Abstract
Tumor cells were first shown to exhibit a distinct metabolic phenotype over 80 years ago. Since then, it has become clear that multiple oncogenic events contribute to the development of a metabolic phenotype which supports rapid proliferation. Because this phenotype represents an essential component of tumorigenesis and disease progression it also represents a potential source of biomarkers associated with aggressive disease. In addition, the addiction of tumor cells to specific nutrients, and the up-regulation of key metabolic enzymes provide unique opportunities for pharmacologic manipulation. Despite the use of multimodality treatment, survival rates for patients with advanced HNSCC remain low, partially due to the development of drug resistance. In this review, we evaluate the role of altered HNSCC metabolism as both a source of novel biomarkers and a means to bypass resistance mechanisms to conventional forms of therapy.
HNSCC statistics and current treatment
HNSCC accounts for approximately 45,000 new patients per year in the US and represents the 5th most common form of cancer worldwide.(1) Though prognosis varies significantly based on disease stage as well as the site from which primary tumors arise within the upper aerodigestive tract, survival rates for patients with stage III-IV disease remains in the range of 35-50%.(2-4) Current management of advanced HNSCC consists of multi-modality treatment which includes some combination of surgery, chemotherapy and external beam radiation (XRT).(2-4) Despite an improved understanding of HNSCC tumor cell biology and the development of targeted therapies (growth factor signaling inhibitors), the single modality efficacy of chemotherapy in HNSCC remains low and disease resistance is a significant concern.(5, 6) Ongoing strategies are aimed at improving response rates through the development of additional therapeutic windows, optimization of drug combinations and the use of rational molecular markers of tumor resistance or sensitivity to specific drug combinations.
Below we discuss the basic principles of tumor cell metabolism and mechanisms by which oncogenic events can lead to altered metabolic activity. Due to the scarcity of primary studies aimed at HNSCC, a significant portion of the discussion relies on data obtained in other tumor types.
Tumor cell metabolism is designed to support increased proliferation
Since Otto Warburg first demonstrated in the 1920s that cancer cells are highly glycolytic compared to normal cells, it has become clear that cellular metabolism in neoplastic cells differs significantly from that supporting normal eukaryotic homeostasis.(7-9) Indeed, many refer to tumor cells as having become “addicted” to glycolysis despite the presence of alternative nutrients and oxygen.(8) Normal eukaryotic cells maximize energy production from a limited nutrient supply through an optimal combination of traditional energetic pathways which include glycolysis, the tricarboxylic acid cycle (TCA), and the mitochondrial electron transport chain. In this manner, cells efficiently convert a glucose molecule into CO2 and H20, while maximizing production of ATP and reducing potential in the form of NAD(P)H. In the absence of oxygen, normal cells lack the final acceptor in the electron transport chain, and rely primarily on anaerobic glycolysis, whose product, lactate is exported in the extra-cellular space leading to increased tissue acidosis.(10) In contrast, tumor cells maximally utilize glycolysis even in the presence of oxygen, maintaining a high rate of lactate production.(7) Essentially, the Warburg effect can be thought of as the loss of the Pasteur effect (inhibition of carbohydrate metabolism and anaerobic conversion of pyruvate into lactate by oxygen).
An important un-resolved issue in the field of cancer metabolism is the impetus driving cancer cells to engage in an inefficient energetic process. Vander Heiden et al (2009) offer a plausible explanation for this phenomenon. Under conditions of rapid aerobic glycolytic flux (sufficient to maintain intra-cellular energy levels) mitochondrial activity can be diverted from fulfilling energetic demands into a biosynthetic pathway in which TCA cycle intermediates are used to generate nucleotides, proteins and fatty acids. This increased biomass generation potential, though it comes at the expense of optimal energy generation, fulfills an important requirement of tumor cell proliferation. (8, 9) Although this paradigm does not represent a truly testable hypothesis, it is supported by circumstantial evidence such as tumor cell utilization of glutamine. Independent lines of investigation have demonstrated that cancer cells metabolize substantial amounts of extra-cellular glutamine and that a subset of cells may be addicted to glutamine.(11-13) Glutaminolysis fulfills both aspects of the metabolic paradigm described above by generating: 1) anabolic carbon through TCA cycling, and 2) reducing equivalents via malic enzyme and isocitrate dehydrogenase.(11) Experimental data regarding the contributions of both glucose and glutamine to TCA cycle intermediates strongly suggests that an important driving force behind tumor cell metabolism is indeed the generation of anabolic intermediates and reducing potential in the form of NADPH.(11, 12, 14)
Integration of altered metabolism into a highly proliferative tumor cell metabolism appears to be a driven by two overlapping mechanisms. The first consists of changes in canonical signaling cascades resulting in increased metabolic flux through metabolic pathways. The second is driven by the signaling activity of metabolites themselves, which supports an altered transcriptional program that further enhances tumor cell proliferation. Both mechanisms are described in more detail below.
