Abstract
Purpose
The optimal management for clinical stage T3 and T4 (N0, M0) prostate cancer is uncertain. Herein we update the results with ten-year data of a phase II prospective trial of neoadjuvant hormonal therapy with goserelin acetate and flutamide followed by radical prostatectomy for locally-advanced prostate cancer (SWOG 9109).
Materials and Methods
62 patients with clinical stage T3 and T4 (N0, M0) prostate cancer were enrolled. Cases were classified by stage T3 versus T4 and by volume of disease (bulky > 4 cm and non-bulky ≤ 4 cm).
Results
A total of 55 of 61 eligible patients completed the trial with radical prostatectomy after neoadjuvant androgen deprivation therapy (ADT). The median pre-operative PSA value was 19.8 ng/ml, and 67% of patients had a Gleason score of 7 or higher. Among 41 patients last known to be alive, median follow-up is 10.6 years (range 5.1–12.6 years). In all, 38 patients have had disease progression (30/55, 55%) or died without progression (8/55, 15%) for a ten-year PFS estimate of 40% (95% CI, 27–53%). Median progression-free survival (PFS) was 7.5 years, and median survival has not been reached. The ten-year overall survival (OS) estimate is 68% (95% CI, 56–80%).
Conclusions
In this small, prospective phase II study, neoadjuvant hormonal therapy with goserelin acetate and flutamide followed by radical prostatectomy achieves long-term PFS and OS comparable to alternative treatments. This approach is feasible and may be an alternative to a strategy of combined radiation and ADT.
Keywords: prostate cancer, PSA, radical prostatectomy, survival, locally-advanced
Introduction
Locally advanced prostate cancer can be treated in a number of different ways, and it is most commonly treated with combined radiation therapy and androgen deprivation therapy (ADT), or, on occasion, with surgery. With level 1 evidence confirming the effectiveness of neoadjuvant hormonal ablation followed by external beam radiation therapy yielding a 5-year PFS rate of 74%, most patients with locally advanced disease undergo the combination of ADT and radiation rather than surgery.1 Indeed, a review of the SEER Medicare database found that 54% of locally advanced prostate cancer patients undergo radiation therapy, while only 6% of these patients undergo surgery.2 Surgical series, which are largely retrospective in nature, show 5-year progression-free survival (PFS) in the 70–85% range,3 with 10-year PFS rates in the 15–73% range, which, when combined with secondary therapies such as ADT and adjuvant radiation, lead to a 15-year cause-specific survival (CSS) rate of 84%.4–6 Current National Comprehensive Cancer Network (NCCN) Guidelines for prostate cancer recommend ADT plus radiation, radical prostatectomy, or ADT alone for the treatment of locally advanced prostate cancer.7 A number of other treatments have been used with published 5-year PFS rates in the 40–70% range, including cryoablation of the prostate, high dose radiation, and combined interstitial seed implant brachytherapy plus external beam radiation therapy.8, 9
In 2002, we published the results of a phase II feasibility study of neoadjuvant hormonal therapy with goserelin acetate and flutamide followed by radical prostatectomy for T3 and T4 (N0, M0) prostate cancer with a median follow-up of 6.1 years.10 We found 5-year PFS and overall survival (OS) rates of 70% and 90%, respectively, in these patients, many of whom would have otherwise been considered unresectable and referred for radiation therapy. Herein we update these results with a median follow-up of 10.6 years with estimated 10-year outcomes. As the optimal long-term approach to treating these locally advanced tumors is unknown, these represent unique prospective data for long-term surgical cases with a median follow-up greater than 10 years and in the context of concomitant androgen deprivation therapy.
