Abstract
Purpose
To determine associations between specific colonoscopy patient characteristics, exam characteristics and patients’ perception of colonoscopy reducing their risk of dying from colorectal cancer.
Methods
A cross-sectional analysis was conducted using data (2004–2008) from the New Hampshire Colonoscopy Registry, consisting of a self-report questionnaire, colonoscopy report form, and a follow-up questionnaire, which included the question, “Having a colonoscopy decreased my chances of dying from colon cancer”. Chi-square tests and logistic regression were used to assess differences in patient responses by patient and colonoscopy characteristics.
Results
A majority of patients (N=5672, 81%) agreed that having a colonoscopy decreased their chances of dying from colon cancer. Patients with a personal history of polyps were more likely to agree that colonoscopy reduced their chances of dying compared to patients without prior polypectomy [OR (95% CI) = 1.34 (1.06, 1.69)] and patients with a family history of colorectal cancer were 33% more likely to agree to the statement than those without a family history [OR (95% CI) = 1.33 (1.12, 1.58)].
Conclusions
Personal history of polyps and family history of colorectal cancer are significant predictors of patients’ positive perception of colonoscopy, suggesting that personal experience may influence the perceived benefit of colonoscopy. Intervention efforts should be made to effectively disseminate knowledge of the benefit of colonoscopy.
Keywords: colonoscopy, colorectal cancer, screening, patient perception
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States, but only half of the recommended population receives CRC screening.[1] The US Preventive Services Task Force (USPSTF) has given CRC screening a grade of A in terms of evidence to support this type of screening, and recommends fecal occult blood testing, sigmoidoscopy, or colonoscopy among adults beginning at age of 50 (for those at average risk) and continuing until age 75.[2] Screening guidelines have also been published by the American Cancer Society and the Multi-Society Task Force;[3] these outline options for both screening and surveillance. The American Society of Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology recommends colonoscopy as the optimal method for CRC screening;[4, 5] this is currently the most widely used method.
Currently, colonoscopy is the only screening method able to prevent CRC through polypectomy, or the removal of polyps. Primary care providers (PCPs) are responsible for appropriately screening and educating their patients about CRC, including understanding the preventive benefit of colonoscopy. Improved understanding of patients’ perceptions, particularly in relationship to motivation to undergo colonoscopy, would be essential to increasing patient compliance with screening.
Studies have investigated the relationships between specific patient characteristics- such as family and health history- and knowledge about screening in general[6] as well as understanding of CRC risk.[7, 8] However, the relationship between individual colonoscopy patients’ characteristics, exam characteristics (including polyp findings), and their perception of the benefit of colonoscopy screening is still unclear. Studies addressing patient perceptions are useful in understanding motivations to undergo colonoscopy and would inform the widespread efforts to increase colorectal cancer screening currently underway in the US.
Objective
The aim of this study was to examine the relationship between specific patient characteristics and colonoscopy results to their perceptions of having reduced their risk of dying of CRC following a colonoscopy. Importantly, this study provides information about patients’ perceptions of the benefit of their recent colonoscopy, which will help identify areas in need of targeted education programs.
Research Methods
Overview
Data for this study was provided by the New Hampshire Colonoscopy Registry (NHCR) - a statewide colonoscopy registry funded by the National Cancer Institute that began (initially through a pilot project) in 2004 and consists of consenting colonoscopy patients at participating registry sites. The registry collects data from a patient self-report questionnaire at the time of colonoscopy, an endoscopist colonoscopy report form, and a patient follow-up letter. The information collected is then linked to individual pathology reports. Details about this ongoing registry have been previously published.[9]
For this analysis, data was collected between November 1, 2004 and September 30, 2008 from 2 sites with 14 participating endoscopists. During this time period, 17,095 follow-up questionnaires were sent to consenting patients approximately 30 days post colonoscopy. A total of 9,101 questionnaires (51%) were returned within 2 weeks of sending. A second mailing was not done.
