Abstract
Periinterventional echocardiographic guidance is considered desirable in order to make interventional closure of interatrial communications safe and straight forward. Transesophageal echocardiography (TEE) including real-time three-dimensional (RT-3D) imaging, later complemented and in part replaced by intracardiac echocardiography (ICE), has become established as the standard approach to guide those procedures. Especially in atrial septal defect (ASD) closure, which tends to be more challenging than patent foramen ovale (PFO) closure, a certain risk of severe complications remains and may result from suboptimal device performance. Other complications may be related to discontinuous use of echocardiographic monitoring. Image fusion and RT-3D ICE are currently under clinical testing and might be suitable to facilitate spatial orientation. Nowadays, two-dimensional ICE is the method of choice for guiding percutaneous device closure, especially of ASDs and “complex” PFOs. Uninterrupted TEE under deep sedation is an alternative. In contrast, closure of “simple” PFOs will often require nothing but final confirmation of the result, and therefore, short echocardiographic viewing is sufficient in many cases.
Key Words: Echocardiographic guidance, transesophageal echocardiography (TEE), atrial septal defect (ASD) closure, patent foramen ovale (PFO) closure
Introduction
Percutaneous closure of interatrial communications has become a well-established technique for preventing paradoxical embolism in patients with a PFO, a secundum-type ASD, and for treating severe left-to-right shunts associated with secundum-type ASDs up to a diameter of 35 mm. During the last two decades, transesophageal echocardiography (TEE) including real-time three-dimensional (RT-3D) imaging, later complemented and in part replaced by intracardiac echocardiography (ICE), has become established as the standard approach to prepare for and to guide the interventional treatment of interatrial communications (1-5). Contrast transthoracic echocardiography (TTE) has a role as a screening tool in patients with unclear right heart overload or after systemic embolic events (6). Yet TTE is neither capable of differentiating between patent foramina ovalia (PFO) and various kinds of atrial septal defects (ASDs), nor is it suitable for guiding percutaneous device closure of interatrial communications. Cardiac MRI and CT have a role for pre-interventional diagnosis in patients being unable to swallow a TEE probe or if TEE findings are inconclusive. In particular, estimation of rim size prior to ASD device closure might be challenging using echocardiography, only.
Although percutaneous device closure of interatrial communications has by now become well accepted, a certain risk of severe complications remains (7). Accurate imaging of the anatomic features of the ASD is critical for case selection, planning, and intraprocedural guidance. Especially in ASD closure, which tends to be more challenging than PFO closure, some complications are known to result from suboptimal device performance. Other complications may be related to the typically discontinuous use of TEE monitoring required because supine patients do not tolerate continuous TEE monitoring well during a longer period unless they are deeply sedated or under general anesthesia. Image fusion currently being under clinical testing might be suitable to facilitate spatial orientation by transferring structural markers from the echocardiographic image into the fluoroscopic one. Rotational angiography might make a sense after device deployment and before releasing it to ensure that the occluder has been deployed properly. That way, image fusion might be helpful to accelerate closure procedures but needs further evaluation. However, it is our aim to keep radiation exposure as low as possible, since many of our patients are at reproductive age.
Experience gained over about 20 years makes us believe that several specific complications of transcatheter closure can be potentially avoided by more sophisticated echocardiographic monitoring. ICE is the method of choice for guiding percutaneous device closure, especially of ASDs and represents the preferred approach in the United States. Uninterrupted TEE under deep sedation is an alternative used in many places in Europe. In contrast, PFO closure will often require nothing but intermittent monitoring or final confirmation of the result, and therefore, short echocardiographic viewing is sufficient in many cases, while continuous monitoring is more appropriate in patients with a complex PFO, e.g., if associated with a large septal aneurysm, or with multifenestrated interatrial septum (IAS).
ICE guided closure of interatrial communications
Equipments and general considerations
Visualizing the IAS, interatrial communications, and the surrounding structures from within the heart offers clear advantages, especially since the inferior rim of the septum can be imaged with high resolution in patients with an ASD. The 8F AcuNav® catheter (Siemens Inc. Mountain View, U.S.A.) equipped with a miniaturized 64 element 5.5-10.0 MHz phased-array transducer is by far the most frequently used system worldwide. The ViewFlex-Plus® (St. Jude Medical, St. Paul, U.S.A.) is another commercially available phased-array system. The latter is based on a 9F catheter. Rotational ICE devices, e.g., the UltraICE®-catheter (Boston Scientific, Natick, U.S.A.) and the 10F AcuNav®-catheter still on the market are no longer significant for guiding transcatheter device closure of interatrial communications, especially not for ASDs and “complex” PFOs. Because of the costs, all these devices are used as pure guiding tools. Periinterventional diagnostics and follow-up remain a domain of TEE.