Mechanisms of altered tumor cell metabolism and relevance to disease processes
Glucose entry into the cell is facilitated by a family of solute transporters (GLUTs) whose membrane availability and activity can vary dramatically.(15) Over-expression of GLUT-1 and GLUT-3 is associated with poorly differentiated solid tumors, and worse overall survival.(16-18) Glucose conversion into pyruvate is rate limited by three steps catalyzed respectively by hexokinase, phosphofructokinase, and pyruvate kinase. Tumor cells exhibit alterations in the regulatory mechanisms controlling each of these steps. Transcription of hexokinase (HK) II is regulated by p53 binding to its promoter as well as changes in glucose, oxygen and hormone levels.(19, 20) In contrast to normal tissue, tumors and tumor cells express the embryonic M2 isoform (PKM2) of this enzyme resulting in higher rates of aerobic glycolysis and cell proliferation.(21) PKM2 activity is closely coordinated with expression of the phosphofructokinase-2 isoform present in tumor cells.(22) Overall, the expression of these key regulatory enzymes is coordinated by multiple oncogenic events in a manner designed to support maximal tumor cell proliferation and survival.(9, 12, 22-25)
The transition between glycolysis and the TCA cycle is primarily controlled by the activity of lactate dehydrogenase and the pyruvate dehydrogenase complex (PDC). The coordinated activity of these two enzyme complexes can coordinate carbon flux at this junction and balance the 2 pathways according to temporary fluxes. McFate et al (2008) demonstrated that regulation of PDC activity by pyruvate dehydrogenase kinase -1 (PDK-1) partially accounts for the Warburg phenomenon in HNSCC cells, making the PDC and more specifically, PDK, potential therapeutic targets to reverse this metabolic phenotype.(26) Wigfield et al (2008) further demonstrated that down-regulation of hypoxia induced PDK-1 decreases lactate and pyruvate secretion.(27) More recently, experiments conducted on HNSCC cell lines containing mitochondrial mutations suggest that there exists a level of coordination between mitochondrial activity, accumulation of the hypoxia inducible factor (HIF) -1alpha and down-regulation of pyruvate dehydrogenase activity via PDK.(28) The integration of nutrient flux and oxygen driven signaling at this particular metabolic nexus suggests that key decisions regarding overall control of metabolism and cell proliferation are coordinated. Specifically, changes in enzyme and transporter activity needed to support an altered metabolic phenotype require the integration of multiple intra-cellular pathways and coordination with decisions regarding cell cycle, arrest and programmed cell death. Evidence from existing studies suggests that this is accomplished using both traditional (growth factor – kinase) and metabolite/nutrient driven signaling. Multiple oncogenic events including TP53 mutation, myc and ras over-expression and persistent activation of growth factor signaling cascades such as PI3K-Akt participate in the regulation of tumor cell metabolism.(8, 9, 12, 13, 22, 24, 29)
The best described canonical, growth factor driven signaling cascade which can regulate tumor cell metabolism is the PI3K-Akt pathway. Akt can drive enhanced glucose catabolism by triggering localization of hexokinase to the mitochondrial membrane (which enhances its activity) and activating ATP citrate lyase, a key enzyme in fatty acid synthesis.(30, 31) Indirectly, Akt can regulate metabolic flux through regulation of Forkhead Box subclass O (FOXO) transcription factors.(32) In the cytosol, Akt can phosphorylate tuberous sclerosis (TSC) factor 2 and mammalian target of rapamycin (mTOR) complex activity regarding glucose metabolism and protein translation.(33) In addition to PI3K-Akt, other traditional oncogenic events can directly regulate metabolic enzymes. Specifically, p53 transcriptional activity has been linked to increased expression of hexokinase II and myc over-expression is closely linked to glutaminolytic flux through glutamate dehydrogenase.(11, 12, 19)
Metabolic fluxes can feed back onto traditional signaling cascades providing a second regulatory loop. The clearest example of this mechanism is regulation of mTOR activity by multiple metabolic cues including availability of amino acids, intra-cellular ATP levels, changes in oxygen tension and reactive oxygen species.(34-36) In a similar manner, changes in intra-cellular ATP levels activate AMP-activated protein kinase (AMPK) which not only regulates mTOR activity, but participates in p53 dependent cell cycle arrest and death.(37)
An important metabolic signaling nexus is provided by activation/stabilization of hypoxia –inducible factor 1 (HIF-1) alpha. Although HIF-1alpha is essentially stabilized in the presence of low oxygen tension, it can also be induced following mTOR activation and interestingly, in response to decreased activity of isocitrate dehydrogenase -1 (IDH-1) resulting in lower levels of alpha-ketoglutarate, a TCA cycle intermediate.(38, 39) Defects in the activity of 2 other TCA cycle enzymes, fumarate hydratase and succinate dehydrogenase have been linked to stabilization of HIF-1alpha via inhibition of prolyl hydroxylases by their products.(40, 41) HIF-1alpha transcriptional targets include the glucose transporters, hexokinase, lactate dehydrogenase A (LDH A) and monocarboxylate tranporter 4 (MCT4) and pyruvate dehydrogenase kinase (PDK).(42, 43) HIF-1alpha is therefore an important contributor to a global transcriptional program which responds to metabolic cues and can regulate survival and proliferation. The importance of HIF-1alpha in the regulation of metabolism and cell proliferation/survival raises the possibility that altered tumor cell metabolism is in fact designed to drive, through altered transcription, tumor growth. This “signaling-transcription” paradigm and the “energy-biomass” paradigm described earlier represent putative explanations for an essentially irrational choice made by tumor cell to produce energy inefficiently. Although which paradigm is correct represents an interesting scientific question in and of itself, the more clinically important point is that both paradigms establish key issues that must be addressed when evaluating metabolic markers of HNSCC disease and developing metabolically based pharmacologic approaches.
