Abstract
Background
Serosorting is increasingly assessed in studies of MSM. Most research studies have measured serosorting by combining reported unprotected anal intercourse (UAI) and the occurrence of participant and partner same HIV status (seroconcordance). The CDC definition of serosorting also incorporates intent to be in such a partnership, although few studies incorporate both intent and behavior into their measures.
Methods
Using data from a national, online survey of 3,519 US MSM, we assessed the role of intention in seroconcordant partnerships, as measured by participant rating of the importance of shared serostatus when selecting a sex partner.
Results
For HIV+ men, 30% of partnerships were seroconcordant; of these, 48% reported intent to be in such a partnership (intentional seroconcordance). For HIV− men, 64% of partnerships were seroconcordant; of these, 80% reported intentional seroconcordance. Intentional seroconcordance was associated with UAI for HIV+ partnerships (OR 1.9; CI: 1.3, 2.9), but not significant for HIV− partnerships (OR 1.1; CI: 0.99, 1.3). In separate models where intent was not considered, seroconcordance was associated with UAI for HIV+ partnerships (odds ratio (OR) 3.2; 95% confidence interval (CI): 2.2, 4.6) and for HIV− partnerships (OR 1.2; CI: 1.0, 1.3; p = 0.03).
Conclusions
Regardless of intentionality, seroconcordance was strongly associated with UAI for HIV+ men and weakly associated with UAI for HIV− men. Intentional seroconcordance was not associated with UAI more strongly than was seroconcordance in absence of consideration of intent. Intentionality may not be a critical element of the relationship between seroconcordance and UAI.
Keywords: HIV prevention, MSM, serosorting, measurement
Introduction
Among 49,273 HIV diagnoses in the United States in 2011, 62% are estimated to have occurred due to sexual transmission among men who have sex with men (MSM).1 Some MSM engage in seroadaptive strategies such as serosorting, which CDC defines as “choosing a sexual partner known to be of the same HIV serostatus, often to engage in unprotected sex, in order to reduce the risk of acquiring or transmitting HIV.”2 Inherent in the construct of CDC’s definition is that the choice of a same status (seroconcordant) partner is made “in order to reduce the risk” – i.e., that serosorting is not defined just by having a partner of the same serostatus, but that serosorting requires that the reason for this was the intention to reduce risk of HIV transmission.
Serosorting is a complex behavior occurring across time and multiple partnerships, making it difficult to operationalize a definition. A literature review of 51 studies identified two main definitions being used in studies of serosorting: a ‘behavioral-based’ definition that considers serosorting solely based on sexual behaviors (i.e. having a seroconcordant UAI partner), and an ‘identity-based’ definition that involves an explicit statement of intent to serosort. 3 Both behavioral- and identity-based definitions may be problematic. Behavioral definitions4–11 are limited to the extent that the proportion of behavior classified as serosorting is unintentional, due to chance. This may be particularly true for HIV negative (HIV−) MSM. A majority of their partnerships would be expected to be seroconcordant negative based solely on chance, because 81% of MSM are estimated to be HIV−.12 Alternately, intent-focused definitions7,13–17 are limited due to imperfect matches between intention and behavior. Studies of serosorting have not sought to explore the relative benefits and differences of these two types of measures, which are usually not implemented simultaneously. Therefore, it is unclear which of the two methods, or a combination thereof, should be used in future studies.
In San Francisco, an innovative approach used a baseline ‘identity-based’ intention statement, and assessed a ‘behavior-based’ definition at 12-month follow. 16,18 For HIV− men who stated intention to only have seroconcordant partners (pure serosorting) at baseline, 38% had enacted that behavior at a 12-month follow-up, compared to 15% of HIV− men who had not stated such intention. The authors of this pioneering study note that the results may not generalize to other settings beyond San Francisco, and that for the case of condom serosorting, only 3% of HIV− men behaviorally enacted their stated intent. Moreover, even for pure serosorting, a majority of HIV− men did not behaviorally enact their stated intent.
Whether serosorting is to be encouraged, discouraged or ignored involves determination of whether it is an effective HIV prevention strategy. The literature on this question is mixed, with one study that assessed HIV incidence indicating serosorting is protective against HIV transmission,19 while two other studies that assessed HIV positive cases among men previously undiagnosed with HIV found that serosorting had limited or no protective value.6,20 All of these studies used behavioral definitions of serosorting, and generalization of their estimates is limited by misclassification bias, to the extent that some men may not be intentionally serosorting. Moreover, understanding serosorting intention levels would provide information on the degree to which serosorting is a preconceived strategy that merits attention in the design of HIV prevention programs.
The present investigation seeks to fill several key gaps currently absent from the literature by using a national, internet-based sample to explore measurement of seroconcordance both with and without a statement of intention. Specifically, to our knowledge, no prior study has provided data of national scope regarding (1) the prevalence of intentional serosorting among seroconcordant partnerships, and its distribution among all sexual partnerships, (2) socio-demographic correlates of both seroconcordance and intentional serosorting and (3) the association between seroconcordance, intentional seroconcordance and UAI. We hypothesized that men who described their seroconcordance as intentional would be more likely to practice UAI, therefore supporting the CDC definition of serosorting as a behavior that should be primarily considered as an intentional phenomenon.
Methods
Study design
Data were analyzed from the baseline assessment of a prospective study of home HIV testing among MSM.21,22 Internet-using MSM with a reported US residence were recruited by banner advertising, from August-December 2010. Respondents eligible to participate in the study were men ≥18 years old that reported sex with ≥1 male partner in the prior 12 months. Zip code data were available from a subset of participants in the present study who were also enrolled in a follow-up study (n=930). This group reported residence in the Northeast (15%), the Midwest (19%), the South (41%), the West (26%) and Puerto Rico (1%). The study was approved by Emory University’s Institutional Review Board (Protocol IRB00031326).
