Abstract
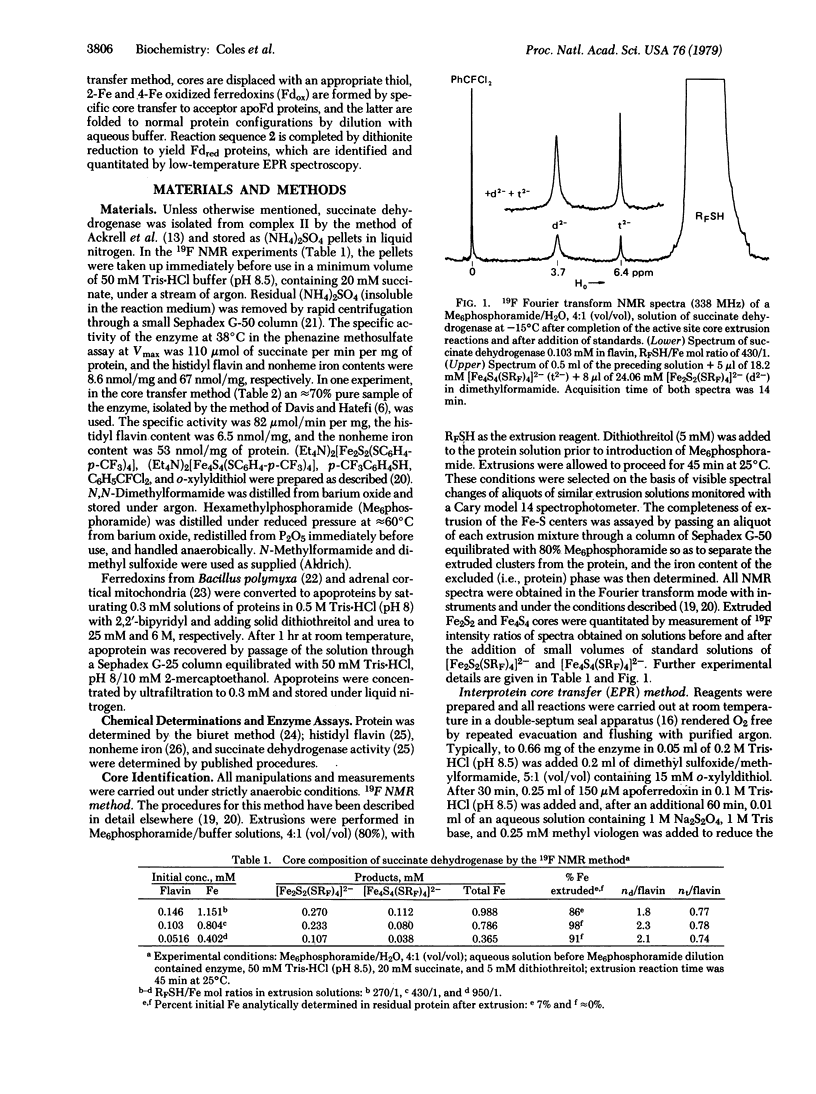
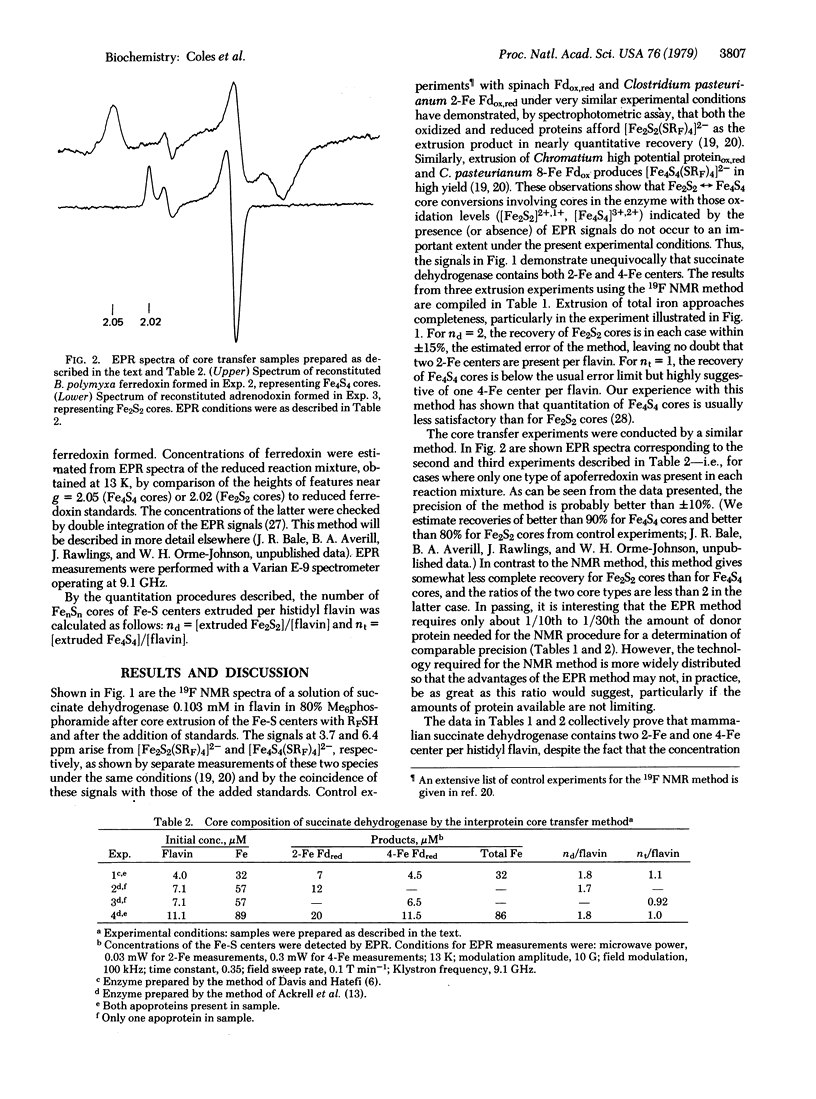
Two techniques have been applied to the determination of the number and type (2-Fe, 4-Fe) of iron-sulfur centers in the iron-sulfur flavoprotein succinate dehydrogenase [succinate:(acceptor) oxidoreductase, EC 1.3.99.1]. One procedure uses p-CF3C6H4SH as an extrusion reagent and Fourier transform 19F nuclear magentic resonance as the method of detection and quantitation of extruded cores of these centers in the form of [Fe2S2(SRF)4]2- and [Fe4S4(SRF)4]2- (RF = p-C6H4CF3). The second procedure, interprotein core transfer, involves thiol displacement of iron-sulfur cores followed by specific core transfer to the apoproteins of Bacillus polymyxa ferredoxin and adrenodoxin. Detection and quantitation are accomplished by electron paramagnetic resonance of reduced proteins at low temperatures. Both procedures clearly show that succinate dehydrogenase contains two dimeric (Fe2S2) and one tetrameric (Fe4S4) centers per mole of histidyl flavin, accounting for all eight nonheme iron and eight labile sulfur atoms found by chemical analysis. These results remove uncertainties created by the less than stoichiometric amounts of binuclear centers detected by electron paramagnetic resonance after dithionite reduction and provide secure characterization of the iron-sulfur centers in this enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Kearney E. B., Coles C. J. Isolation of reconstitutively active succinate dehydrogenase in highly purified state. J Biol Chem. 1977 Oct 25;252(20):6963–6965. [PubMed] [Google Scholar]
- Beinert H., Ackrell B. A., Kearney E. B., Singer T. P. Iron-sulfur components of succinate dehydrogenase: stoichiometry and kinetic behavior in activated preparations. Eur J Biochem. 1975 May;54(1):185–194. doi: 10.1111/j.1432-1033.1975.tb04128.x. [DOI] [PubMed] [Google Scholar]