Markers of altered metabolism in HNSCC
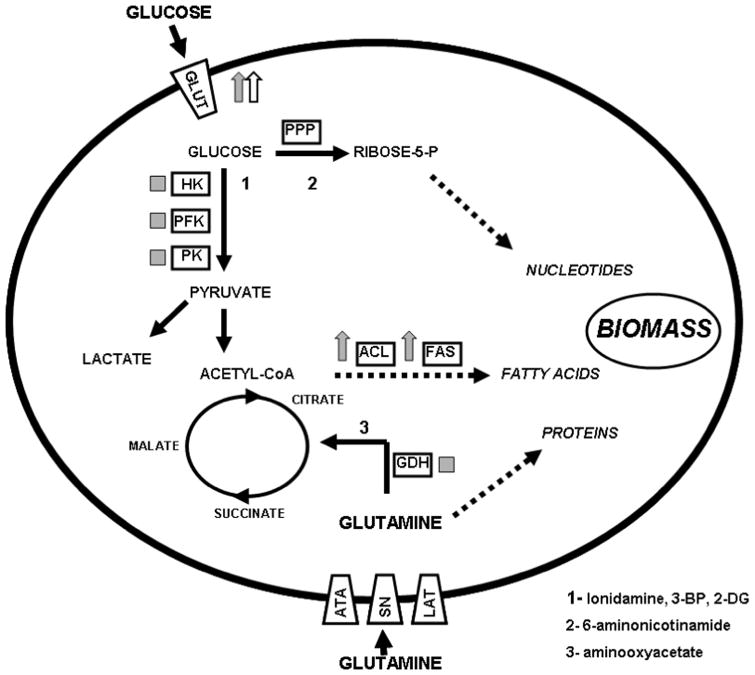
To date, few studies have attempted to clarify the metabolic phenotype of HNSCC from a biochemical perspective (Figure 1). Brizel et al (2001) found that in biopsies of 40 patients with HNSCC, intra-tumor lactate levels were not correlated with presenting T stage or N stage, but high tumor lactate concentrations were correlated with decreased 2 year metastasis free survival.(44) Prospective analysis in an in vivo xenograft model of HNSCC indicates that increased tumor lactate levels may be predictive of relative radioresistance.(45) Most recently, Ziebart et al (2010) found that, although HNSCC tumors maintain higher lactate levels than normal tissue and that lactate tumor levels correlate with survival, tumor lactate levels are not predicted by lactate dehydrogenase (LDH) levels.(46) This discrepancy represents an important though somewhat expected observation which will be discussed in more detail later in the review.
Figure 1. Metabolic perturbations associated with tumorigenesis.
Tumor cell metabolism represents a trade-off between energy and biomass. This is accomplished by diverting glucose from efficient energy generating mechanisms (TCA cycle and oxidative phophorylation) into aerobic glycolysis, and utilizing the resulting acetyl-CoA and citrate molecules into fatty acid generation. Secondary pathways such as the pentose phosphate shunt (PPP) and glutaminolysis are often activated to further augment biomass generation and balance glycolytic losses. Blue arrows indicate over-expression in tumors; blue boxes indicate altered activity in tumors (increased expression, altered localization, epigenetic modification, switches among isozymes); red arrows indicate over-expression in HNSCC cell lines or tumors; red boxes indicate altered activity in HNSCC cell lines or tumors. HK- hexokinase, PFK- phosphofructokinase, PK- pyruvate kinase, ACL- ATP citrate lyase, FAS- fatty acid synthase, GDH- glutamate dehydrogenase, GLUT- glucose transporters, ATA- amino acid transporter A or sodium-coupled neutral amino acid transporter, SN- system N or sodium-coupled neutral amino acid transporter, LAT-system L or sodium independent amino acid transporter.