Measures
For this analysis, we used data from measures of demographics, participant sexual experiences, and behaviors with recent sexual partners. Measures of participant demographics and aggregate sexual behaviors were based on the instrument used in the first MSM cycle of the CDC’s National HIV Behavioral Surveillance System (NHBS).23 Participant sexual experiences were assessed based on a 6-month recall period to minimize recall bias due to the detailed nature of the items. For up to five recent sex partners, participants completed a partner module that collected partner demographic and sexual risk behavior information. The partner module was developed based on questions in the NHBS, and adapted based on findings from focus groups and a Facebook.com pilot with over 1,000 participants.
Each sexual partnership’s (hereafter termed ‘partnership’) questions assessed sexual repertoire, relationship type, where the respondent met the partner, partner’s HIV status, and participant’s intent to be in a seroconcordant relationship. Sexual repertoire was assessed with, “Have you had anal sex with (partner name) in the past six months?” and “Have you had unprotected anal sex with (partner name) in the last six months?” A single variable was created based on answers to these questions, with categories of ever UAI and all other sex. Relationship type was categorized into one-time casual, repeated casual or main based on two items: “Did you have sex with (partner name) once, or more than once during the last 6 months?” and “Is (partner name) someone you feel committed to above all others (someone you might call your boyfriend or significant other, life partner or husband)?” Meeting venue was measured with, “Where did you first meet (partner name)?” This variable was categorized into online, through friends/work/school and other. Partner’s HIV status before first sex was determined with, “Did you and (partner’s name) share your HIV statuses before having sex?” and “What was (partner’s name) status?” Partnerships were classified as seroconcordant if the participant and partner’s HIV statuses were the same. To assess intent to serosort, the participant rated on a five-point Likert scale, “How important was the knowledge that (partner’s name) was (partner’s HIV status) in deciding to first have sex with (partner).” Likert scale points were: “Not important at all”, “Slightly important”, “Moderately important”, “Very important”, and “Extremely important.” We considered seroconcordance to be intentional when “Very important” or “Extremely important” options were selected.
Analysis
Respondent demographic data were analyzed at the individual level; data related to respondents’ sexual partners were analyzed at the partner level. All analyses were stratified by reported participant HIV status. Participants with unknown HIV status (22%: 774 out of the total sample of 3519) were excluded from analyses involving serosorting, because awareness of one’s own HIV status is requisite for the act of serosorting.
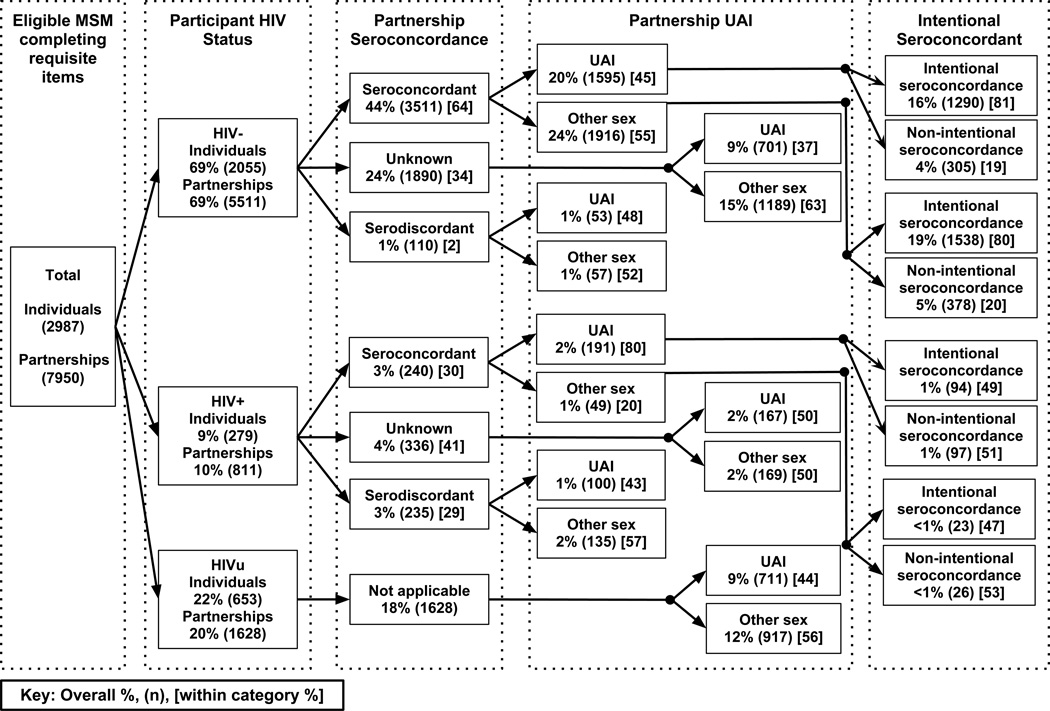
In Figure 1, we used unadjusted percents and counts to explore the distribution of intentional seroconcordance in our sample, stratified by participant HIV status, partner seroconcordance, and UAI practice. Table 1 describes differences in the distributions of participant demographic characteristics (age, income, education, sexual orientation and race/ethnicity) by participants’ HIV status (HIV+, HIV− or unknown), using Pearson’s χ2 values. Table 2 describes differences in distributions of partner characteristics (partner type, sex type, and meeting locale) by participants’ HIV status, using odds ratios (OR). In Table 3, we used OR to assess associations between demographic and partnership characteristics and (1) partnership seroconcordance and (2) partnership intentional seroconcordance. All OR were generated from generalized estimating equation (GEE) regressions with an exchangeable correlation matrix and logit link function. GEE regressions were used to account for repeated observations of participants within the partnership data.
Figure 1.
HIV status, seroconcordance, sexual behavior, and serosorting intentionality reported by MSM in an online HIV prevention study, United States, 2010. UAI: unprotected anal intercourse; other sex: oral sex or condom protected anal sex.
Table 1.