Methods
Patient population
Eligible patients had histologically proven prostate cancer stage T3 or T4 prostate cancer (T3-4N0M0). Either computerized tomography, magnetic resonance imaging, or lymphangiogram were required to exclude pelvic lymphadenopathy. Bidimensionally measurable disease by transrectal ultrasound and a baseline PSA were required prior to registration. Patients were stratified into bulky versus nonbulky disease using transrectal ultrasound, digital rectal examination (DRE) and/or cystoscopy. Bulky disease was defined as a tumor greater than 4 cm in diameter, invasion of the bladder on transrectal ultrasound or cystoscopy, or involvement of more than 50% of the gland with cancer. Transrectal ultrasound was performed prior to treatment, and at 9 and 17 weeks after study entry. Digital rectal examinations were performed prior to study entry and at 5, 9, 13, and 17 weeks of follow-up.
No prior treatment was allowed prior to study enrollment. Those patients who ultimately underwent radical prostatectomy could have subsequent treatment with radiation or ADT only after biochemical failure, which was a postoperatively measured PSA of ≥ 0.1 ng/ml or clinical and/or radiologic evidence of local recurrence or distant metastasis. A resectable prostate was defined as clear delineation of the lateral borders of the prostate on DRE, the margin at the bladder neck had to be distinguishable on transrectal ultrasound or cystoscopy, and at the prostatic apex no disease involvement of the urethra or external sphincter was allowed.
Treatment
Neoadjuvant total androgen blockade was initiated within 1 month of histologic diagnosis of prostate cancer. Goserelin 3.6 mg subcutaneously monthly and flutamide 250 mg twice daily were administered for 4 months. The flutamide dose could be reduced to 125 mg daily for grade II diarrhea. For severe hot flashes, the goserelin could be withheld until symptoms diminished to grade 2 or less and was then resumed. Subjects were removed from protocol treatment for unacceptable toxicity or disease progression defined as a new or enlarging tumor mass in the prostate, ureteral obstruction with hydronephrosis, biopsy proven pelvic metastases, increase in PSA, and development of bony metastasis or histologic evidence of extrapelvic prostate cancer metastasis.
Statistics
The primary end point of the original study was resectability rate. We assumed that a true resectability rate less than 40% would not be of further interest, while a true resectability rate of 60% or greater in these locally advanced cases would be of considerable interest. A 2-stage design was used with an initial 30 cases accrued, and if less than 12 cases were resectable, the study would be closed. An additional 25 patients were accrued with that end point met, and of the 55 accrued, 29 or more being resectable without toxicity and with favorable survival, further study would be warranted. The design had a significance level of 3.8% and a power of 89%. A total of 55 cases were sufficient to estimate the resectability rate and probability of response, which was 1-year PFS or a particular toxicity within ± 13% (95% CI). PFS was defined from the date of registration to the first evidence of biochemical, local, or distant disease progression, or death in the absence of disease progression. Those alive were censored at their last contact date. Biochemical progression was defined as 2 PSA levels at least 3 months apart indicating an increase of 50% or more above the nadir PSA level following radical prostatectomy. All eligible patients are included in the PFS and OS analyses regardless of surgery status.10
Results
A total of 62 patients were accrued to SWOG 9109 from December 1993 to October 1996. Of these patients, 1 patient was ineligible due to a CT scan being performed outside of the specified time frame, 2 ultimately refused surgery after ADT, and 4 were no longer surgical candidates after the 4 months of ADT. Patient characteristics both pre- and post-operatively are displayed in Table 1. The median subject age at enrollment was 62.0 years (range 50–70), with 72% white, 20% non-Hispanic black, 7% Hispanic, and 2% Asian or Pacific Islander. The clinical stage distribution was cT3 in 97% and cT4 in 3%, and disease was classified as bulky in 39% and non-bulky in 61%. Median pre-study PSA was 19.8 ng/ml (range 2.6–407.8), and biopsy Gleason score was 6 or less in 33%, 7 in 33%, 8 in 14%, and 9–10 in 20%. After 4 months of combined ADT, 98% (54) of patients had a subjective tumor size reduction by DRE, and 55% (30) had an undetectable PSA (defined as < 0.1 ng/ml).
TABLE 1.