A second line of evidence regarding the importance of metabolism in HNSCC is derived from the utility of fluoro-deoxy-glucose (FDG) - positron emission tomography (PET) imaging in the diagnosis and staging of primary and recurrent disease. The mechanism of FDG avidity is based on preferential uptake of a fluorinated glucose derivative into tumor cells, and conversion into a non-metabolizable intermediate. As such, FDG avidity reflects primarily the cells capacity for glucose transport and the activity of the first glycolytic step, driven by HK.(47) Despite some limitations, FDG-PET is currently employed in the diagnosis of new HNSCC as well as the monitoring of response to therapy and post-treatment surveillance for recurrence/ metastasis.(48) When combined with anatomic imaging (CT), FDG-PET can be particularly sensitive for distant metastasis.(49)
The primary component of FDG avidity is preferential glucose uptake primarily via GLUT-1. Increased GLUT-1 expression has been found in pre-neoplastic and neoplastic mucosal lesions as well as loco-regional metastasis.(50) Over-expression of this transporter appears to be confined to neoplastic tissue, and absent from benign squamous cell lesions.(51) Two retrospective pathologic studies have demonstrated clinical utility of GLUT-1 as a marker of HNSCC disease.(52, 53) Li et al (2008) found that recurrent HNSCC had a higher staining index than primary disease and in poorly differentiated tumors compared to well differentiated tumors.(52) A detailed analysis of GLUT-1 in HNSCC indicated that, in a retrospective pathologic analysis (IHC) of 118 patients with oral cavity SCC, a low staining index for GLUT-1 correlated with longer median survival compared to patients with a high index (138 months vs 60 months). Thus, GLUT-1 expression was found to be an independent marker of prognosis. Similar data were obtained for relative FDG avidity (SUV >5.6 vs SUV <5.6).(53) Although the correlation between FDG avidity and GLUT-1 expression is not perfect, in general both parameters appear to correlate with poorer prognosis, secondary to less differentiated tumors.(52, 53)
GLUT-1 is the glucose transporter most often associated with HNSCC, but not the only member of the GLUT family which may contribute to its pathogenesis and/or progression. In a study of 48 cases of laryngeal SCC, Baer et al (2002) found that although all samples expressed GLUT-1, GLUT-3 positive cases were associated with worse survival and more poorly differentiated tumors.(18) Gene expression analysis conducted by Estilo et al (2009) which found that GLUT3 over-expression correlated with increased depth of tumor invasion, larger tumor size, advanced pathologic stage and recurrence.(54) An important caveat for these retrospective studies stems from the role of glucose transporters in supporting rapid tumor cell proliferation. It is quite possible that over-expression of GLUTs is simply a reflection of rapidly proliferating cells and that its expression may in fact be identical with many other non-specific markers of proliferation such as Ki-67 or proliferating cell nuclear antigen (PCNA). In order to determine whether GLUT over-expression represents a truly independent predictor of disease progression, prospective biomarker studies and evaluating the impact of perturbing GLUT expression on tumor progression in pre-clinical models are required. It is important to note, that unlike most other tumor markers (altered protein, phospho-protein or mRNA levels), GLUT over-expression has a biochemical correlate (FDG-PET) which strongly suggests that protein levels are indeed linked to a functional tumor phenotype (increased glucose uptake).
In addition to GLUTs, few other metabolic markers have been characterized in HNSCC. Wigfield et al (2008) found that PDK-1 expression, though low in normal mucosal tissues, is up-regulated in HNSCC. Increased expression generally correlated with worse overall and disease free survival, suggesting that high levels of PDK-1 potentiate increased glycolytic flux and allow tumor cells to thrive.(27) Data regarding PKM2 are mixed. Though PKM2 is over-expressed in some oral tongue specimens, a more detailed quantitative analysis of OSCC tumor specimens found a poor correlation between PKM2 levels and clinical parameters.(55, 56) More recently, transketolase-like 1 (TKTL1) has been demonstrated to enhance HNSCC cell line tumorigenicity in mouse models and correlate with poorer survival in patients with laryngeal SCC.(57, 58) Combined with work demonstrating a role for mitochondrial mutations in regulating HIF-1alpha these data suggest that elucidating the coordination between glycolytic and non-glycolytic pathways will be key to a better understanding of the role of altered metabolism in HNSCC pathogenesis.
Although the presence of stabilized HIF-1alpha could be considered an indicator of altered metabolism, that would represent an oversimplification of its esquisitely complex role in tumor formation. Similarly, though a role for mTOR in HNSCC pathogenesis has been established by multiple authors, mTOR represents much more than a metabolic marker, given its role as a key regulator of all protein translation.(59) The intrinsically close relationship between activation of Akt and mTOR function make it difficult to determine which effects are due to changes in tumor cell metabolism and which result from altered signaling cues. As described above, it is quite likely that both signaling streams are closely inter-connected.
Implications of altered metabolism for HNSCC response to therapy
Non-surgical approaches to the management of HNSCC generally employ external beam radiation (XRT) coupled with cytotoxic chemotherapy, wherein cisplatin is the most commonly used agent.(5) The anti-tumorigenic effects of XRT are generated through a DNA damage mechanism which relies on the formation of free radicals. This is the likely reason why in the absence of normal oxygen tension (hypoxia), the efficacy of XRT is diminished.(60) Cisplatin, though not itself a free radical, acts in a similar fashion, in so far as it is readily inactivated by thiol containing proteins both in the extra-cellular and intra-cellular compartments.(61, 62) The remaining free cisplatin molecules can enter the nucleus, bind DNA and induce damage through a variety of mechanisms.(62) It is therefore clear that the toxicity of an XRT-cisplatin based therapy is dependent on the intracellular reducing potential, generally reflected in the balance of three molecule pairs: NAD+/NADH, NADP+/ NADPH and oxidized glutathione/ reduced glutathione. Under conditions of stress, which may deplete reducing potential, both XRT and cisplatin effects would be potentiated. In addition, tumor cell recovery from XRT and cisplatin induced damage is energetically demanding (cisplatin export, activation of DNA damage repair pathways). These two lines of reasoning suggest that targeting metabolic pathways which generate reducing potential and replenish intra-cellular energy stores should significantly potentiate the toxicity of conventional therapies.