Demographic characteristics and serostatus of MSM in an online HIV prevention study, United States, 2010–2011
| HIV− | HIV+ | HIVu | Total | |
|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | |
| Age | ||||
| 18–24 | 33 (796) | 16 (56) | 68 (528) | 39 (1380) |
| 25–29 | 21 (492) | 15 (53) | 13 (101) | 18 (646) |
| 30–39 | 23 (549) | 22 (77) | 9 (68) | 20 (694) |
| >=40 | 23 (550) | 47 (162) | 10 (77) | 22 (789) |
| Income | ||||
| $0–$14,999 | 28 (635) | 24 (82) | 48 (333) | 32 (1050) |
| $15,000–$39,999 | 31 (700) | 29 (99) | 28 (194) | 30 (993) |
| $40,000 – $74,999 | 23 (522) | 24 (82) | 15 (106) | 22 (710) |
| >=$75,000 | 18 (408) | 22 (73) | 8 (58) | 16 (539) |
| Education | ||||
| High school or less | 16 (386) | 18 (61) | 36 (277) | 21 (724) |
| Some college | 38 (909) | 41 (142) | 44 (343) | 40 (1394) |
| College or more | 46 (1086) | 42 (145) | 20 (152) | 40 (1383) |
| Sexual orientation | ||||
| Homosexual | 81 (1944) | 90 (313) | 70 (542) | 80 (2799) |
| Bisexual | 16 (379) | 8 (29) | 24 (186) | 17 (594) |
| Other/heterosexual | 3 (63) | 2 (6) | 6 (45) | 3 (114) |
| Race/Ethnicity | ||||
| White, non-Hispanic | 54 (1300) | 46 (160) | 52 (401) | 53 (1861) |
| Black, non-Hispanic | 16 (376) | 31 (107) | 16 (126) | 17 (609) |
| Hispanic | 13 (321) | 10 (35) | 18 (143) | 14 (499) |
| Other | 16 (390) | 13 (46) | 13 (104) | 15 (540) |
HIV status based on participant self-report of HIV negative (HIV−), HIV positive (HIV+) or unknown (HIVu) serostatus.
Table 2.
Characteristics MSM partnerships by participant serostatus in an online HIV prevention study, United States, 2010–2011
| HIV− | HIV+ | Unknown | Total | |
|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | |
| Partner type | ||||
| Main | 24 (1446) | 17 (150) | 23 (402) | 23 (1998) |
| Casual, repeated | 31 (1817) | 36 (306) | 30 (536) | 31 (2659) |
| Casual, one time | 45 (2668) | 47 (403) | 47 (830) | 46 (3901) |
| Sex type | ||||
| UAI | 43 (2404) | 56 (461) | 44 (711) | 44 (3576) |
| CAI | 31 (1742) | 25 (201) | 28 (463) | 30 (2406) |
| Oral only | 27 (1500) | 19 (155) | 28 (454) | 26 (2109) |
| How encountered | ||||
| Online | 52 (3058) | 59 (508) | 48 (839) | 52 (4405) |
| Friend/Work/School | 24 (1399) | 12 (101) | 30 (519) | 24 (2019) |
| Other | 24 (1440) | 29 (245) | 23 (399) | 24 (2084) |
HIV status based on participant self-report of HIV negative (HIV−), HIV positive (HIV+) or unknown (HIVu) serostatus.
UAI: unprotected anal intercourse; CAI: condom protected anal intercourse.
Table 3.
Seroconcordant and intentional seroconcordant MSM partnerships by demographic and partnership characteristics in an online HIV prevention study, United States, 2010–2011
| HIV Negative Participants (HIV−) | HIV Positive Participants (HIV+) | |||||||
|---|---|---|---|---|---|---|---|---|
| Seroconcordant / All Partnerships |
Intentional / Seroconcordant Partnerships |
Seroconcordant / All Partnerships |
Intentional / Seroconcordant Partnerships |
|||||
| % (n) | OR (95% CI) | % (n) | OR (95% CI) | % (n) | OR (95% CI) | % (n) | OR (95% CI) | |
| Total | 64 (3567) | 80 (2863) | 30 (248) | 48 (120) | ||||
| Age | ||||||||
| 18–24 | 63 (1149) | ref | 84 (966) | ref | 18 (16) | ref | 69 (11) | ref |
| 25–29 | 63 (699) | 0.93 (0.75, 1.15) | 81 (566) | 0.93 (0.68, 1.28) | 20 (26) | 1.31 (0.52, 3.31) | 42 (11) | 0.35 (0.07, 1.93) |
| 30–39 | 64 (858) | 1.06 (0.86, 1.31) | 80 (683) | 0.73 (0.55, 0.98)* | 28 (59) | 2.19 (0.96, 4.98) | 46 (27) | 0.53 (0.12, 2.27) |
| >=40 | 65 (861) | 1.01 (0.82, 1.24) | 75 (648) | 0.56 (0.42, 0.74)‡ | 36 (147) | 3.26 (1.52, 7.01)† | 48 (71) | 0.51 (0.13, 2.03) |
| Sexual orientation | ||||||||
| Homosexual | 63 (2900) | ref | 79 (2290) | ref | 30 (234) | ref | 48 (112) | ref |
| Bisexual | 70 (581) | 1.29 (1.03, 1.61)* | 87 (505) | 1.69 (1.22, 2.35)† | 23 (11) | 0.66 (0.28, 1.57) | 64 (7) | 1.01 (0.22, 4.59) |
| Other/heterosexual | 66 (85) | 1.12 (0.67, 1.86) | 79 (67) | 0.82 (0.43, 1.57) | 18 (3) | 0.35 (0.05, 2.30) | 33 (1) | 1.18 (0.08, 18.23) |
| Race/Ethnicity | ||||||||
| White, non-Hispanic | 66 (2090) | ref | 79 (1653) | ref | 32 (144) | ref | 38 (55) | ref |
| Black, non-Hispanic | 56 (470) | 0.72 (0.58, 0.89)† | 82 (385) | 1.32 (0.95, 1.84) | 25 (54) | 0.72 (0.44, 1.15) | 61 (33) | 2.53 (1.11, 5.76)* |
| Hispanic | 58 (448) | 0.73 (0.59, 0.92)† | 82 (366) | 1.18 (0.85, 1.65) | 29 (21) | 0.85 (0.41, 1.77) | 90 (19) | 9.57 (1.95, 47.1)† |