Patient Characteristics
| Goserelin Acetate + Flutamide | |
|---|---|
| No. Patients | 61 |
| Median Patient Age (range) | 62.0 (50–70) |
| No. race (%): | |
| White | 44 (72) |
| Black (non-Hispanic) | 12 (20) |
| Hispanic | 4 (7) |
| Asian or Pacific Islander | 1 (2) |
| No. stage (%): | |
| T3 | 59 (97) |
| T4 | 2 (3) |
| No. bulky disease (%): | |
| Yes | 24 (39) |
| No | 37 (61) |
| Median pre-study PSA (range) | 19.8 (2.6–407.8) |
| Undetectable PSA (<0.1 ng/ml) pre-op (%) | 30 (55) |
| No. pre-study Gleason sum (%): | |
| 6 or less | 19 (33) |
| 7 | 19 (33) |
| 8 | 8 (14) |
| 9–10 | 11 (20) |
Of the 61 eligible patients, 55 (90%) went on to undergo radical prostatectomy. Of those patients, 54 had pathologic data available for assessment of extent of disease, with 50 having complete information. 9/50 (18%) had Gleason score < 7, 26/50 (52%) had Gleason score 7–10, and 15/50 (30%) had no Gleason score assigned after treatment recorded, as neoadjuvant ADT often renders prostate cancer histologically uninterpretable. Organ confined disease was present in 28/50 (56%) of cases, while 21/50 (42%) had extraprostatic extension, 14/50 (28%) had seminal vesicle invasion, and 9/50 (18%) had lymph node metastasis. One intra-operative complication (2%) occurred with a recognized and repaired rectal injury and 42/54 patients (78%) of patients were continent at last follow-up. One post-operative death for unknown reasons occurred.
Of the 61 eligible patients, 20 have died. Of the deaths, 1/20 (3%) occurred perioperatively (in the recovery room post-prostatectomy) and 2/20 (5%) were confirmed deaths due to prostate cancer. 5/20 (25%) were died of unrelated causes (unrelated cancers 3/20, fire 1/20, auto accident 1/20). 12/20 (55%) were confirmed deaths from unknown causes. Of the 41 patients last known to be alive as of January 24, 2008, the median follow-up is 10.6 years (range 5.1–12.6 years; interquartile range 9.3, 11.2 years).
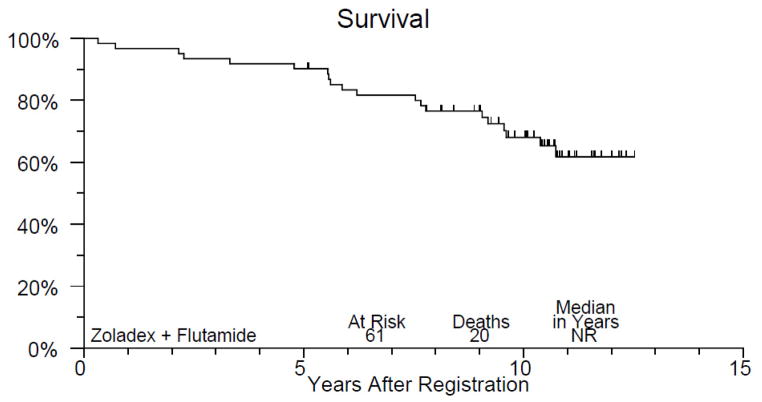
Figure 1 displays PFS of study patients. A total of 38 patients have had disease progression and the 10-year PFS estimate is 40% (95% CI, 27–53%). The median time to progression or death without progression was 7.5 years. Figure 2 displays OS of study patients. A total of 20 patients have died and the 10-year overall survival estimate is 68% (95% CI, 56–80%). Median survival in this group has not yet been reached.
Figure 1.
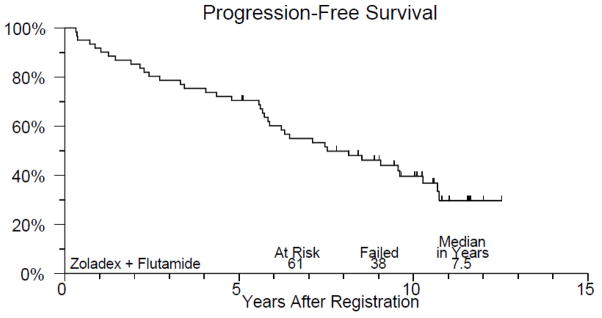
Kaplan Meier progression-free survival plot. Median time to progression or death without progression 7.5 years; 10-year progression-free survival estimate 40%.