Over the last 50 years, a significant number of compounds with anti-metabolic activity have been identified. Most of these compounds exhibit specific enzymatic inhibitory activity through a combination of competitive and non-competitive inhibitory mechanisms. A comprehensive review of these agents is beyond the scope of this review. However, the most well known and most thoroughly tested compounds include: 2-deoxy-D-glucose (2-DG), 3-bromopyruvate (3-BP) and lonidamine.(25) Among these, 2-DG (a non-metabolizable D-glucose analog) and lonidamine, both agents with hexokinase inhibiting activity, have received substantial pre-clinical and clinical attention. Both agents have been shown to improve the response of tumor cells and tumor xenografts to a wide variety of conventional chemotherapeutic agents.(63-66) In the clinical setting, 2-DG has been used as an adjuvant to XRT treatment of glioblastoma multiforme with reasonable safety.(67, 68) Lonidamine has been studied in several clinical trials addressing multiple solid tumor types, with promising results.(69, 70) To date, 3-BP, a hexokinase inhibitor chemically unrelated to 2-DG or lonidamine has been used exclusively in the laboratory and pre-clinical setting, and shown to possess single agent activity and enhance cisplatin cytotoxicity.(71) A variety of other drugs, targeted to enzymatic steps throughout glycolysis, the TCA cycle and secondary metabolic pathways have been described and tested in the laboratory setting. In general, inhibition of major energy producing pathways has been shown to improve the cytotoxicity of traditional chemotherapeutic approaches.
Two clinical trials originating in Italy in the early 1990s evaluated the role of metabolic targeting in HNSCC and suggested that the addition of lonidamine to either external beam radiation or chemotherapy (methotrexate) improved clinical outcomes.(72, 73) In the setting of phase III double blind, randomized, placebo controlled trial of 97 patients with stage II-IV disease, the addition of lonidamine resulted in a lower treatment failure rate (50% vs 77% for placebo), improved locoregional control (63% vs 37% at 5 years) and disease free survival (40% vs 19%).(73) With these exceptions, metabolic targeting in HNSCC has been primarily limited to a small number of pre-clinical studies and focused on 2-DG. As in other solid tumor types, 2-DG has been shown to potentiate cisplatin's anti-tumor effects on tumor cell proliferation and survival and inhibit growth of HNSCC xenografts.(64, 65) It remains unclear whether 2-DG as a single agent is cytotoxic or merely cytostatic in the context of HNSCC cell lines.(74) Interestingly, combining 2-DG with 6-aminonicotinamide, an inhibitor of the pentose phosphate pathway (a secondary source of reducing potential) results in an enhanced cytostatic/ cytotoxic effect suggesting that pharmacologic targeting of multiple metabolic pathways may represent a viable means of potentiating the effectiveness of traditional therapy.(75) These data support the observation that glucose catabolism via the pentose phosphate pathway is indeed an important contributor to HNSCC tumorigenicity.(57)
A substantial body of literature has demonstrated a role for PI3K/Akt/mTOR inhibition in reducing HNSCC tumorigenicity. Rapamycin has potent anti-tumorigenic activity in HNSCC animal models. Another mTOR inhibitor (CCI-779) can inhibit HNSCC proliferation and tumorigenicity when combined with XRT and conventional chemotherapeutic agents.(76-79) It is important to note however, that these studies generally do not focus on, nor demonstrate that a metabolic effect drives the anti-proliferative and anti-tumorigenic effects of mTOR inhibition. Such a distinction would be extremely difficult due to the broad cellular implications of altered mTOR activity.
The therapeutic index of traditional cytotoxic agents is largely driven by the inherently faster proliferative rate of tumor cells and an altered ability to cope with activation of damage signals. The lack of actual tumor specificity of agents such as cisplatin results in significant drug toxicity. Most anti-metabolic agents studied to date suffer from a similar lack of tumor cell specificity. Although rapidly proliferative cells (intestinal epithelial lining, hematopoietic cells) may be more susceptible to the effects of anti-metabolic agents, anti-glycolytic agents can also affect cells which are primarily dependent on glucose catabolism such as red blood cells and neurons (non-proliferative). As such, there is every reason to be skeptical that therapeutic gain will be obtained without a significant toxicity cost. Unfortunately, the limited number of clinical trials conducted to date on this drug class makes it difficult to predict the full range of side effects without additional data.