| Other | 69 (559) | 1.12 (0.89, 1.40) | 83 (459) | 1.15 (0.85, 1.55) | 30 (29) | 0.88 (0.47, 1.64) | 45 (13) | 1.54 (0.55, 4.26) |
| Partner type | ||||||||
| Main | 75 (1003) | ref | 81 (806) | ref | 34 (50) | ref | 52 (26) | ref |
| Casual, repeated | 66 (1128) | 0.60 (0.52, 0.69)‡ | 78 (883) | 0.99 (0.84, 1.17) | 30 (90) | 0.77 (0.52, 1.14) | 41 (37) | 0.80 (0.52, 1.24) |
| Casual, one time | 56 (1422) | 0.43 (0.38, 0.49)‡ | 82 (1162) | 1.08 (0.93, 1.27) | 27 (105) | 0.67 (0.46, 0.98)* | 54 (57) | 0.99 (0.64, 1.53) |
| How encountered | ||||||||
| Other offline | 50 (669) | ref | 78 (517) | ref | 20 (48) | ref | 42 (20) | ref |
| Friend/Work/School | 63 (816) | 1.58 (1.37, 1.83)‡ | 81 (659) | 1.02 (0.84, 1.25) | 26 (25) | 1.45 (0.87, 2.42) | 28 (7) | 1.67 (0.91, 3.08) |
| Online | 70 (2074) | 2.07 (1.82, 2.35)‡ | 81 (1682) | 1.20 (1.00, 1.44) | 35 (175) | 1.72 (1.19, 2.49)† | 53 (93) | 1.89 (1.22, 2.93)† |
p<.05,
p<.01,
p<.001.
OR: Odds ratios adjusted for significant participant demographic and partnership characteristics.
HIV status based on participant self-report of HIV negative (HIV−), HIV positive (HIV+) or unknown (HIVu) serostatus.
In Table 4, we conducted a series of multivariable logistic regressions with UAI as the dependent variable and either seroconcordance or intentional seroconcordance as the independent variable, with all models based on the GEE parameters mentioned above. The purpose of developing these models was to understand the direction and magnitude of association between serosorting intention and UAI. To achieve less biased estimates, we controlled for a set of participant demographic variables and partnership variables shown to be significant predictors of UAI in prior research, which may also be associated with the two seroconcordance outcomes.14,24–28 Sensitivity analyses assessed whether changes in how we dichotomized serosorting intent impacted the direction or significance of study findings regarding its relation to UAI. All analyses were conducted in STATA 11.2.29
Table 4.
Adjusted associations between unprotected anal intercourse and serosorting measurement definition, stratified by reported HIV status, among MSM partnerships from an online HIV prevention study, United States, 2010–2011
| HIV Negative Participants (HIV−) | HIV Positive Participants (HIV+) | |||
|---|---|---|---|---|
| Model 1: Seroconcordance |
Model 2: Serosorting Intent |
Model 1: Seroconcordance |
Model 2: Serosorting Intent |
|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Seroconcordance | ||||
| No | ref | ref | ||
| Yes | 1.15 (1.01, 1.31)* | 3.21 (2.24, 4.61)‡ | ||
| Serosorting Intent | ||||
| No | ref | ref | ||
| Yes | 1.13 (0.99, 1.28) | 1.93 (1.28, 2.9)† | ||
| Partner type | ||||
| Casual, one time | ref | ref | ref | ref |
| Casual, repeated | 1.85 (1.63, 2.11)‡ | 1.86 (1.63, 2.11)‡ | 1.38 (1.00, 1.89)* | 1.40 (1.03, 1.90)* |
| Main | 5.83 (5.03, 6.75)‡ | 5.87 (5.08, 6.8)‡ | 2.12 (1.40, 3.22)‡ | 2.11 (1.41, 3.15)‡ |
| How encountered | ||||
| Offline | ref | ref | ref | ref |
| Online | 0.94 (0.83, 1.06) | 0.95 (0.84, 1.07) | 1.09 (0.79, 1.50) | 1.12 (0.82, 1.52) |
| Race/Ethnicity | ||||
| White, non-Hispanic | ref | ref | ref | ref |
| Black, non-Hispanic | 0.97 (0.79, 1.20) | 0.97 (0.79, 1.20) | 0.48 (0.30, 0.76)† | 0.45 (0.28, 0.73)† |
| Hispanic | 0.90 (0.73, 1.11) | 0.90 (0.73, 1.11) | 0.71 (0.35, 1.46) | 0.66 (0.32, 1.36) |
| Other | 0.80 (0.65, 0.98)* | 0.80 (0.65, 0.98)* | 0.47 (0.26, 0.88)* | 0.46 (0.25, 0.86)† |
| Age | ||||
| 18–24 | ref | ref | ref | ref |
| 25–29 | 0.98 (0.80, 1.19) | 0.98 (0.80, 1.19) | 1.26 (0.61, 2.59) | 1.52 (0.73, 3.19) |
| 30–39 | 0.96 (0.79, 1.17) | 0.97 (0.79, 1.17) | 1.66 (0.84, 3.27) | 2.11 (1.05, 4.22)* |
| >=40 | 0.90 (0.74, 1.10) | 0.91 (0.74, 1.11) | 1.41 (0.75, 2.65) | 1.83 (0.97, 3.47) |
| Education | ||||
| High school or less | ref | ref | ref | ref |
| Some college / Associates degree | 0.98 (0.79, 1.21) | 0.98 (0.79, 1.21) | 1.29 (0.73, 2.27) | 1.34 (0.76, 2.39) |
| College or more | 0.73 (0.59, 0.91)† | 0.73 (0.59, 0.91)† | 1.52 (0.86, 2.67) | 1.54 (0.87, 2.72) |
| Sexual orientation | ||||
| Homosexual | ref | ref | ref | ref |
| Bisexual | 1.09 (0.89, 1.34) | 0.91 (0.74, 1.11) | 0.99 (0.45, 2.20) | 0.88 (0.40, 1.95) |
| Other/heterosexual | 0.70 (0.41, 1.18) | 0.64 (0.39, 1.05) | 1.43 (0.31, 6.64) | 1.31 (0.34, 5.11) |
p<.05,
p<.01,
p<.001,
aOR=adjusted odds ratio.