The patient self-report questionnaire, which is completed prior to colonoscopy, is comprised of patient demographics (age, education, race or ethnic background, marital status, health insurance), health history (personal and family history of CRC or polyps, inflammatory bowel syndrome or other cancers), and additional health history (vitamins and supplements, cigarette smoking, alcohol, and exercise), as well as endoscopy history. Immediately after colonoscopy, endoscopists recorded data using an NHCR standardized colonoscopy report form, including exam indication, findings of the exam including number, location, and method of removal for all polyps, and location of suspected cancer (if any), preparation type, qualitative score of preparation success, type of sedation medications used, end of procedure status, withdrawal time, complications, and follow-up recommendations including “pending pathology results”. The self-administered follow-up questionnaire was mailed to NHCR participating patients within one month after the procedure. The questionnaire consisted of questions about the overall perceptions of CRC risk, overall quality of care received and attitudes towards the procedure itself, including self assessment of quality of procedure, willingness to recommend colonoscopies to family and friends, and whether having a colonoscopy decreased the risk of dying from colon cancer. All data collection and procedures were approved by the Committee for Protection of Human Subjects. The protocol was developed in accordance with research guidelines and approved by the Institutional Review Board.
Patients
Clinically, patients undergoing diagnostic colonoscopies (for symptoms or signs) would not be expected to enhance their knowledge or acceptance of screening: therefore 1,402 (15%) of the 9,101 patients with a diagnostic exam indication were removed from the analysis. An additional 42 (<1%) patients were removed due to lack of current consent at the time of analysis and 418 (5%) because we did not have both a patient self report questionnaire and a matching endoscopist’s colonoscopy report form. Following the American Cancer Society and American College of Gastroenterology guidelines for colorectal cancer screening minimum age recommendations [4, 5] 123 patients who were under the age of 40 were excluded. These exclusions resulted in a screening and surveillance population of 7,116 (78%) patients included in the final analysis.
Outcome
We assessed colonoscopy patients’ perception about whether they had reduced their risk of dying of CRC by using the results of survey responses to the following statement, “Having a colonoscopy decreased my chances of dying from colon cancer.” The measured responses were based on a Likert scale: “strongly agree”, “moderately agree”, “neutral”, “moderately disagree”, and “strongly disagree”. We created a dichotomized outcome by combining strongly and moderately agree responses as a single “agree” response and the neutral, strongly and moderately disagree responses as a single “disagree” response.
Patient Characteristics
Age, sex, race, personal history of CRC, family history of CRC, personal history of polyps, education and information that this exam was the first colonoscopy were taken from the patient self report used in the analysis. Race was dichotomized into two groups: white and non-white. The non-white group consisted of Hispanic, Spanish, Latin, African American, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander and Other. Personal history of CRC was determined by a positive response of “exam for personal history of colon cancer” to the question, ‘What is the reason for your visit today?’ or a positive result of colon or rectal cancer from a previous colonoscopy or sigmoidoscopy. Family history of CRC was derived from responses to a series of health history questions about first-degree relatives’ diagnoses of CRC. Information about personal history of polyps was taken from patients’ reply to ‘Have you ever had polyps?’. Information that this was a first colonoscopy was created from a positive response of ‘Never, this is my first’ to the question ‘When was your last colonoscopy?’.
From the endoscopist’s colonoscopy report form, indication for exam was classified as screening (average risk), surveillance (personal history), diagnostic, and no indication given for exam. We defined a screening exam if the endoscopist indicated a screening exam for family history of colon cancer or polyps or a screening exam with no symptoms or family history. An indication for exam that included either a personal history of colon cancer or personal history of polyps, or familial adenomatous polyposis (FAP) or hereditary non-polyposis colon cancer (HNPCC) was defined as surveillance. Indications including possible gastrointestinal (GI) bleeding, change in bowel habits (diarrhea/constipation), abdominal pain, biopsy of suspected cancer, positive fecal occult blood test, polypectomy of known polyp, iron deficiency anemia or other signs or symptoms were classified as diagnostic exams. When no indication was given then a ‘No indication’ category was defined. We defined current polypectomy if the endoscopist reported reaching the cecum or terminal ileum and at least one polyp, indicated by polyp location, size and treatment was found.