Future directions
Tumor cell metabolism is receiving increasing attention as a potential source of both novel biomarkers and putative targets for pharmacologic intervention. In addition to the studies mentioned above, new metabolic targets continue to be identified in a variety of tumor types. Most recently, Ward et al (2010) demonstrated that mutations in isocitrate dehydrogenase, a mitochondrial enzyme, result in altered tumor cell metabolism and lead to increased production of the onco-metabolite 2-hydroxyglutatrate (2-HG).(80) This type of analysis can be subsequently used to develop both novel diagnostic tools and new targeted therapies. In this article, we review existing data regarding the role of altered metabolism in HNSCC pathogenesis and response to treatment. In general, there is convincing evidence that, like other solid tumors, HNSCC requires high rates of glucose uptake and conversion in order to support tumorigenesis. This appears to make HNSCC tumors susceptible to combination therapy employing anti-glycolytic agents such as 2-DG. However, information about the importance of secondary energetic pathways, key regulatory mechanisms and the potential of other anti-metabolic agents in the treatment of HNSCC remains lacking.
Two technological developments provide researchers with new and potentially important tools in the continued study of HNSCC metabolism. The first represents a conversion of liquid-gas chromatography and mass spectrometry into an integrated, high throughput, broad based metabolomic platform. This technology makes it possible to obtain a metabolic tumor signature which, when combined with clinical parameters, could lead to a metabolically based algorithm predictive of disease progression and response to treatment.(81) The second is the development of sensitive sensor of metabolic activity (pH changes and oxygen consumption) with real time capability. This technology can help elucidate not only subtle endogenous metabolic differences among tumor cell lines, but also identify the precise sequence and timing of metabolic effects of tested pharmacologic agents.(82)
In order to better understand the role of altered metabolism in HNSCC pathogenesis and response to treatment we suggest three specific lines of investigation based on studies performed in other tumor types. First, we suggest using a comprehensive genomic, epigenetic and proteomic algorithm to identify specific metabolic alterations associated with HNSCC development. This analysis would not only confirm the importance of metabolic pathways identified in other tumor types (such as glycolysis), but more importantly, identify secondary energetic pathways that allow HNSCC tumors in particular, to progress and develop resistance to conventional therapeutic strategies. An entire new class of markers of aggressive and metastatic disease centered on nutrient transporters and metabolic enzymes would then be developed. A broad based metabolomic analysis (using the platforms described above) will need to be integrated into this data set in order to validate the phenotype of putative metabolic markers. Second, we suggest using a similar type of analysis to identify the key metabolic regulatory mechanisms contribution to HNSCC tumorigenesis. This is important, because although most oncogenic events can impact metabolic activity in some way, not all are equally relevant to this particular tumor type. Identifying the primary driving oncogenic events behind altered HNSCC metabolism may have important therapeutic implications. From a therapeutic perspective, the most logical next step is a comprehensive investigation of known anti-metabolic agents in a more relevant pre-clinical setting using HNSCC cell lines representative of disease heterogeneity and orthotopic animal models which allow for the study of effects both on the primary tumor and loco-regional metastasis, the most important predictor of mortality and morbidity in HNSCC. We believe that by combining these approaches we will significantly improve our understanding of HNSCC pathogenesis, resulting in significant clinical advances.
Acknowledgments
Grant Support: This work is supported in part by the National Institutes of Health National Research Science Award Research Training Grant (NIDCD) T32DC007367 (VCS).
References
- 1.Society AC. Cancer Facts & Figures 2009. 2009 [Google Scholar]
- 2.Forastiere A, Weber R, Ang K. Treatment of head and neck cancer. N Engl J Med. 2008;358(10):1076. doi: 10.1056/NEJMc073274. author reply 1077-8. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J Surg Oncol. 2008;97(8):701–7. doi: 10.1002/jso.21012. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Trotti A, Pfister DG, Grandis JR. Head and neck cancer: recent advances and new standards of care. J Clin Oncol. 2006;24(17):2603–5. doi: 10.1200/JCO.2006.07.1464. [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Bernier J, Bentzen SM, Vermorken JB. Molecular therapy in head and neck oncology. Nat Rev Clin Oncol. 2009;6(5):266–77. doi: 10.1038/nrclinonc.2009.40. [DOI] [PubMed] [Google Scholar]
- 7.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 8.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang CV. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 70(3):859–62. doi: 10.1158/0008-5472.CAN-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson DL, C M. Lehninger principles of biochemistry. 5th. W.H. Freeman and Company; 2008. [Google Scholar]
- 11.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104(49):19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105(48):18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178(1):93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69(20):7986–93. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8(2):113–28. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80(6):1046–51. doi: 10.1002/(sici)1097-0142(19970915)80:6<1046::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Noguchi Y, Satoh S, Hayashi H, Inayama Y, Kitamura H. Expression of facilitative glucose transporter isoforms in lung carcinomas: its relation to histologic type, differentiation grade, and tumor stage. Mod Pathol. 1998;11(5):437–43. [PubMed] [Google Scholar]