HIV status based on participant self-report of HIV negative (HIV−), HIV positive (HIV+), or unknown status. Participants unaware of their HIV status are excluded from the models, as it is not possible for them to engage in serosorting. Seroconcordance determined by reporting a partner of same serostatus; intentional seroconcordance determined by presence of seroconcordance and endorsement of a statement of intent to be in a seroconcordant partnership.
Results
Overall, 6,104 men began the online survey, and 4,138 began the section regarding sex partners, a response rate of 68%. Of this group, 3,519/4138 (85%) had sex with a man in the previous six months. Table 1 shows demographic features of the 3,519 respondents who had sex with men in the last six months, by reported HIV status. At the individual level, MSM unaware of their status (HIVu) were younger, poorer and less educated than HIV− and HIV+ MSM. HIVu men were less likely than HIV− and HIV+ men to identify as homosexual (70, 81 and 90% respectively; p<0.001). Racial composition among the groups was similar, with the exception of non-Hispanic blacks being over-represented in the HIV+ group (p<0.001).
From 3,519 respondents who reported sex with a man in the previous six months, we collected participant HIV status and partnership type data on 8,558 partnerships in the prior six months (i.e., each man reported, on average, data on 2–3 sexual partners). Table 2 shows characteristics of these partnerships by participant’s HIV status. Nearly half were UAI partners, and less than one-quarter were classified as main partners. Approximately half of partners were met online. A higher proportion of HIV+ men reported UAI than HIV− and HIVu men (56%, 43% and 44%, respectively; p<0.001). Also, a lower proportion of HIV+ men reported main partnerships than HIV− and HIVu men (17%, 24% and 23%, respectively; p<0.001). A higher proportion of HIV+ men’s partners were met online than among HIV− and HIVu men (59%, 52% and 48%, respectively; p<0.05).
Figure 1 details classification of eligible individuals, and their partnerships, by respondent serostatus, partnership seroconcordance, partnership UAI in the last six months and intentionality of partnership seroconcordance. It includes data from 7,950/8,558 respondent partnerships in which all items were completed regarding HIV status, partner HIV status, UAI and intentionality of seroconcordance. Based on CDC’s definition of serosorting (intention and seroconcordance and practice of UAI), 17% of all partnerships (16% HIV−, 1% HIV+) indicated serosorting.
Nearly half of all partnerships involved an unknown serostatus (HIVu) respondent or partner: 20% of partnerships involved HIVu respondents and any partners (R:HIVu, P:any) and 28% involved known status participants and HIVu partners (R:HIV−, P:HIVu or R:HIV+, P:HIVu). For all partnerships with an HIVu member, seroconcordance or serodiscordance could not have been established at the time of the partnership, and therefore serosorting was not possible. The reason for unknown HIV status differed for HIVu respondents and HIVu partners. Most HIVu respondents (90%) reported never having received an HIV test, but nearly all respondents with HIVu partners reported never discussing their partner’s serostatus (98%), an issue further explored in a separate analysis.30
Levels of UAI were high across all subgroups in the analysis: the lowest level was over one-third (37%) for R:HIV−, P:HIVu partnerships, and the highest level was over three-quarters (80%) for R:HIV+, P:HIV+ partnerships. For all other partnership groupings, the range of UAI levels was remarkably narrow (43–50%) given that this figure includes diverse partnerships such as R:HIV−, P:HIV+; R:HIV−, P:HIV−; and R:HIV+, P:HIVu partnerships.
Among HIV− respondent partnerships, known serodiscordance (R:HIV−, P:HIV+) was rare (2%). This level is less than would be expected by chance, given that 10% of the overall sample was HIV+. Although HIV− respondents reported fewer than expected serodiscordant partnerships based on random mixing alone, one-third (34%) of their partnerships involved HIVu partners.
Among HIV+ respondent partnerships, fewer were known serodiscordant (R:HIV+, P:HIV−) (29%) than would be expected based on the prevalence of HIV− men (69%) in our sample. Similarly, more HIV+ respondent partnerships were serconcordant (30%) than would be expected based on random partnering based on the 10% HIV prevalence in our sample.
Table 3 shows proportions of partnerships which were seroconcordant, and proportions of these seroconcordant partnerships classified as intentionally seroconcordant. Overall, the proportion of seroconcordant partnerships was 64% for HIV− respondents and 30% for HIV+ respondents; within seroconcordant partnerships, intention to choose a seroconcordant partner was 80% for HIV− respondents and 48% for HIV+ respondents. The intersection of seroconcordance and stated intention shows that 51% of HIV− partnerships and 15% of HIV+ partnerships were intentionally seroconcordant.