Figure 2.
Kaplan Meier overall survival plot for study patients. 10-year survival estimate 68%; median survival has not yet been reached.
Discussion
In the PSA era, radical prostatectomy has achieved excellent cancer control rates in low-risk patients (clinical stage T1 and T2) with impressive long-term PFS and OS.11
The management of clinical stage T3 and T4 disease (N0, M0), remains more challenging, with competing approaches showing mixed results. Across treatment modalities, as would be expected, recurrence rates are high and OS is compromised by cancer recurrence. Radiation therapy remains the most frequently used modality in this challenging setting. Data from a phase 3 clinical trial show that the combination of 3 years of ADT with radiation therapy yields superior outcomes than radiation alone for patients with clinical stage T3-4, N0-1, M0 prostate cancer. A statistically significant improvement in PFS and OS over external beam radiation therapy alone was noted with 5-year rates of 74% and 78% and 8-year 55% and 70%, respectively.1
One negative consequence to this approach is the prolonged exposure to ADT with its recognized negative metabolic consequences such as impaired bone health, obesity, and diabetes.12
Radical prostatectomy is an accepted alternative according to NCCN guidelines and is being offered more frequently to patients with local disease yielding comparable PFS and OS rates to combined ADT and external beam radiation therapy.3 In the series of Loeb and colleagues, 123 high risk patients with cT2b and T3 underwent radical prostatectomy with 7- and 10-year PFS of 39% and 35% respectively with CSS of 92% and 88%. Interestingly, cT3 patients fared worse in this setting, with a 10-year PFS of 15% and OS of 63%. Preservation of potency and continence was favorable in this series at 64% and 92%, but more than half of all patients required adjuvant ADT and radiation therapy at recurrence.4 In the series of Freedland 15-year follow-up of patients with cT3a disease who were treated with radical prostatectomy, PFS was 49% and CSS was 84%, with 46% of patients requiring adjuvant ADT or radiation therapy.5 Evidence from a large, phase 3 clinical trial has demonstrated the such adjuvant radiation therapy for high risk pathologic features does improve PFS and OS outcomes (HR 0.71 and 0.72), but at the cost of increased Grade II and III side effects.13, 14
Our data, a prospective phase II clinical trial of neoadjuvant combined ADT for 4 months with goserelin acetate and flutamide followed by radical prostatectomy, demonstrate comparable 10-year PFS and OS to other approaches with 40% and 68% respectively. The shorter interval of ADT as compared to the approach in Bolla et al may provide metabolic advantages over the longer course of ADT required by that approach. Furthermore, the subjective reduction in disease by DRE (98%) and biochemically (55%) may render some unresectable disease resectable and less likely to have positive surgical margins, recurrence, and ultimately the requirement for adjuvant therapy. Unfortunately, the use of adjuvant and salvage radiation therapy was not captured in this dataset, so outcomes related to additional therapies are not available.
The weakness of this study is the inherent one of a phase 3, pilot study: it is small and, although we have presented long-term data, its purpose was primarily to assess feasibility of this approach. Prior surgical series on locally advanced patients have similar long-term follow-up but suffer from their retrospective nature and heterogeneous patient selection.3–6 To truly assess the appropriateness of this approach, it will almost certainly require a head-to-head comparison of this hormone-surgery approach to the more commonly-used radiation therapy with prolonged hormonal therapy. Given the growing evidence of risk of cardiac and metabolic disease associated with such longer-term exposure to androgen deprivation, such a study would appear warranted.
Conclusions
In this small, prospective phase II study, neoadjuvant hormonal therapy with goserelin acetate and flutamide followed by radical prostatectomy achieves long-term PFS and OS comparable to alternative treatments. This approach is feasible and should be considered as an alternative to combined radiation and ADT.