Standard approach
A procedural standard approach has been established during the last decade. Prior to passing any instruments through the interatrial communication, the ICE-catheter is advanced through the inferior vena cava (IVC) into the right atrium (RA). The transducer is aimed at the left atrium (LA) to obtain the transatrial longitudinal view (Figure 1). It is not recommended to advance the catheter into the left atrium. Initially, the adequate position of a long guide wire inserted into the left upper pulmonary vein is demonstrated by angulating the probe from the longitudinal view inferiorly. The stretched size of ASDs can be adequately measured by ICE. The use of sizing balloons is still mandatory for estimating the size of the communication prior to ASD device closure. The most frequently used “waist” method (Figure 2) and the “stop flow” method are alternate approaches (9).
Figure 1.
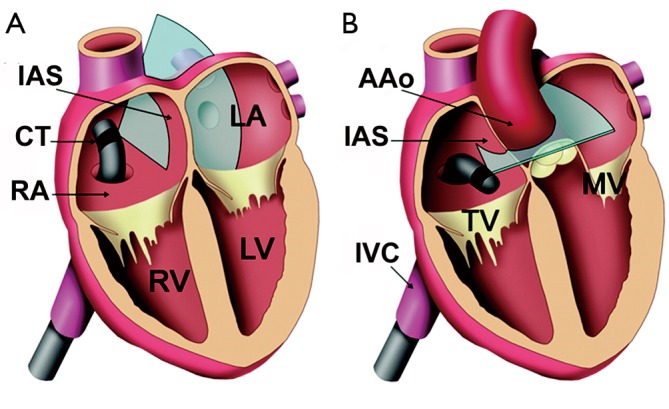
Graphic showing intracardiac echocardiographic standard views for guidance of device closure of interatrial communications [modified from Bartel et al. (8)]. A. transatrial longitudinal view; B. transatrial short-axis view; AAo, ascending aorta; CT, catheter tip; IAS, interatrial septum; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; MV, mitral valve; RA, right atrium; RV, right ventricle; TV, tricuspid valve
Figure 2.
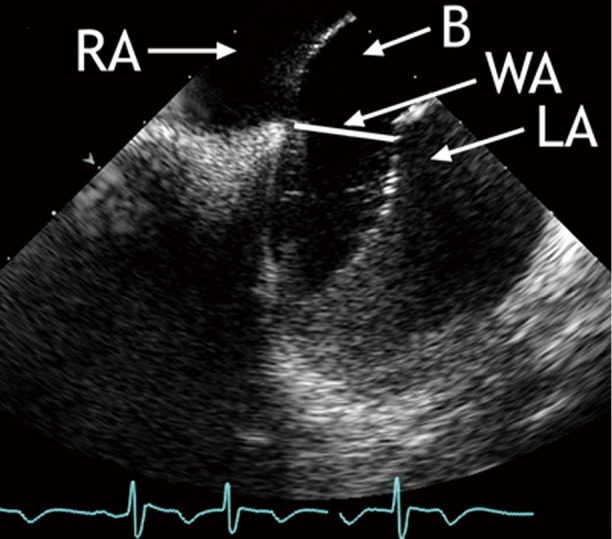
ICE-guided balloon sizing prior to ASD device closure: the sizing balloon is inflated in the LA and then withdrawn into the RA until a waist forms in the balloon. The diameter of the waist is taken as the stretched diameter of the ASD. (B, balloon; LA, left atrium; RA, right atrium; WA, waist)
Next, the long access sheath required for occluder device application is inserted over the guide wire. Fluoroscopic imaging during that part of the procedure can be limited to brief intermittent checks because ICE can primarily guide and document placement of the tip of the sheath in the LA. Echocardiographic guidance complemented by fluoroscopic viewing is recommended during deployment of the closure device:
ICE depicts release of the left-sided disc which opens inside the LA near the mouth of the left upper pulmonary vein. The whole system including the long access sheath with the delivery cable inside and the opened left-sided disc is slightly pulled back until the open disc exerts some traction on the IAS. ICE depicts when the left-sided disc matches the IAS closely.