Demographic and behavioral factors associated with being in a seroconcordant partnership were largely similar for HIV− and HIV+ respondents, except for age. HIV+ respondents aged ≥ 40 years were significantly more likely to be in seroconcordant partnerships than their age 18–24 counterparts (odds ratio (OR) 3.3; 95% confidence interval (CI): 1.5, 7.0), but age had no significant association with seroconcordance for HIV− partnerships (OR 1.0; CI: 0.8, 1.2). Relative to white respondent partnerships, point estimates indicated decreases in odds of seroconcordance for HIV− black (OR 0.72; CI: 0.58, 0.89) and HIV+ black (OR 0.72; CI: 0.44, 1.2) respondent partnerships. One-time casual partners were less likely to be seroconcordant than main partners for HIV− (OR 0.43; CI: 0.38, 0.49) and HIV+ (OR 0.67; CI: 0.46, 0.98) respondents. Online partnerships were more likely than offline partnerships to be seroconcordant for HIV− (OR 2.1; CI: 1.8, 2.4) and HIV+ (OR 1.7; CI: 1.2, 2.5) respondents.
Among HIV− seroconcordant partnerships, intention to be in such a partnership was associated with being ≥ 40 years of age (OR 0.56; CI: 0.42, 0.74) and bisexuality (OR 1.7; CI: 1.2, 2.4). Among HIV+ seroconcordant partnerships, intention was associated with participant’s black race (OR 2.5; CI: 1.1, 5.8), Hispanic ethnicity (OR 9.6; CI: 2.0, 47) and meeting a partner online (OR 1.9; CI: 1.2, 2.9). Thus, seroconcordance and intentional seroconcordance shared few demographic associations.
Models of associations between UAI and the independent variable, any seroconcordance or intended seroconcordance, were stratified by participant serostatus (Table 4). For HIV− men, a small but significant association was found between seroconcordance and UAI (OR 1.2; CI: 1.0, 1.3; p = 0.03). However, UAI was not associated with intended seroconcordance (OR 1.1; CI: 0.99, 1.3). For HIV+ men, seroconcordance was strongly associated with UAI (OR 3.2; CI: 2.2, 4.6), and intended seroconcordance was similarly associated (OR 1.9; CI: 1.3, 2.9). Across all four models, main partnership was strongly associated with UAI. For HIV− men, the association (OR 5.8; CI: 5.0, 6.8) was substantially stronger than the association between seroconcordance and UAI described above. Indicating a potential dose-response relationship, casual repeated partnerships, compared to one-time partnerships, were also associated with UAI across the models. The only other significant control variable across models of UAI was ethnicity, with “other” ethnicity members less likely to report UAI than White, non-Hispanics. Sensitivity analyses indicated that using different cut-points on the Likert scale to dichotomize the serosorting intention variable did not alter the direction or significance of study findings regarding serosorting and its relation to UAI.
Discussion
We found high levels of stated intention to be in a seroconcordant relationship among HIV− seroconcordant partnerships (80%), and moderate levels (48%) among HIV+ seroconcordant partnerships. For HIV− men, nearly two-thirds of all their partnerships were seroconcordant, and most of that seroconcordance was intended. High rates of stated intention indicate that seroconcordance was not solely due to social or sexual network structures, but instead may be the product of a deliberate harm-reduction strategy. Supporting this hypothesis was the rarity of known serodiscordant relationships (2%) among HIV− respondents relative to HIV prevalence in our sample of 10%. Some HIV− men might avoid the cognitive dissonance of known serodiscordant partnerships, instead taking on undefined risk by not ascertaining their partner’s serostatus (34% of HIV− men’s partnerships were with partners of unknown serostatus).
About one-third of HIV+ men’s partnerships were seroconcordant, with just under half of these reported as intentionally concordant. Seroconcordant HIV+ partnerships made up a higher proportion of HIV+ men’s partnerships than would be expected by chance, but the absolute level was low (30%). Like the present study, other research has found seroconcordance among both HIV− and HIV+ men to occur at higher than expected levels.16
Associations between UAI and different measures of serosorting did not support our hypothesis that men who described their seroconcordance as intentional would be more likely to practice UAI. Our findings regarding the limitations of seroconcordance intent in predicting UAI behavior, however, echo a meta-analysis that concluded that across multiple domains, health behavior intentions accounted for only 22% of the variance in health behaviors.31
Measurement of the ‘intent to serosort’ construct has been fraught with difficulties relating to time order, improperly aggregated behavior, and the degree to which pre-imposed strategies are formulated and subsequently influence sexual decisions (in addition to other, more common behavioral measurement limitations such as recall bias). Our measures attempted to mitigate problems relating to time order by assessing participant’s knowledge of partner’s HIV status prior to first sex. Further, our measures sought to mitigate the need for participants to aggregate behavioral strategy across different partnerships, because in actuality men may use different strategies in different situations. Yet our measures were limited to the extent that participants either 1) did not approach sexual partnerships with a conscious, pre-formulated HIV prevention plan or 2) did not consistently implement within partnerships such HIV prevention plans. A separate study found that over half of HIV− MSM practicing UAI outside of their main partnership reported that they had no specific HIV risk reduction strategy;32 this may indicate that, for a substantial proportion of individuals, conscious and pre-formulated plans may not accurately describe their HIV−risk reduction behaviors.
Several possible scenarios could explain why measurement of intention did not lead to stronger associations with UAI. First, although seroconcordance was associated with UAI, intention might have no impact on this relationship. If this were the case, using a stated intention measure would effectively reduce sensitivity of the “any seroconcordance” measure and would misclassify respondents. Another possibility is that our measure of intent is not adequate to detect a true relationship between seroconcordance intentionality and UAI. The most probable scenario is that some combination of the above factors influenced our findings regarding intent and UAI. Also, it is possible that, despite our large number of respondents and partnerships, we did not have sufficient power to detect differences between the magnitude of the associations between UAI and the two definitions of seroconcordance. Despite uncertainty regarding intent, this study yields clear implications for future research. The CDC’s definition of serosorting requiring intent may reduce sensitivity unnecessarily. Upcoming studies should always include a behavioral measure of serosorting, based on seroconcordance and UAI, and not rely solely on identity measures of intentional serosorting.
