Abstract
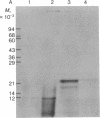
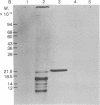
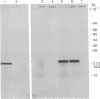
The biosynthetic origin of the 10,000 molecular weight neurophysins, carriers of the peptide hormones oxytocin and vasopressin, has been studied by cell-free synthesis, Poly(A)-RNA was isolated from bovine hypothalamus and translated in a wheat germ system containing 35S- or 3H-labeled amino acids. A number of unique [35S]cysteine- but few [35S]-methionine-labeled proteins were coded by hypothalamic mRNA. A single, major, isotopically labeled protein (molecular weight 23,000-25,000) was immunoprecipitated from these translation mixtures by addition of purified antibodies against bovine neurophysin II and subsequent addition of Cowan I strain of Staphylococcus aureus. Specificity of the immunoprecipitation was demonstrated by competition with unlabeled authentic neurophysins and the absence of competition with structurally unrelated ovalbumin. Furthermore, neither nonimmune serum nor purified antibodies against ribonuclease immunoprecipitated the protein. The [35S]cysteine-labeled protein that was specifically immunoprecipitated was oxidized with performic acid and digested with trypsin in the presence of unlabeled, authentic bovine neurophysin II. Peptide mapping revealed that most of the major [35S]cysteine-labeled peptides (of the translation product) were identical to major cysteine-containing peptides of authentic neurophysin. The data show that hypothalamic mRNA directs the translation of several unique cysteine-rich proteins in an in vitro cell-free system. Furthermore, one of these proteins, which has a higher molecular weight than authentic neurophysin, is recognized by purified antibodies to bovine neurophysin II and has cysteine-containing tryptic peptides in common with those of authentic neurophysin. The data suggest that this protein is the primary translation product, pre-pro-neurophysin.
Keywords: biosynthetic precursor, cell-free translation, specific immunoprecipitation, polyacrylamide gel electrophoresis, peptide mapping
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Breslow E. On the mechanism of binding of neurohypophyseal hormones and analogs to neurophysins. Ann N Y Acad Sci. 1975 Feb 21;248:423–441. doi: 10.1111/j.1749-6632.1975.tb34203.x. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Gainer H. Neurophysin biosynthesis in normal rats and in rats with hereditary diabetes insipidus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4046–4049. doi: 10.1073/pnas.74.9.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein M. J., Robinson A. G., Gainer H. Immunological identification of rat neurophysin precursors. Nature. 1977 Sep 15;269(5625):259–261. doi: 10.1038/269259a0. [DOI] [PubMed] [Google Scholar]
- Cohen P., Camier M., Wolff J., Alazard R., Cohen J. S., Griffin J. H. Studies of bovine neurophysin-neurohypophyseal hormone interactions. Ann N Y Acad Sci. 1975 Feb 21;248:463–479. doi: 10.1111/j.1749-6632.1975.tb34207.x. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Fischer E. A., Curd J. G., Chaiken I. M. Preparation of biologically active conjugates of bovine neurophysins and other polypeptides with multi-(poly-D,L-alanyl)--poly-L-lysine and their use to elicit antibodies. Immunochemistry. 1977 Aug;14(8):595–602. doi: 10.1016/0019-2791(77)90155-0. [DOI] [PubMed] [Google Scholar]
- Gainer H., Sarne Y., Brownstein M. J. Biosynthesis and axonal transport of rat neurohypophysial proteins and peptides. J Cell Biol. 1977 May;73(2):366–381. doi: 10.1083/jcb.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Lauber M., Camier M., Cohen P. Immunological and biochemical characterization of distinct high molecular weight forms of neurophysin and somatostatin in mouse hypothalamus extracts. FEBS Lett. 1979 Jan 15;97(2):343–347. doi: 10.1016/0014-5793(79)80118-0. [DOI] [PubMed] [Google Scholar]
- Loh Y. P. Immunological evidence for two common precursors to corticotropins, endorphins, and melanotropin in the neurointermediate lobe of the toad pituitary. Proc Natl Acad Sci U S A. 1979 Feb;76(2):796–800. doi: 10.1073/pnas.76.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell M. J., Joklik W. K., Villa-Komaroff L., Lodish H. F. Translation of reovirus messenger RNAs synthetesized in vitro into reovirus polypeptides by several mammalian cell-free extracts. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2649–2653. doi: 10.1073/pnas.69.9.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4826–4830. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- SACHS H., TAKABATAKE Y. EVIDENCE FOR A PRECURSOR IN VASOPRESSIN BIOSYNTHESIS. Endocrinology. 1964 Dec;75:943–948. doi: 10.1210/endo-75-6-943. [DOI] [PubMed] [Google Scholar]
- Sachs H., Fawcett P., Takabatake Y., Portanova R. Biosynthesis and release of vasopressin and neurophysin. Recent Prog Horm Res. 1969;25:447–491. doi: 10.1016/b978-0-12-571125-8.50013-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Audhya T. K., Walter R. Complete amino acid sequence of bovine neurophysin-I. A major secretory product of the posterior pituitary. J Biol Chem. 1978 Jul 25;253(14):5019–5024. [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R., Audhya T. K., Schlesinger D. H., Shin S., Saito S., Sachs H. Biosynthesis of neurophysin proteins in the dog and their isolation. Endocrinology. 1977 Jan;100(1):162–174. doi: 10.1210/endo-100-1-162. [DOI] [PubMed] [Google Scholar]
- Wuu T. C., Crumm S. E. Amino acid sequence of bovine neurophysin-II: a reinvestigation. Biochem Biophys Res Commun. 1976 Jan 26;68(2):634–639. doi: 10.1016/0006-291x(76)91192-x. [DOI] [PubMed] [Google Scholar]