While the delivery cable is still under traction, the long access sheath is further pulled back to release the right-sided disc. ICE demonstrates release of the right-sided disc inside the RA and the left-sided disc remaining on the left side lined-up with the IAS.
While monitoring the opening of the right-sided disc in the RA, the delivery cable is carefully advanced until the disc fits snugly into the IAS, but from the right side. Confirmation requires two perpendicular imaging plains (transatrial longitudinal and short-axis views), which are also used to rule out jamming of one of the discs at the level of the interatrial communication.
When the occluder device is positioned but still connected to the delivery cable, the “wiggle-maneuver” is monitored. Aiming to ensure that neither disc can tilt or slip to the contralateral side, the occluder device is pushed and pulled before release. This will confirm that the IAS is optimally engaged. Details of this maneuver, which is considered the best technique to avoid unsatisfactory device orientation or embolization, is nicely shown by ICE. The so-called “wiggle-maneuver” should be visualized in the transatrial longitudinal view and again in the transatrial short-axis view (Figure 1). In the short-axis view, compression of the aortic bulb by the occluder device can be ruled out. It makes sense to monitor device disconnection from the delivery cable by ICE as well.
When released, the device will fully line up with the IAS (Figure 3), reaching its definitive orientation. The final position of the device should be visualized to ascertain that the discs completely enclose the rims of the interatrial communication on both sides. After device deployment, color flow imaging can depict any residual shunt. During deployment of the occluder device, device and ICE catheter do not interfere with each other. As a precaution, ICE catheter and occluder device should be kept at least 1 cm apart. A closer position would impair optimal imaging of the occluder device. As elucidated, continuous ICE imaging during each stage of the procedure allows optimal device positioning, a prerequisite for safe percutaneous closure of interatrial communications.
Figure 3.
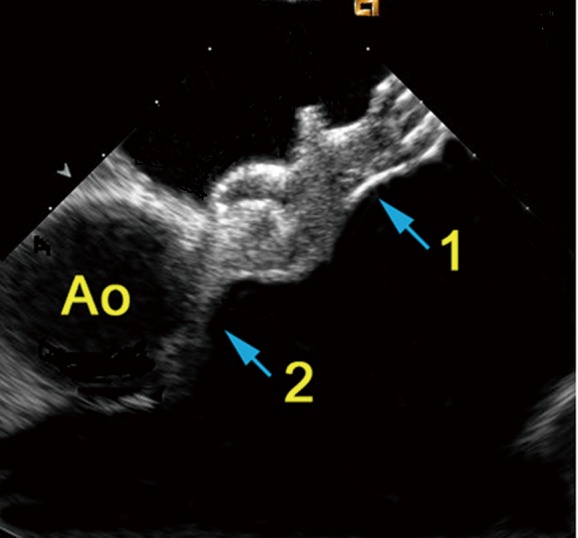
ASD-occluder in place seen from a short-axis intracardiac view. Ao, aorta; 1, aortic root; 2, closure device
Several investigators have demonstrated that ICE can safely guide interventional treatment of interatrial communications and that ICE is superior to conventional TEE monitoring (8,10-12). TEE requires general anesthesia with or without endotracheal intubation, whereas ICE permits unlimited echocardiographic viewing in fully conscious and compliant patients. This is of utmost importance since malposition and migration of the device into the systemic or pulmonary circulation or perforation of the cardiac wall and rapid thrombus formation on the device are known to occur (7,13,14). ICE provides better image resolution than TEE, thereby facilitating the procedure, particularly when long continuous or repeated echocardiographic viewing is required or when complications develop. ICE results in much less procedural stress to the patient, and fluoroscopic and procedural times can be shortened (8,12). Since many patients with interatrial communications are of reproductive age or younger, reduction of radiation exposure has to be considered a major advantage of ICE. In that respect, patients who undergo ASD closure benefit more from ICE than patients undergoing PFO closure, because ASD closure tends to be more complex and more time-consuming.
ICE can also be recommended for pediatric patients, especially in view of the unresolved issue of potential esophageal injury from TEE probe insertion. Several years experience has shown that ICE is a practical and straightforward approach for guiding device closure in children and adolescents (2,11). The overall risk related to ICE appears to be low. There is no reported increase in vascular complications from the additional venous puncture. After market release of the smaller 8F catheter, this has become even less of a concern. Transient atrial arrhythmias during manipulation of the ICE catheter in the RA have been reported. It is likely that ICE improves the safety of interventional device closure in interatrial communications, even though that has not been approved yet.