Regardless of intentionality, seroconcordance was strongly associated with UAI for HIV+ men and weakly associated with UAI for HIV− men, findings very similar to those from other studies of serosorting.10,33 Except the small (2%) set of HIV+ seroconcordant partnerships, there was remarkably little variation in levels of UAI across all other partnerships grouped by seroconcordance in the study: 37%–50%. This translated to very different partnership types having similar levels of UAI. This could be seen as indicating continued and high overall UAI, but also could be interpreted as a lack of substantial increase in UAI among HIV− men in seroconcordant relationships (45%) relative to HIV− men in sero-unknown (37%) or serodiscordant (48%) relationships. This finding, combined with failure of intention to improve the association between seroconcordance and UAI, calls into question the plausibility of incorporating serosorting behaviors among HIV− men into HIV prevention interventions.
Out of all factors in our models of UAI, main partnership had the strongest correlation, indicating the importance of relationship commitment as a factor in sexual practices. Data from the NHBS and other surveys of MSM also show main partnership to be highly associated with UAI, a contributor to the estimate that a substantial proportion of incident HIV transmissions occur within main partnerships.24,25 The implication of small effects of seroconcordance and large effects of main partnership on UAI among HIV− men indicates prevention programs are likely to benefit from increased focus on factors that develop during relationships, such as trust and commitment.
Our study had a number of important limitations. Our respondents comprised a sample of Internet-using men who opted into a banner-ad based survey, making our findings subject to selection bias. Study findings therefore are not representative of all US MSM, or all internet-using MSM. The cross-sectional nature of our data also limited our assessments of relationships to associations. Because we asked participants to report retrospectively on their intent, data are subject to recall bias; it is not clear what impact such bias might have on our results. The median interval from relationship initiation to interview was less than six months, however, which somewhat mitigates this concern. Additionally, we relied on self-reported data for all measures, which could result in social desirability bias. We excluded HIVu participants only from analyses directly related to serosorting, because serosorting is not a meaningful strategy without knowledge of one’s own serostatus. Exclusion of this group is non-trivial, because HIVu respondents were younger, lower-income, less educated and less likely to identify as homosexual than their known serostatus peers. A similar limitation is that all partnerships men avoided, such as an HIV− man avoiding an HIV+ partner, were by definition not captured in our measures of sexual behavior. Despite these limitations, the present study’s methodology also has some advantages. First, by conducting an online survey, we were able to collect data from across the United States. Second, our measurement of intent and seroconcordance sought to resolve a long-standing question regarding the definition of serosorting, and the HIV prevention significance of intentional versus unintentional seroconcordance.
According to our findings, understanding whether seroconcordance is intentional is not necessary to understand the relationship between seroconcordance and UAI. We found a small, but significant, association between HIV− partnership seroconcordance and higher levels of UAI. For men living with HIV infection, having an HIV+ partner was strongly associated with UAI, despite the reality that these men less often expressed that their serosorting was intentional than did their HIV− counterparts. These results indicate that intention to be in a seroconcordant partnership may be less determinant of UAI than seroconcordance itself.
Acknowledgments
Source of Funding:
This study was supported by the National Institute on Minority Health and Health Disparities RC1MD004370, National Institute of Mental Health R01MH085600, Eunice Kennedy Shriver National Institute for Child Health and Human Development R01HD067111, and was facilitated by the Emory Center for AIDS Research P30AI050409. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data presentation:
Parts of data in this manuscript were presented at the 2011 National HIV Prevention Conference in Atlanta, GA: August 14–17.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention. [Accessed May 25, 2013];HIV Surveillance Report, 2011. 2013 :23. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/
- 2.Centers for Disease Control and Prevention. [Accessed May 25, 2013];Meeting Summary: “Consultation on Serosorting Practices among Men who Have Sex with Men”. 2009 http://www.cdc.gov/hiv/topics/research/resources/other/serosorting.htm.
- 3.Eaton LA, Kalichman SC, O'Connell DA, Karchner WD. A strategy for selecting sexual partners believed to pose little/no risks for HIV: serosorting and its implications for HIV transmission. AIDS Care. 2009 Oct;21(10):1279–1288. doi: 10.1080/09540120902803208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lattimore S, Thornton A, Delpech V, Elford J. Changing patterns of sexual risk behavior among London gay men: 1998–2008. Sex Transm Dis. 2011 Mar;38(3):221–229. doi: 10.1097/OLQ.0b013e3181f2ebe1. [DOI] [PubMed] [Google Scholar]
- 5.Bruce D, Harper GW, Suleta K. Sexual Risk Behavior and Risk Reduction Beliefs Among HIV-Positive Young Men Who have Sex with Men. AIDS Behav. 2012 Feb 15; doi: 10.1007/s10461-012-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr. 2008 Oct 1;49(2):212–218. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- 7.Eaton LA, Kalichman SC, Cain DN, et al. Serosorting sexual partners and risk for HIV among men who have sex with men. Am J Prev Med. 2007 Dec;33(6):479–485. doi: 10.1016/j.amepre.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velter A, Bouyssou-Michel A, Arnaud A, Semaille C. Do men who have sex with men use serosorting with casual partners in France? Results of a nationwide survey (ANRS-EN17-Presse Gay 2004) Euro Surveill. 2009;14(47) doi: 10.2807/ese.14.47.19416-en. [DOI] [PubMed] [Google Scholar]