Statistical Analysis
Descriptive statistics and frequency distributions were used to evaluate the associations between patient and colonoscopy characteristics and patients’ follow-up questionnaire responses to perception of reducing risk of dying from CRC. Chi-square tests assessed differences between patient perception responses by patient and colonoscopy characteristics and a p-value = 0.05 or lower was considered statistically significant. A Spearman rank correlation was used to measure collinearity among several covariates and indication for exam. We modeled the odds ratio (OR) for ‘Agree’ v. ‘Disagree’ and 95% confidence intervals (CI) of patient’s response of reduced risk of dying from CRC using logistic regression to examine the influence of particular patient and colonoscopy factors of interest. All analyses were conducted using SAS 9.2 (Cary, NC).
Results
The median age of the study population was 59 years with ages ranging from 40 to 94 years and a calculated mean (±SD) age of 60 (±9). More patients were female (55%) and the majority were highly educated, with 78% of patients having some college or a college/post graduate degree. As is typical of New Hampshire, race was predominantly white (98%) with 20% of patients reporting a family history of colorectal cancer, 3% stating a personal history of colorectal cancer and 27% indicating a personal history of polyps. Forty percent of patients stated that this was their first colonoscopy. Endoscopists indicated 63% of the exams were screening, followed by 22% surveillance and 15% had no indication for the exam given. Within the surveillance group, 86% of the patients reported a history of polyps (data not shown). For those patients whose colonoscopy reached the cecum, 34% had a current polypectomy (see Table I).
Table I.
Distribution of Patient and Colonoscopy Characteristics: Total and by Patient Agreement that Colonoscopy Reduces Chance of Dying of CRC
| Total (N=7116) N (Col %) | Having a colonoscopy has decreased my chances of dying from colon cancer* | |||
|---|---|---|---|---|
| Disagree (N=1358) N (Row %) | Agree (N=5672) N (Row %) | p-value | ||
| Patient Characteristic* | ||||
| Age | 0.02 | |||
| 40–49 | 439 (6.2) | 98 (22.5) | 338 (77.5) | |
| 50–59 | 3277 (46.1) | 662 (20.4) | 2583 (79.6) | |
| 60–69 | 2128 (29.9) | 368 (17.5) | 1732 (82.5) | |
| 70 + | 1272 (17.9) | 230 (18.4) | 1019 (81.6) | |
| Sex | 0.67 | |||
| Female | 3856 (54.9) | 741 (19.5) | 3069 (80.6) | |
| Male | 3165 (45.1) | 596 (19.1) | 2533 (80.9) | |
| Race | 0.20 | |||
| Non-White | 161 (2.4) | 37 (23.0) | 124 (77.0) | |
| White | 6571 (97.6) | 1234 (19.0) | 5258 (81.0) | |
| Personal History CRC | 0.12 | |||
| No | 6864 (97.0) | 1319 (19.4) | 5471 (80.6) | |
| Yes | 215 (3.0) | 31 (15.1) | 174 (84.9) | |
| Family History of CRC | <0.0001 | |||
| No | 5643 (79.7) | 1128 (20.3) | 4441 (79.8) | |