Life-threatening acute congestive left ventricular failure complicating surgical or transcatheter closure of ASDs in patients with impaired left ventricular function has been repeatedly reported (15). Nevertheless, device closure is an alternative to surgery in these patients, provided (I) there is no right-to-left shunting, (II) LA pressure is markedly reduced due to echocardiography guided preconditioning, (III) LA pressure remains below the critical level of 20 mmHg on temporary ASD closure, and (IV) the operator is familiar with the procedure and can complete it in a straightforward manner, preferably under ICE-guidance without general anesthesia and without endotracheal intubation (16). ICE is particularly helpful in device closure of multiple IAS fenestrations requiring either simultaneous device implantation or staged deployment of closure devices. ICE is the ideal method to make sure the bigger occluder encompasses the smaller one and both devices are well aligned with the IAS.
From an economic point of view, savings from shorter procedural time and from avoiding general anesthesia need to be weighed against the cost of the ICE catheter (17). One has to take into consideration that in many countries, health insurance agencies do not cover the costs of ICE. Reuse of the catheter is permitted in some European countries (Germany, Eastern Europe) but not in the U.S. Technically, the ICE-catheter can be resterilized up to five times. After a brief learning curve, interventional cardiologists who are familiar with echocardiography can fully benefit from the advantages of ICE. Cardiologists not familiar with echocardiography will benefit from a team approach, i.e. the presence of a non-invasive cardiologist in the catheterization laboratory.
Real-time three-dimensional ICE
The new AcuNav® V catheter (Siemens Inc. Mountain View, U.S.A.) was recently CE-certified and represents the only commercially available RT-3D ICE system. The 10F catheter carries a matrix transducer providing a 22°×90° real time volume image from inside the heart or from neighboring vessels. There are no experimental or clinical data available on this system yet. Unfortunately, the manufacturer does not intend to conduct a randomized trial at this time. Consequently, it will remain unclear for a while if RT-3D ICE may be superior to conventional ICE and under which particular circumstances 3D-RT ICE might be more suitable. The small image volume in the near field represents the main limitation of this interesting approach. That is way 3D-RT ICE appears to gain less of an impact on the guidance of closure procedures than 3D-RT TEE. The benefits of imaging are related to the distance between transducer and target region (Figure 4). That distance can be increased by flexing the tip of the catheter inside the RA far towards the right hepatic lobe. Nevertheless, it is difficult to completely depict a PFO occluder or even a bigger ASD occlude (Figure 5). Any such attempts fail if the transducer is positioned close to the IAS. Nevertheless, the technique may facilitate spatial alignment between occluder and IAS, what makes 3D-RT ICE potentially interesting for beginners in the interventional treatment of interatrial communications, especially when treating ASDs. Important spatial relationships, e.g., between tip of the access sheath and LA wall or the junction between occluder and delivery cable, are more likely to remain within the imaging volume than within a 2D imaging plane and are therefore easier to display than with 2D ICE. In contrast, reverberation artifacts associated with 3D ICE make structures behind the occluder more difficult to visualize (Figure 5). Higher costs in comparison to conventional ICE are another limitation. A randomized multicenter trial and systematic experimental studies are desperately needed before one can justify the additional expense of 3D guidance, especially in view of the many years of proven experience with the current equipment.
Figure 4.

Principles of RT-3D ICE
Figure 5.