- 18.Baer S, Casaubon L, Schwartz MR, Marcogliese A, Younes M. Glut3 expression in biopsy specimens of laryngeal carcinoma is associated with poor survival. Laryngoscope. 2002;112(2):393–6. doi: 10.1097/00005537-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 19.Mathupala SP, Heese C, Pedersen PL. Glucose catabolism in cancer cells. The type II hexokinase promoter contains functionally active response elements for the tumor suppressor p53. J Biol Chem. 1997;272(36):22776–80. doi: 10.1074/jbc.272.36.22776. [DOI] [PubMed] [Google Scholar]
- 20.Mathupala SP, Rempel A, Pedersen PL. Aberrant glycolytic metabolism of cancer cells: a remarkable coordination of genetic, transcriptional, post-translational, and mutational events that lead to a critical role for type II hexokinase. J Bioenerg Biomembr. 1997;29(4):339–43. doi: 10.1023/a:1022494613613. [DOI] [PubMed] [Google Scholar]
- 21.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 22.Telang S, Yalcin A, Clem AL, et al. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene. 2006;25(55):7225–34. doi: 10.1038/sj.onc.1209709. [DOI] [PubMed] [Google Scholar]
- 23.Murugan AK, Hong NT, Fukui Y, Munirajan AK, Tsuchida N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int J Oncol. 2008;32(1):101–11. [PubMed] [Google Scholar]
- 24.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24(50):7435–42. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 25.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 26.McFate T, Mohyeldin A, Lu H, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283(33):22700–8. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer. 2008;98(12):1975–84. doi: 10.1038/sj.bjc.6604356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W, Zhou S, Chang SS, McFate T, Verma A, Califano JA. Mitochondrial mutations contribute to HIF1alpha accumulation via increased reactive oxygen species and up-regulated pyruvate dehydrogenease kinase 2 in head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15(2):476–84. doi: 10.1158/1078-0432.CCR-08-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong X, Zhao F, Thompson CB. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19(1):32–7. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majewski N, Nogueira V, Bhaskar P, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16(5):819–30. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277(37):33895–900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- 32.Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300(5619):644–7. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- 33.Dan HC, Sun M, Yang L, et al. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277(38):35364–70. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 34.Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278(32):29655–60. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 35.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammaliana TOR: homeostatic ATP sensor. Science. 2001;294(5544):1102–5. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 36.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273(23):14484–94. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 37.Jones RG, Plas DR, Kubek S, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18(3):283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324(5924):261–5. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14(15):2231–9. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 42.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281(14):9030–7. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 43.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12(2):149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(2):349–53. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 45.Quennet V, Yaromina A, Zips D, et al. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol. 2006;81(2):130–5. doi: 10.1016/j.radonc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Ziebart T, Walenta S, Kunkel M, Reichert TE, Wagner W, Mueller-Klieser W. Metabolic and proteomic differentials in head and neck squamous cell carcinomas and normal gingival tissue. J Cancer Res Clin Oncol. doi: 10.1007/s00432-010-0875-y. [DOI] [PubMed] [Google Scholar]
- 47.Koukourakis G, Maravelis G, Koukouraki S, Padelakos P, Kouloulias V. Overview of positron emission tomography chemistry: clinical and technical considerations and combination with computed tomography. J BUON. 2009;14(4):575–80. [PubMed] [Google Scholar]
- 48.Stokkel MP, ten Broek FW, van Rijk PP. The role of FDG PET in the clinical management of head and neck cancer. Oral Oncol. 1998;34(6):466–71. doi: 10.1016/s1368-8375(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 49.Gourin CG, Watts TL, Williams HT, Patel VS, Bilodeau PA, Coleman TA. Identification of distant metastases with positron-emission tomography-computed tomography in patients with previously untreated head and neck cancer. Laryngoscope. 2008;118(4):671–5. doi: 10.1097/MLG.0b013e3181625737. [DOI] [PubMed] [Google Scholar]
- 50.Reisser C, Eichhorn K, Herold-Mende C, Born AI, Bannasch P. Expression of facilitative glucose transport proteins during development of squamous cell carcinomas of the head and neck. Int J Cancer. 1999;80(2):194–8. doi: 10.1002/(sici)1097-0215(19990118)80:2<194::aid-ijc6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 51.Chandan VS, Faquin WC, Wilbur DC, Khurana KK. The utility of GLUT-1 immunolocalization in cell blocks: An adjunct to the fine needle aspiration diagnosis of cystic squamous lesions of the head and neck. Cancer. 2006;108(2):124–8. doi: 10.1002/cncr.21714. [DOI] [PubMed] [Google Scholar]