| Yes | 1436 (20.3) | 222 (15.6) | 1204 (84.4) | |
| Personal History of Polyps | <0.0001 | |||
| No | 3169 (47.1) | 637 (20.3) | 2494 (79.7) | |
| Yes | 1791 (26.6) | 255 (14.4) | 1520 (85.6) | |
| Unknown | 1767 (26.3) | 383 (22.0) | 1359 (78.0) | |
| Education | 0.47 | |||
| Less than High School | 200 (3.0) | 45 (23.1) | 150 (76.9) | |
| High School graduate | 1218 (18.5) | 222 (18.5) | 981 (81.6) | |
| Some college | 1483 (22.6) | 276 (18.8) | 1189 (81.2) | |
| College or post-graduate | 3673 (55.9) | 704 (19.4) | 2929 (80.6) | |
| Total (N=72116) N (Col %) | Having a colonoscopy has decreased my chances of dying from colon cancer* | |||
|---|---|---|---|---|
| Disagree (N=1358) N (Row %) | Agree (N=5672) N (Row %) | p-value | ||
| First colonoscopy | <0.0001 | |||
| No | 4058 (59.7) | 701 (17.5) | 3310 (82.5) | |
| Yes | 2736 (40.3) | 600 (22.2) | 2102 (77.8) | |
| Colonoscopy Characteristic* | ||||
| Exam Indication | <0.0001 | |||
| Screening | 4496 (63.2) | 919 (20.7) | 3527 (79.3) | |
| Surveillance | 1540 (21.6) | 224 (14.7) | 1296 (85.3) | |
| No Indication | 1080 (15.2) | 215 (20.2) | 849 (79.8) | |
| Current Polypectomy | 0.07 | |||
| No | 4666 (65.6) | 918 (19.9) | 3687 (80.1) | |
| Yes | 2450 (34.4) | 440 (18.1) | 1985 (81.9) | |
Missing (N, %): Sex (95, 1%), Race (384, 5%), Personal History of CRC (37, <1%), Family History of CRC (37, <1%), Personal History of Polyps (389, 5%), Education (542, 8%), First Colonoscopy (322, 5%). Having a colonoscopy has decreased my chances of dying from colon cancer (86, 1%).
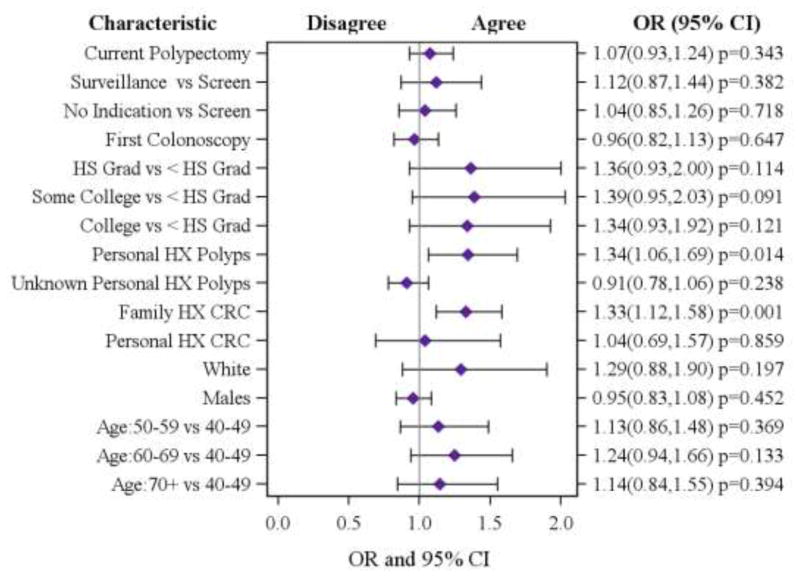
Of the 7,116 patients in the study population, a majority (N=5,672, 81%) agreed that having a colonoscopy decreased the chances of dying from CRC. Significant differences were found in patient agreement among age groups, family history of CRC, personal history of polyps, first colonoscopy and exam indication (see Table I). The fully adjusted model revealed that patients with a personal history of polyps were more likely to agree that a colonoscopy reduced their chances of dying from CRC compared to those patients without prior polyps (OR (95% CI) =1.34 (1.06, 1.69); p=0.01, see Figure 1). Patients reporting a family history of CRC were 33% more likely to agree that having a colonoscopy reduces chances of dying of CRC than those without a family history (OR (95% CI) =1.33 (1.12, 1.58); p=0.001). Patients having a current polypectomy (polyps removed at the time of the colonoscopy) were just as likely to agree and/or disagree about reduced risk of dying from CRC after having a colonoscopy as those without a current polypectomy (OR (95% CI) =1.07 (0.93, 1.24); p=0.34). While significant in the unadjusted analysis, age, exam indication and self-reported first colonoscopy were not significant in the fully adjusted model (Figure 1).