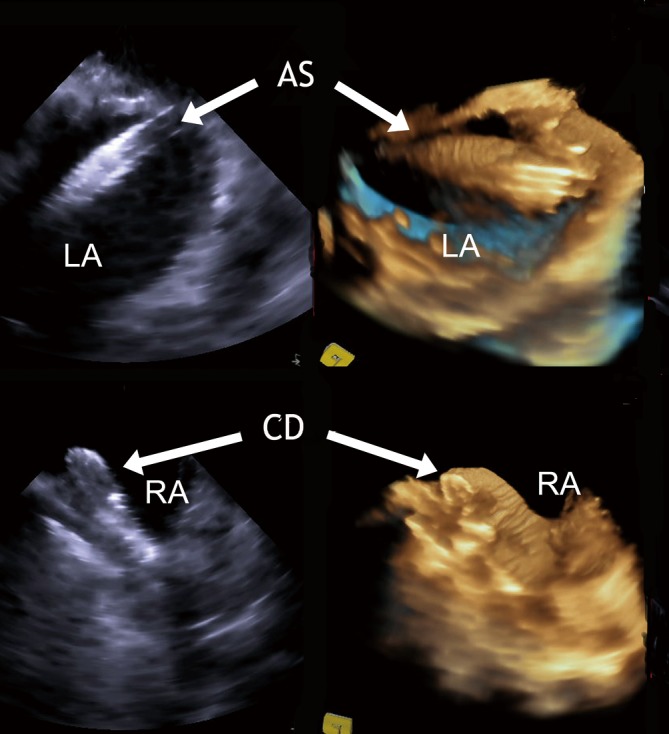
RT-3D ICE: comparative imaging of a access sheath passing a PFO (upper panel) and a fully deployed PFO device (lower panel) by 2D and RT-3D ICE. LA, left atrium; RA, right atrium; ES, access sheath; CD, closure device
TEE guided closure of interatrial communications
Conventional two-dimensional TEE
Traditionally, percutaneous ASD and PFO closures are performed under two-dimensional (2D) TEE guidance using general anesthesia and endotracheal intubation (9,10,18). In contrast to ICE-guidance, a non-invasive cardiologist has to be present in the catheterization laboratory. Independent of the guiding tool used, TEE is employed prior to device closure to assess defect size, position, and any associated left-to-right and right-to left shunting. It is particularly important to characterize the ASD rims defined as the IAS tissue surrounding the ASD. The rims are named after the respective adjacent structures: superior vena cava rim (superior), aortic rim (anterosuperior), coronary sinus rim (anteroinferior), inferior vena cava rim (inferior), and posterior rim (posterior). If the inferior rim is less than 5 mm wide, device closure is not an option. Absence of an aortic rim is no contraindication for device closure but may require a certain degree of over-sizing of the ASD-occluder encompassing the aortic bulb. In PFO closure, tunnel length, associated aneurysms of the IAS and septal pouch are important factors (19). A PFO is categorized as “complex” if any of the following findings is present: tunnel length beyond 7 mm, multiple openings into the LA, aneurysm of the IAS or septal pouch, IAS thickness of 10 mm or more, presence of a Chiari network or a Eustachian valve, and hybrid defects (IAS with multiple fenestrations). If no such finding is demonstrated, the PFO is considered “simple” (20). During the last decade, TEE has been used to characterize patient subgroups with a high risk of cerebral embolism. PFO size and the presence of embryonic recesses are important factors (21). A large body of circumstantial evidence, including case reports of an embolus traversing a PFO, epidemiologic data, and case control series links PFO to cryptogenic stroke (22) what has been currently confirmed by the results of the RESPECT-trial (TCT 2012). On this background, TEE can be considered the method of choice for a systematic evaluation of the IAS and its anatomical details (23,24).
Continuous TEE viewing is dispensable in many PFO closure procedures, which can be fast and straightforward interventions. Especially “simple” PFOs present no real challenge to interventional cardiologists experienced in interventions for congenital heart disease. Such cases do not necessarily require continuous echocardiographic monitoring. When the device is deployed but still connected to the delivery cable, one short inspection by TEE under monitored anesthesia is absolutely adequate to check for proper device position, to perform the “wiggle-maneuver”, and to observe the device while it is being released from the cable. For “complex” PFOs, continuous ICE guidance is nowadays considered the first choice. Continuous RT-3D TEE under general anesthesia remains an alternative of almost equal value.
As described for ICE-guidance, ASD sizing is an important aspect of intraprocedural TEE evaluation. Sizing is usually accomplished by either the balloon “waist” method or by the “stop flow” method rather than by direct measurement of the non-stretched size. The “stop flow” method is particularly suitable in ASDs too large to inflate a balloon to the point where a waist appears. Despite of the oval shape of many ASDs single plane measurements are sufficient since the balloon rounds the defect. Direct TEE measurements have been shown to underestimate stretched ASD size even more than direct ICE measurements (9). TEE is also used to verify proper placement of long access sheaths in the LA, and to monitor device deployment and its release from the delivery system. Short-axis (45-70°), bicaval (90°), and 4-chamber (0°) TEE views are used to safely deploy the occluder, to check for its proper position and to rule out thrombus formation during the procedure. Uninterrupted TEE is required to reliably detect rapid thrombus formation as quickly as possible. Thrombus formation occurs in about 2% of interventional device closure procedures and is related to hypercoagulable states. Prolonged TEE-monitoring under conscious sedation is associated with a risk of aspiration. From the current perspective, conventional TEE has historically improved the results of ASD closure (25). Today 2D TEE is no longer considered the most appropriate guiding tool for device closure of “complex” interatrial communications. TEE remains adequate for the follow-up evaluation of interventional results (Figure 6).