- Beinert H., Ackrell B. A., Vinogradov A. D., Kearney E. B., Singer T. P. Interrelations of reconstitution activity, reactions with electron acceptors, and iron-sulfur centers in succinate dehydrogenase. Arch Biochem Biophys. 1977 Jul;182(1):95–106. doi: 10.1016/0003-9861(77)90287-9. [DOI] [PubMed] [Google Scholar]
- Coles C. J., Tisdale H. D., Kenney W. C., Singer T. P. Studies on succinate dehydrogenase: XXI. Quaternary structure of succinate dehydrogenase. Physiol Chem Phys. 1972;4(4):301–316. [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Dus K., De Klerk H., Sletten K., Bartsch R. G. Chemical characterization of high potential iron proteins from Chromatium and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1967 Jun 27;140(2):291–311. doi: 10.1016/0005-2795(67)90470-9. [DOI] [PubMed] [Google Scholar]
- Gillum W. O., Mortenson L. E., Chen J. S., Holm R. H. Quantitative extrusions of the Fe4S4 cores of the active sites of ferredoxins and the hydrogenase of Clostridium pasteurianum. J Am Chem Soc. 1977 Jan 19;99(2):584–595. doi: 10.1021/ja00444a044. [DOI] [PubMed] [Google Scholar]
- KEARNEY E. B., SINGER T. P. On the prosthetic group of succinic dehydrogenase. Biochim Biophys Acta. 1955 Aug;17(4):596–597. doi: 10.1016/0006-3002(55)90432-7. [DOI] [PubMed] [Google Scholar]
- LUSTY C. J., MACHINIST J. M., SINGER T. P. STUDIES ON THE RESPIRATORY CHAIN-LINKED REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE DEHYDROGENASE. VII. "LABILE" SULFIDE GROUPS IN THE DEHYDROGENASE AND IN RELATED PROTEINS. J Biol Chem. 1965 Apr;240:1804–1810. [PubMed] [Google Scholar]
- MASSEY V. Studies on succinic dehydrogenase. VII. Valency state of the iron in beef heart succinic dehydrogenase. J Biol Chem. 1957 Dec;229(2):763–770. [PubMed] [Google Scholar]
- Ohnishi T., Lim J., Winter D. B., King T. E. Thermodynamic and EPR characteristics of a HiPIP-type iron-sulfur center in the succinate dehydrogenase of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2105–2109. [PubMed] [Google Scholar]