Table 2.
Pathologic Features at Prostatectomy (n=50*)
| Gleason score | |
| pT0 | 1/50 (2%) |
| 6 | 8/50 (16%) |
| 7 | 8/50 (16%) |
| 8–10 | 18/50 (36%) |
| Treatment effect/not given | 15/50 (30%) |
| Pathologic Stage | |
| Organ confined | 28/50 (56%) |
| EPE | 21/50 (42%) |
| SV invasion | 14/50 (28%) |
| LN involvement | 9/50 (18%) |
| Positive Surgical Margin | 13/50 (26%) |
although 55 underwent RP, only 50 had complete pathologic information reported
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA22433, CA14028, CA76447, CA46441, CA42777 CA35178, CA46113, CA58658, CA20319, CA76132, CA04919, CA35176, CA16385, CA35090, CA13612, CA04919 and CA58348
Abbreviations
- ADT
androgen deprivation therapy
- CI
confidence interval
- CSS
cancer specific survival
- DRE
digital rectal examination
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- PFS
progression free survival
- PSA
prostate specific antigen
- SEER
Surveillance Epidemiology and End Results
- SWOG
Southwest Oncology Group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002 Jul 13;360(9327):103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer D, Egleston B, Abdalla I, et al. Patterns of prostate cancer treatment by clinical stage and age. Am J Public Health. 2001 Jan;91(1):126–8. doi: 10.2105/ajph.91.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berglund RK, Jones JS, Ulchaker JC, et al. Radical prostatectomy as primary treatment modality for locally advanced prostate cancer: a prospective analysis. Urology. 2006 Jun;67(6):1253–6. doi: 10.1016/j.urology.2005.12.003. Epub 2006 may 6. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Smith ND, Roehl KA, et al. Intermediate-term potency, continence, and survival outcomes of radical prostatectomy for clinically high-risk or locally advanced prostate cancer. Urology. 2007 Jun;69(6):1170–5. doi: 10.1016/j.urology.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Partin AW, Humphreys EB, et al. Radical prostatectomy for clinical stage T3a disease. Cancer. 2007 Apr 1;109(7):1273–8. doi: 10.1002/cncr.22544. [DOI] [PubMed] [Google Scholar]
- 6.Ward JF, Slezak JM, Blute ML, et al. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005 Apr;95(6):751–6. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 7.Prostate Cancer. V.1.2009, 2008.
- 8.El Hayek OR, Alfer W, Jr, Reggio E, et al. Percutaneous prostate cryoablation as treatment for high-risk prostate cancer. Clinics (Sao Paulo) 2007 Apr;62(2):109–12. doi: 10.1590/s1807-59322007000200003. [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka Y, Konishi K, Oh RJ, et al. High-dose-rate brachytherapy without external beam irradiation for locally advanced prostate cancer. Radiother Oncol. 2006 Jul;80(1):62–8. doi: 10.1016/j.radonc.2006.06.011. Epub 2006 Jul 25. [DOI] [PubMed] [Google Scholar]
- 10.Powell IJ, Tangen CM, Miller GJ, et al. Neoadjuvant therapy before radical prostatectomy for clinical T3/T4 carcinoma of the prostate: 5-year followup, Phase II Southwest Oncology Group Study 9109. J Urol. 2002 Nov;168(5):2016–9. doi: 10.1016/S0022-5347(05)64285-1. [DOI] [PubMed] [Google Scholar]
- 11.Makarov DV, Humphreys EB, Mangold LA, et al. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 vs 4.1 to 6.0 ng/ml. J Urol. 2006 Aug;176(2):554–8. doi: 10.1016/j.juro.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 12.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009 May;181(5):1998–2006. doi: 10.1016/j.juro.2009.01.047. Discussion 2007–8.Epub 2009 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009 Mar;181(3):956–62. doi: 10.1016/j.juro.2008.11.032. Epub 2009 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005 Aug 13–19;366(9485):572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]