Figure 6.
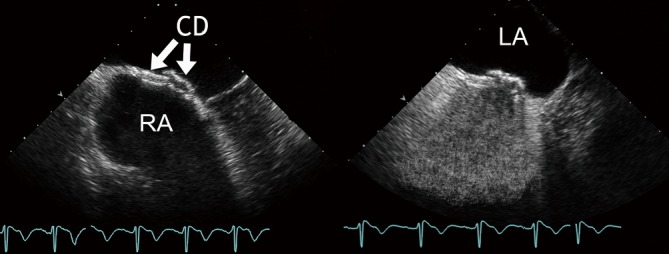
Follow-up examination after implantation of two devices in a multifenestrated IAS using conventional TEE (native – left panel; contrast – right panel). CD, closure devices; LA, left atrium; RA, right atrium
Real-time three-dimensional TEE
RT-3D TEE offers improved spatial orientation and a substantial information gain regarding the characteristics of an ASD, “complex” PFO, or an IAS with multiple fenestrations (26). It is particularly useful for interventionalists learning interatrial device closure procedures. This group will benefit from continuous or at least prolonged and repeated viewing throughout the intervention. To make the most of RT-3D TEE, general anesthesia and endotracheal intubation are required, just as with 2D TEE. The major advantage of RT-3D TEE in comparison with 2D ICE or conventional TEE is its capability of demonstrating the dynamic morphology of interatrial communications, particularly in ASDs with complex geometries, i.e. elliptical, oblong, or fenestrated shapes (27-29). RT-3D TEE is therefore an alternative to ICE. Until a decade ago, rendering three-dimensional reconstructions from multiple two-dimensional images provided (at the time) unprecedented en-face dynamic views of the IAS and interatrial communications for diagnostic purposes (30-32). In contrast to this now outdated approach, RT-3D TEE is applicable to on-line and point-of-care analysis (33), including preinterventional assessment of the particular morphology, wire placement, balloon-sizing, deployment of a suitable access sheath, device implantation, and detection of complications, e.g., device malposition and dislodgement, cardiac perforation and intrapericardial hemorrhage, device thrombosis, and device failure, erosion or arm fracture (34).
During the procedure, the “three-dimensional zoom” mode is most useful to observe the position of guide wire, sheath, and device in real time (18). The spatial relation between any interatrial communication and the occluder device to be implanted can be easily depicted at any time and from each side, e.g., on en-face views from the LA (Figure 7) and RA. Thereby, any tilting of the device or sliding of one of the discs into the communication can be ruled out with certainty. If the device is seen to impinge on a native structure, e.g., on the aortic bulb, late perforation of the aortic wall or atrial wall are distinct possibilities. In such cases, the device needs to be retrieved and redeployed. All these events are much easier to depict by RT-3D TEE than by two-dimensional imaging tools. In contrast to RT-3D ICE, RT-3D TEE (both using a three-dimensional matrix array) has been fully established as a periinterventional diagnostic and guiding tool in device closure of ASDs and “complex” PFOs. Lower costs are the main advantage of RT-3D TEE over 2D and 3D ICE. This needs to be weighed against the costs for general anesthesia, and the advantages of en-face views, which facilitate device alignment during implantation and confirmation of proper device position (33). RT-3D TEE is not only very useful for patient selection and for guiding device closure, but also for follow-up examinations. En-face views from both atria offer the best confirmation of tissue ingrowth and for displaying endotheliazation after 6 months.
Figure 7.
