI and dyslipidemia) are in the model. Additionally, the cohort evaluated were all women with invasive confirmation of MCD, therefore, our findings may not be generalizable to all men or women with IHD. CSX. Finally, our best multivariate model that predicted 27% of the variance in MPRI used mostly traditional cardiac RFs. We did not measure novel RFs such as inflammatory markers, oxidative stress, circulating progenitor cells which may play a role in CMRI MPRI variance and are currently under further investigation.
Summary and implications
In summary, impaired MPRI measured by CMRI is related to dyslipidemia and elevated BMI in women with MCD. These results combined with prior studies suggest that traditional RFs contribute modestly to MCD. Overall, the RFs of dyslipidemia, obesity, hypertension, family history of CAD, and history of irregular menses explained 27% of the observed variance in MPRI. Because MCD subjects are at elevated risk of adverse outcomes, development and testing of therapeutic strategies is needed. These results call for additional work in a larger dataset in order to confirm and extend these initial observations. Furthermore, research evaluating optimal medical therapy to control traditional RFs should be explored as treatment targets in MCD.
Acknowledgements
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources UN55ES6580F and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, Cardium Therapeutics, San Diego, CA, Siemens, Munich, Germany, GE/Amersham, Pittsburg, PA, Astellas, Northbrook, IL, Lantheus, North Billericas, MA, Spectrum Dynamics, San Jose, CA, Iba Molecular, Somerset, NJ.
Disclosure: C. Noel Bairey Merz- Gilead research support; Puja Mehta- Gilead research support; Chrisandra Shufelt- Gilead research support. All other authors declare no conflict of interest.
References
- 1.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol 1999;33:1453-61 [DOI] [PubMed] [Google Scholar]
- 2.Humphries KH, Pu A, Gao M, et al. Angina with “normal” coronary arteries: sex differences in outcomes. Am Heart J 2008;155:375-81 [DOI] [PubMed] [Google Scholar]
- 3.Davis KB, Chaitman B, Ryan T, et al. Comparison of 15-year survival for men and women after initial medical or surgical treatment for coronary artery disease: a CASS registry study. Coronary Artery Surgery Study. J Am Coll Cardiol 1995;25:1000-9 [DOI] [PubMed] [Google Scholar]
- 4.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 2000;342:829-35 [DOI] [PubMed] [Google Scholar]
- 5.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993-9 [DOI] [PubMed] [Google Scholar]
- 6.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reis SE, Holubkov R, Lee JS, et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol 1999;33:1469-75 [DOI] [PubMed] [Google Scholar]
- 8.Wessel TR, Arant CB, McGorray SP, et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE). Clin Cardiol 2007;30:69-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, et al. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation 2000;102:1233-8 [DOI] [PubMed] [Google Scholar]
- 10.Brush JE, Jr, Cannon RO, 3rd, Schenke WH, et al. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med 1988;319:1302-7 [DOI] [PubMed] [Google Scholar]
- 11.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948-53 [DOI] [PubMed] [Google Scholar]
- 12.Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010;3:1030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishimori ML, Martin R, Berman DS, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging 2011;4:27-33 [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Jerosch-Herold M, Jacobs DR, Jr, et al. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2006;47:565-72 [DOI] [PubMed] [Google Scholar]
- 15.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722-5 [DOI] [PubMed] [Google Scholar]
- 17.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv 2012;5:646-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shufelt C, Thomson L, Goykhman P, et al. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovas Diagn and Ther 2013;3:153-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med 1997;157:2413-46 [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486-97 [DOI] [PubMed] [Google Scholar]
- 21.Gierach GL, Johnson BD, Bairey Merz CN, et al. Hypertension, menopause, and coronary artery disease risk in the Women’s Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol 2006;47:S50-8 [DOI] [PubMed] [Google Scholar]
- 22.Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 2006;47:S4-S20 [DOI] [PubMed] [Google Scholar]
- 23.Shaw LJ, Bairey Merz CN, Azziz R, et al. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health--National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 2008;93:1276-84 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Shufelt CL, Bairey Merz CN. Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol 2009;53:221-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wackers FJ, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care 2004;27:1954-61 [DOI] [PubMed] [Google Scholar]