- Ohnishi T., Salerno J. C. Thermodynamic and EPR characteristics of two ferredoxin-type iron-sulfur centers in the succinate-ubiquinone reductase segment of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2094–2104. [PubMed] [Google Scholar]
- Onishi T., Leigh J. S., Winter D. B., Lim J., King T. E. EPR studies on two ferredoxin-type iron-sulfur centers in reconstitutively active, inactive, and reactivated soluble succinate dehydrogenases. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1026–1035. doi: 10.1016/0006-291x(74)90258-7. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Reductive titrations of iron-sulfur proteins containing two to four iron atoms. J Biol Chem. 1969 Nov 25;244(22):6143–6148. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B., ZASTROW N. Isolation and properties of succinic dehydrogenase. Biochim Biophys Acta. 1955 May;17(1):154–155. doi: 10.1016/0006-3002(55)90340-1. [DOI] [PubMed] [Google Scholar]
- Salerno J. C., Ohnishi T. Tetranuclear and binuclear iron-sulfur clusters in succinate dehydrogenase: a method of iron quantitation by formation of paramagnetic complexes. Biochem Biophys Res Commun. 1976 Dec 6;73(3):833–840. doi: 10.1016/0006-291x(76)90884-6. [DOI] [PubMed] [Google Scholar]
- Singer T. P. Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem Anal. 1974;22:123–175. doi: 10.1002/9780470110423.ch3. [DOI] [PubMed] [Google Scholar]
- Strombaugh N. A., Burris R. H., Orme-Johnson W. H. Ferredoxins from Bacillus polymyxa. Low potential iron-sulfur proteins which appear to contain single four iron, four sulfur centers accepting a single electron on reduction. J Biol Chem. 1973 Nov 25;248(22):7951–7956. [PubMed] [Google Scholar]
- Walker W. H., Singer T. P. Identification of the covalently bound flavin of succinate dehydrogenase as 8-alpha-(histidyl) flavin adenine dinucleotide. J Biol Chem. 1970 Aug 25;245(16):4224–4225. [PubMed] [Google Scholar]