Figure 1. Patient Agreement that Colonoscopy Reduces Chance of Dying of CRC According to Patient and Colonoscopy Characteristics*.
*Model is fully adjusted for patient and colonoscopy characteristics; HS: High School, HX: History.
Discussion
This study shows that personal history of polyps and family history of CRC are moderately strong predictors of patients’ positive perception of the benefits of colonoscopy. However, patients who had a polypectomy at the time of their current colonoscopy were not any more likely than those who did not have a polyp to agree that having a colonoscopy had reduced their chances of dying from CRC. It appeared that post (30 days) polypectomy patients did not yet understand the preventive effect of polypectomy. These results suggest that patients without a prior experience of a polypectomy or a family history of CRC may not fully understand the key benefit of colonoscopy screening. In contrast, individuals with a previous polyp(s) and/or have a family history of CRC have had the opportunity to receive education from a variety of sources (including their health care provider during discussion of the need for a follow-up surveillance exam), therefore may have more strongly agreed that having a colonoscopy reduced their risk of dying of CRC, while those with a current polypectomy have not had this longitudinal opportunity for patient education. Since the primary benefit of colonoscopy is the prevention of CRC through polypectomy, our study results suggest that patients without personal history of polyps and/or a family history of CRC, which is approximately two-thirds of our study population, do not initially fully understand (or are not being adequately informed) that having a colonoscopy had the potential to reduce their risk of dying from CRC through the removal of polyps. Fortunately, over time this important recognition of the ability for polypectomy to prevent cancer appears to evolve. Efforts to ensure early and consistent understanding of the benefits of prevention could have major impact on patient compliance with both screening and surveillance; future studies may elucidate effective interventions to accomplish this goal.
In our study, we assessed patient perception by determining patients’ attitudes towards colonoscopy as an effective mortality-reducing procedure, as opposed to individual perceptions and knowledge of CRC risk in general, which has been more clearly studied in the literature.[10–13] Although positive correlations between perception of risk of dying of CRC and patient perception of the benefit of colonoscopy are likely, further research is needed to develop a broader framework to address patient perception. It is important to note, as our study suggests, that patient attitudes about their own experience and risk may be a stronger predictor of motivating behavior than generalized knowledge of benefits of screening. The premise of the perception question in this study relied on the self-perception of the specific individual (“reduced my risk”), and not on the effectiveness of colonoscopy in lowering mortality in general. However, one limitation of the study is that some subjects may have based their answers on the general effectiveness of colonoscopy, unintentionally misrepresenting the data collected.
The perceived benefit of colonoscopy, as shown in our study, varies despite the general consensus of its effectiveness, suggesting the preventive benefit of colonoscopy for CRC screening message has not been adequately disseminated. In order to improve screening behaviors, interventions aimed at changing individual perceptions, such as risk communication methods that employ anecdotal evidence of success[14] and interactive computer-based educational programs[15] can be utilized and have been shown to be effective. However, more importantly, patients need to be better informed about the difference between screening for early detection of cancer such as mammography or prostate testing and colonoscopy screening for early detection and, even more significantly, prevention through polypectomy.
Primary care physician recommendations for screening are powerful predictors for patient screening behaviors.[16–19] Educating providers about colonoscopy screening is a critical step in improving the dissemination of educational efforts to inform patients. With increased provider and patient targeted education interventions, one can expect improved patient colonoscopy referral practices, better patient adherence to screening, and ultimately improvement in screening rates among target populations.