- 52.Li SJ, Guo W, Ren GX, Huang G, Chen T, Song SL. Expression of Glut-1 in primary and recurrent head and neck squamous cell carcinomas, and compared with 2-[18F]fluoro-2-deoxy-D-glucose accumulation in positron emission tomography. Br J Oral Maxillofac Surg. 2008;46(3):180–6. doi: 10.1016/j.bjoms.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Kunkel M, Reichert TE, Benz P, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97(4):1015–24. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 54.Estilo CL, Oc P, Talbot S, et al. Oral tongue cancer gene expression profiling: Identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer. 2009;9:11. doi: 10.1186/1471-2407-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man NgR, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer. 2008;123(2):251–7. doi: 10.1002/ijc.23583. [DOI] [PubMed] [Google Scholar]
- 56.Ervens J, Fuchs H, Niemann VT, Hoffmeister B. Pyruvate kinase isoenzyme M2 is not of diagnostic relevance as a marker for oral cancer. J Craniomaxillofac Surg. 2008;36(2):89–94. doi: 10.1016/j.jcms.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Sun W, Liu Y, Glazer CA, et al. TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res. 16(3):857–66. doi: 10.1158/1078-0432.CCR-09-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volker HU, Scheich M, Schmausser B, Kammerer U, Eck M. Overexpression of transketolase TKTL1 is associated with shorter survival in laryngeal squamous cell carcinomas. Eur Arch Otorhinolaryngol. 2007;264(12):1431–6. doi: 10.1007/s00405-007-0394-x. [DOI] [PubMed] [Google Scholar]
- 59.Clark C, Shah S, Herman-Ferdinandez L, et al. Teasing out the best molecular marker in the AKT/mTOR pathway in head and neck squamous cell cancer patients. Laryngoscope. 120(6):1159–65. doi: 10.1002/lary.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wardman P. The importance of radiation chemistry to radiation and free radical biology (The 2008 Silvanus Thompson Memorial Lecture) Br J Radiol. 2009;82(974):89–104. doi: 10.1259/bjr/60186130. [DOI] [PubMed] [Google Scholar]
- 61.Sedletska Y, Giraud-Panis MJ, Malinge JM. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anticancer Agents. 2005;5(3):251–65. doi: 10.2174/1568011053765967. [DOI] [PubMed] [Google Scholar]
- 62.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007;7(1):3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 63.Maschek G, Savaraj N, Priebe W, et al. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64(1):31–4. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 64.Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67(7):3364–70. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simons AL, Fath MA, Mattson DM, et al. Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-D-glucose correlates with increased 18F-FDG uptake as determined by PET imaging. Int J Radiat Oncol Biol Phys. 2007;69(4):1222–30. doi: 10.1016/j.ijrobp.2007.07.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosbe KW, Brann TW, Holden SA, Teicher BA, Frei E., 3rd Effect of lonidamine on the cytotoxicity of four alkylating agents in vitro. Cancer Chemother Pharmacol. 1989;25(1):32–6. doi: 10.1007/BF00694335. [DOI] [PubMed] [Google Scholar]
- 67.Mohanti BK, Rath GK, Anantha N, et al. Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35(1):103–11. doi: 10.1016/s0360-3016(96)85017-6. [DOI] [PubMed] [Google Scholar]
- 68.Singh D, Banerji AK, Dwarakanath BS, et al. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005;181(8):507–14. doi: 10.1007/s00066-005-1320-z. [DOI] [PubMed] [Google Scholar]
- 69.Oudard S, Carpentier A, Banu E, et al. Phase II study of lonidamine and diazepam in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2003;63(1):81–6. doi: 10.1023/a:1023756707900. [DOI] [PubMed] [Google Scholar]
- 70.De Lena M, Lorusso V, Latorre A, et al. Paclitaxel, cisplatin and lonidamine in advanced ovarian cancer. A phase II study. Eur J Cancer. 2001;37(3):364–8. doi: 10.1016/s0959-8049(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 71.Ihrlund LS, Hernlund E, Khan O, Shoshan MC. 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol. 2008;2(1):94–101. doi: 10.1016/j.molonc.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colella E, Merlano M, Blengio F, et al. Randomised phase II study of methotrexate (MTX) versus methotrexate plus lonidamine (MTX + LND) in recurrent and/or metastatic carcinoma of the head and neck. Eur J Cancer. 1994;30A(7):928–30. doi: 10.1016/0959-8049(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 73.Magno L, Terraneo F, Bertoni F, et al. Double-blind randomized study of lonidamine and radiotherapy in head and neck cancer. Int J Radiat Oncol Biol Phys. 1994;29(1):45–55. doi: 10.1016/0360-3016(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 74.Zhang XD, Deslandes E, Villedieu M, et al. Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res. 2006;26(5A):3561–6. [PubMed] [Google Scholar]
- 75.Varshney R, Dwarakanath B, Jain V. Radiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cells. Int J Radiat Biol. 2005;81(5):397–408. doi: 10.1080/09553000500148590. [DOI] [PubMed] [Google Scholar]
- 76.Nathan CO, Amirghahari N, Rong X, et al. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67(5):2160–8. doi: 10.1158/0008-5472.CAN-06-2449. [DOI] [PubMed] [Google Scholar]
- 77.Ekshyyan O, Rong Y, Rong X, et al. Comparison of radiosensitizing effects of the mammalian target of rapamycin inhibitor CCI-779 to cisplatin in experimental models of head and neck squamous cell carcinoma. Mol Cancer Ther. 2009;8(8):2255–65. doi: 10.1158/1535-7163.MCT-08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amornphimoltham P, Patel V, Sodhi A, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65(21):9953–61. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 79.Aissat N, Le Tourneau C, Ghoul A, et al. Antiproliferative effects of rapamycin as a single agent and in combination with carboplatin and paclitaxel in head and neck cancer cell lines. Cancer Chemother Pharmacol. 2008;62(2):305–13. doi: 10.1007/s00280-007-0609-2. [DOI] [PubMed] [Google Scholar]
- 80.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 17(3):225–34. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–4. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 82.Wu M, Neilson A, Swift AL, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292(1):C125–36. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]