- 9.Jin F, Crawford J, Prestage GP, et al. Unprotected anal intercourse, risk reduction behaviours, and subsequent HIV infection in a cohort of homosexual men. AIDS. 2009 Jan 14;23(2):243–252. doi: 10.1097/QAD.0b013e32831fb51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zablotska IB, Imrie J, Prestage G, et al. Gay men's current practice of HIV seroconcordant unprotected anal intercourse: serosorting or seroguessing? AIDS Care. 2009 Apr;21(4):501–510. doi: 10.1080/09540120802270292. [DOI] [PubMed] [Google Scholar]
- 11.Marks G, Millett GA, Bingham T, Lauby J, Murrill CS, Stueve A. Prevalence and protective value of serosorting and strategic positioning among Black and Latino men who have sex with men. Sex Transm Dis. 2010 May;37(5):325–327. doi: 10.1097/OLQ.0b013e3181c95dac. [DOI] [PubMed] [Google Scholar]
- 12.Prevalence and awareness of HIV infection among men who have sex with men --- 21 cities, United States, 2008. MMWR. Morbidity and mortality weekly report. 2010 Sep 24;59(37):1201–1207. [PubMed] [Google Scholar]
- 13.van den Boom W, Stolte I, Sandfort T, Davidovich U. Serosorting and sexual risk behaviour according to different casual partnership types among MSM: the study of one-night stands and sex buddies. AIDS Care. 2012;24(2):167–173. doi: 10.1080/09540121.2011.603285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois-Arber F, Jeannin A, Lociciro S, Balthasar H. Risk Reduction Practices in Men Who Have Sex with Men in Switzerland: Serosorting, Strategic Positioning, and Withdrawal Before Ejaculation. Arch Sex Behav. 2011 Nov 15; doi: 10.1007/s10508-011-9868-4. [DOI] [PubMed] [Google Scholar]
- 15.McFarland W, Chen YH, Raymond HF, et al. HIV seroadaptation among individuals, within sexual dyads, and by sexual episodes, men who have sex with men, San Francisco, 2008. AIDS Care. 2011 Mar;23(3):261–268. doi: 10.1080/09540121.2010.507748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFarland W, Chen YH, Nguyen B, et al. Behavior, intention or chance? A longitudinal study of HIV seroadaptive behaviors, abstinence and condom use. AIDS Behav. 2012 Jan;16(1):121–131. doi: 10.1007/s10461-011-9936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prestage G, Brown G, Down IA, Jin F, Hurley M. "It's Hard to Know What is a Risky or not a Risky Decision": Gay Men's Beliefs About Risk During Sex. AIDS Behav. 2012 Mar 20; doi: 10.1007/s10461-012-0180-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Vallabhaneni S, Raymond HF, McFarland W. Predictors of serosorting and intention to serosort among men who have sex with men, San Francisco. AIDS Educ Prev. 2012 Dec;24(6):564–573. doi: 10.1521/aeap.2012.24.6.564. [DOI] [PubMed] [Google Scholar]
- 19.Philip SS, Yu X, Donnell D, Vittinghoff E, Buchbinder S. Serosorting is associated with a decreased risk of HIV seroconversion in the EXPLORE Study Cohort. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden MR, Dombrowski JC, Kerani RP, Stekler JD. Failure of serosorting to protect African American men who have sex with men from HIV infection. Sex Transm Dis. 2012 Sep;39(9):659–664. doi: 10.1097/OLQ.0b013e31825727cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khosropour CK, Sullivan PS. Mobile phone-based data collection to enhance retention of racial/ethnic minorities in a longitudinal internet-based HIV behavioral risk study of MSM in the United States; 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 22.Rosenberg ES, Khosropour CK, Sullivan PS. Heterogeneous racial differences in disclosure of HIV status by serostatus and partnership sexual risk among US MSM; The 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; Rome, Italy. 2011. [Google Scholar]
- 23.Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral surveillance among people at risk for HIV infection in the U.S. : the National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(Suppl 1):32–38. doi: 10.1177/00333549071220S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodreau SM, Carnegie NB, Vittinghoff E, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PLoS One. 2012;7(11):e50522. doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan PS, Salazar L, Buchbinder S, Sanchez TH. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS. 2009 Jun 1;23(9):1153–1162. doi: 10.1097/QAD.0b013e32832baa34. [DOI] [PubMed] [Google Scholar]
- 26.Beyrer C. HIV epidemiology update and transmission factors: risks and risk contexts--16th International AIDS Conference epidemiology plenary. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007 Apr 1;44(7):981–987. doi: 10.1086/512371. [DOI] [PubMed] [Google Scholar]
- 27.Grov C, DeBusk JA, Bimbi DS, Golub SA, Nanin JE, Parsons JT. Barebacking, the Internet, and harm reduction: an intercept survey with gay and bisexual men in Los Angeles and New York City. AIDS Behav. 2007 Jul;11(4):527–536. doi: 10.1007/s10461-007-9234-7. [DOI] [PubMed] [Google Scholar]
- 28.Crepaz N, Marks G, Liau A, et al. Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: a meta-analysis. AIDS. 2009 Aug 24;23(13):1617–1629. doi: 10.1097/QAD.0b013e32832effae. [DOI] [PubMed] [Google Scholar]
- 29.Stata Statistical Software: Release 11 [computer program] College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 30.Winter AK, Sullivan PS, Khosropour CM, Rosenberg ES. Discussion of HIV Status by Serostatus and Partnership Sexual Risk among Internet-Using MSM in the United States. J Acquir Immune Defic Syndr. 2012 Apr 30; doi: 10.1097/QAI.0b013e318257d0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armitage CJ, Conner M. Efficacy of the Theory of Planned Behaviour: a meta-analytic review. The British journal of social psychology / the British Psychological Society. 2001 Dec;40(Pt 4):471–499. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell JW. HIV-Negative and HIV-Discordant Gay Male Couples' Use of HIV Risk-Reduction Strategies: Differences by Partner Type and Couples' HIV-Status. AIDS Behav. 2013 May;17(4):1557–1569. doi: 10.1007/s10461-012-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell JJ, Bragg L, Shiboski S, Grant RM. Sexual seroadaptation: lessons for prevention and sex research from a cohort of HIV-positive men who have sex with men. PLoS One. 2010;5(1):e8831. doi: 10.1371/journal.pone.0008831. [DOI] [PMC free article] [PubMed] [Google Scholar]