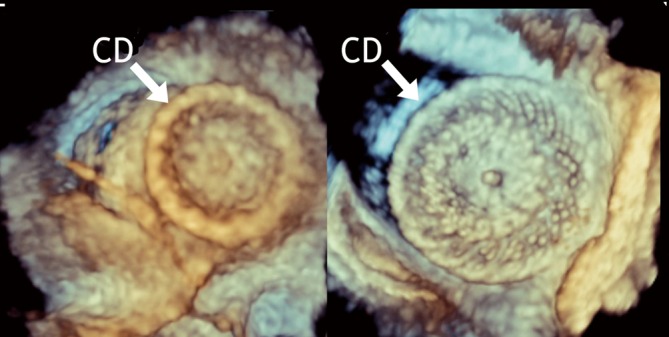
After ASD device closure: RT-3D TEE en-face view of the right-sided disc from the RA (left panel) and of the left-sided disc from the LA (right panel). CD, closure device
Conclusions
Echocardiography plays a critical role prior to, during and after percutaneous device closure of interatrial communications. The ability to visualize interatrial communications and closure devices from within the heart offers clear advantages. More importantly, as the ICE probe can be inserted percutaneously, only local anesthesia is required, sparing the patient the risks and discomfort of general anesthesia. ICE is safe and provides imaging quality beyond that of TEE. ASD and “complex” PFO closure procedures performed under ICE guidance require lower radiation doses, shorter procedure times, and are associated with shorter turnaround times than closures completed under TEE monitoring. Therefore, ICE has now become a standard tool in centers performing transcatheter device closure of “complex” interatrial communications. RT-3D TEE is a valuable alternative, especially because of its ability to provide en-face views. The impact of RT-3D TEE and ICE guidance on the outcome of transcatheter device closure remains to be elucidated. While RT-3D ICE is still under clinical testing, the costs of ICE catheters remain an important limitation. Conversely, the cost advantage is a plus for RT-3D TEE as a periinterventional guiding tool.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Silvestry FE, Kerber RE, Brook MM, et al. Echocardiography-guided interventions. J Am Soc Echocardiogr 2009;22:213-31; quiz 316-7 [DOI] [PubMed] [Google Scholar]
- 2.Luxenberg DM, Silvestry FE, Herrmann HC, et al. Use of a new 8 French intracardiac echocardiographic catheter to guide device closure of atrial septal defects and patent foramen ovale in small children and adults: initial clinical experience. J Invasive Cardiol 2005;17:540-5 [PubMed] [Google Scholar]
- 3.Hijazi ZM, Shivkumar K, Sahn DJ. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation 2009;119:587-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenig PR, Abdulla RI, Cao QL, et al. Use of intracardiac echocardiography to guide catheter closure of atrial communications. Echocardiography 2003;20:781-7 [DOI] [PubMed] [Google Scholar]
- 5.Kim SS, Hijazi ZM, Lang RM, et al. The use of intracardiac echocardiography and other intracardiac imaging tools to guide noncoronary cardiac interventions. J Am Coll Cardiol 2009;53:2117-28 [DOI] [PubMed] [Google Scholar]
- 6.Ha JW, Shin MS, Kang S, et al. Enhanced detection of right-to-left shunt through patent foramen ovale by transthoracic contrast echocardiography using harmonic imaging. Am J Cardiol 2001;87:669-71, A11. [DOI] [PubMed]
- 7.Chessa M, Carminati M, Butera G, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 2002;39:1061-5 [DOI] [PubMed] [Google Scholar]
- 8.Bartel T, Konorza T, Arjumand J, et al. Intracardiac echocardiography is superior to conventional monitoring for guiding closure of interatrial communications. Circulation 2003;107:795-7 [DOI] [PubMed] [Google Scholar]
- 9.Bartel T, Konorza T, Barbieri V, et al. Single-plane balloon sizing of atrial septal defects with intracardiac echocardiography: an advantageous alternative to fluoroscopy. J Am Soc Echocardiogr 2008;21:737-40 [DOI] [PubMed] [Google Scholar]
- 10.Mullen MJ, Dias BF, Walker F, et al. Intracardiac echocardiography guided device closure of atrial septal defects. J Am Coll Cardiol 2003;41:285-92 [DOI] [PubMed] [Google Scholar]
- 11.Koenig P, Cao QL, Heitschmidt M, et al. Role of intracardiac echocardiographic guidance in transcatheter closure of atrial septal defects and patent foramen ovale using the Amplatzer device. J Interv Cardiol 2003;16:51-62 [DOI] [PubMed] [Google Scholar]
- 12.Bartel T, Konorza T, Neudorf U, et al. Intracardiac echocardiography: an ideal guiding tool for device closure of interatrial communications. Eur J Echocardiogr 2005;6:92-6 [DOI] [PubMed] [Google Scholar]
- 13.Nkomo VT, Theuma P, Maniu VC, et al. Patent foramen ovale transcatheter closure device thrombosis. Mayo Clin Proc 2001;76:1057-61 [DOI] [PubMed] [Google Scholar]
- 14.Martín F, Sánchez PL, Doherty E, et al. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Circulation 2002;106:1121-6 [DOI] [PubMed] [Google Scholar]