Although CRC incidence and mortality has decreased in recent years, screening remains underutilized. Efforts need to be made to continually communicate and improve upon the dissemination of knowledge about prevention of CRC through colonoscopy.
Acknowledgments
Grant funding provided by the National Cancer Institute: #5R01CA121141
Footnotes
No financial disclosures were publishable by the authors of this paper
References
- 1.Neugut AI, Lebwohl B. Screening for colorectal cancer: the glass is half full. Am J Public Health. 2009;99(4):592–4. doi: 10.2105/AJPH.2008.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63(4):546–57. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 6.Shokar NK, Vernon SW, Weller SC. Cancer and colorectal cancer: knowledge, beliefs, and screening preferences of a diverse patient population. Fam Med. 2005;37(5):341–7. [PubMed] [Google Scholar]
- 7.Buc E, Kwiatkowski F, Alves A, Panis Y, Mantion G, Slim K. Tobacco smoking: a factor of early onset of colorectal cancer. Dis Colon Rectum. 2006;49(12):1893–6. doi: 10.1007/s10350-006-0704-1. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman DA, Prindiville S, Weiss DG, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959–67. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]
- 9.Carney P, LB, Goodrich M, JW, Dietrich A. Design and Development of a Population-based Colonoscopy Registry. Journal of Registry Management. 2006;33(4):91–99. [Google Scholar]
- 10.McQueen A, Vernon SW, Meissner HI, Klabunde CN, Rakowski W. Are there gender differences in colorectal cancer test use prevalence and correlates? Cancer Epidemiol Biomarkers Prev. 2006;15(4):782–91. doi: 10.1158/1055-9965.EPI-05-0629. [DOI] [PubMed] [Google Scholar]
- 11.Robb KA, Miles A, Wardle J. Demographic and psychosocial factors associated with perceived risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(3):366–72. [PubMed] [Google Scholar]
- 12.Robb KA, Miles A, Wardle J. Subjective and objective risk of colorectal cancer (UK) Cancer Causes Control. 2004;15(1):21–5. doi: 10.1023/B:CACO.0000016567.82368.6c. [DOI] [PubMed] [Google Scholar]
- 13.Stark JR, Bertone-Johnson ER, Costanza ME, Stoddard AM. Factors associated with colorectal cancer risk perception: the role of polyps and family history. Health Educ Res. 2006;21(5):740–9. doi: 10.1093/her/cyl049. [DOI] [PubMed] [Google Scholar]
- 14.Lipkus IM, Green LG, Marcus A. Manipulating perceptions of colorectal cancer threat: implications for screening intentions and behaviors. J Health Commun. 2003;8(3):213–28. doi: 10.1080/10810730305684. [DOI] [PubMed] [Google Scholar]
- 15.Schroy PC, 3rd, Glick JT, Wilson S, Robinson PA, Heeren TC. An effective educational strategy for improving knowledge, risk perception, and risk communication among colorectal adenoma patients. J Clin Gastroenterol. 2008;42(6):708–14. doi: 10.1097/MCG.0b013e3180500318. [DOI] [PubMed] [Google Scholar]
- 16.Fenton JJ, Reid RJ, Baldwin LM, Elmore JG, Buist DS, Franks P. Influence of primary care use on population delivery of colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2009;18(2):640–5. doi: 10.1158/1055-9965.EPI-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009;37(1):8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shieh K, Gao F, Ristvedt S, Schootman M, Early D. The impact of physicians’ health beliefs on colorectal cancer screening practices. Dig Dis Sci. 2005;50(5):809–14. doi: 10.1007/s10620-005-2644-3. [DOI] [PubMed] [Google Scholar]
- 19.Ye J, Xu Z, Aladesanmi O. Provider recommendation for colorectal cancer screening: examining the role of patients’ socioeconomic status and health insurance. Cancer Epidemiol. 2009;33(3–4):207–11. doi: 10.1016/j.canep.2009.07.011. [DOI] [PubMed] [Google Scholar]