- 15.Ewert P, Berger F, Nagdyman N, et al. Masked left ventricular restriction in elderly patients with septal defects: a contraindication for closure? Catheter Cardiovasc Interv 2001;52:177-80 [DOI] [PubMed] [Google Scholar]
- 16.Bartel T, Müller S.To close or not to close an atrial septal defect in ischemic cardiomyopathy. Catheter Cardiovasc Interv 2013;81:651-3 [DOI] [PubMed] [Google Scholar]
- 17.Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am J Cardiol 2004;94:690-2 [DOI] [PubMed] [Google Scholar]
- 18.Vaidyanathan B, Simpson JM, Kumar RK. Transesophageal echocardiography for device closure of atrial septal defects. JACC Cardiovasc Imaging 2009;2:1238-42 [DOI] [PubMed] [Google Scholar]
- 19.Du ZD, Koenig P, Cao QL, et al. Comparison of transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder associated with deficient versus sufficient rims. Am J Cardiol 2002;90:865-9 [DOI] [PubMed] [Google Scholar]
- 20.Rana BS, Shapiro LM, McCarthy KP, et al. Three-dimensional imaging of the atrial septum and patent foramen ovale anatomy: defining the morphological phenotypes of patent foramen ovale. Eur J Echocardiogr 2010;11:i19-25 [DOI] [PubMed] [Google Scholar]
- 21.Fazio G, Ferro G, Carità P, et al. The PFO anatomy evaluation as possible tool to stratify the associated risks and the benefits arising from the closure. Eur J Echocardiogr 2010;11:488-91 [DOI] [PubMed] [Google Scholar]
- 22.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000;55:1172-9 [DOI] [PubMed] [Google Scholar]
- 23.Rana BS, Thomas MR, Calvert PA, et al. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010;3:749-60 [DOI] [PubMed] [Google Scholar]
- 24.Davison P, Clift PF, Steeds RP. The role of echocardiography in diagnosis, monitoring closure and post-procedural assessment of patent foramen ovale. Eur J Echocardiogr 2010;11:i27-34 [DOI] [PubMed] [Google Scholar]
- 25.Hellenbrand WE, Fahcy JT, McGowan FX, et al. Transesophageal echocardiographic guidance of transcatheter closure of atrial septal defect. Am J Cardiol 1990;66:207-13 [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Shen J, Huang Y, et al. En face view of atrial septal defect by two-dimensional transthoracic echocardiography: comparison to real-time three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr 2010;23:714-21 [DOI] [PubMed] [Google Scholar]
- 27.Acar P, Saliba Z, Bonhoeffer P, et al. Influence of atrial septal defect anatomy in patient selection and assessment of closure with Cardioseal device; a three-dimenisonal transoesophageal echocardiographic reconstruction. Eur Heart J 2000;21:573-81 [DOI] [PubMed] [Google Scholar]
- 28.Franke A, Kühl HP, Rulands D, et al. Quantitative analysis of the morphology of secundum-type atrial septal defects and their dynamic change using transesophageal three-dimensional echocardiography. Circulation 1997;96:II-323-7 [PubMed] [Google Scholar]
- 29.Maeno YV, Benson LN, McLaughlin PR, et al. Dynamic morphology of the secundum atrial septal defect evaluated by three-dimensional transesophageal echocardiography. Heart 2000;83:673-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Q, Radtke W, Berger F, et al. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transesophageal echocardiography. Eur Heart J 2000;21:941-7 [DOI] [PubMed] [Google Scholar]
- 31.Magni G, Hijazi ZM, Pandian NG, et al. Two- and three-deminsional transesophageal echocardiography in patient seöection and assessment of atrial septal defect closure by the new DAS-Angel Wings Device. Initial clinical experience. Circulation 1997:96:1722-8 [DOI] [PubMed] [Google Scholar]
- 32.Marx GR, Fulton DR, Pandian NG, et al. Delineation of site, relative size and dynamic geometry of atrial septal defects real-time three-dimensional echocardiography. J Am Coll Cardiol 1995;25:482-90 [DOI] [PubMed] [Google Scholar]
- 33.Lodato JA, Cao QL, Weinert L, et al. Feasibility of real-time three-dimensional transesophageal echocardiography for guidance of percutaneous atrial septal defect closure. Eur J Echocardiogr 2009;10:543-8 [DOI] [PubMed] [Google Scholar]
- 34.Bartel T, Bonaros N, Müller S.Device failure weeks to months after transcatheter closure of secundum type atrial septal defects. Heart 2010;96:1603. [DOI] [PubMed] [Google Scholar]


