Abstract
In this issue of Molecular Cell, Zhang and colleagues (2013) identify a new class of intron-derived circular RNAs (ciRNAs) and show that they have the potential to enhance transcription of their host gene.
The advent of genome-wide approaches has revealed that a much greater fraction of metazoan genomes are transcribed than previously appreciated (Kapranov et al., 2007). Increased sequencing depth and novel RNA profiling strategies have identified a seemingly endless stream of novel classes of non-coding RNA molecules, including large intergenic non-coding RNAs (lincRNAs), intron-derived small nucleolarlincRNAs (sno-lincRNAs), enhancer RNAs (eRNAs) as well as a variety of different small RNAs (miRNAs, piRNAs, etc.) (Rinn and Chang, 2012). The fact that many of the observed non-coding RNAs undergo complex RNA processing and biogenesis, tissue- or cell-specific regulation, distinct subcellular localization patterns, or are associated with various diseases, suggests that they have important, though mostly unknown functions (Wang and Chang, 2011). Among the most interesting classes of non-coding RNAs are the recently described circular RNAs – several groups have reported the existence of circular RNA (circ-RNAs) molecules generated by a process called back-splicing wherein downstream exons are spliced to upstream exons in reverse order (Wilusz and Sharp, 2013). Some of these circ-RNAs have been shown to act as molecular sponges by competing and/or sequestering miRNAs (Wilusz and Sharp, 2013). In this issue of Molecular Cell, Zhang et al. report the identification of yet another class of circular intronic RNAs (ciRNAs) derived from introns of protein coding genes and demonstrate their capacity to enhance transcription of their host genes.
Pre-mRNA splicing occurs via a two-step mechanism catalyzed by the spliceosome wherein the 2’OH of the branch point nucleotide attacks the phosphodiester bond at the 5’ splice site followed by an attack by the free 3’OH of the 5’exon on the 3’ splice site resulting in ligated exons and the excised lariat intron. The vast majority of excised introns are immediately linearized by the debranching enzyme and subsequently degraded.
To search for novel intronic non-coding RNAs, Zhang et al. scoured poly(A)-, ribosomal-depleted RNA sequence data obtained from H9 and HeLa cells and identified nearly 700 introns for which robust signal was observed. Only 6 of these introns corresponded to the sno-lincRNAs previously described by Chen and colleagues (Yin et al., 2012). Strikingly, the authors find that many of the remaining introns are resistant to degradation by RNAse R, anexoribonuclease that digests linear RNAs but not lariat or circular RNAs. As these ciRNAs don't have a unique promoter, they appear to be generated by transcription and splicing from the host pre-mRNA, but are highly stable by escaping debranching.
Though there have been few previously reported cases of stable, circular introns, the mechanism of stability and how they escape debranching has been unknown (Gardner et al., 2012). Zhang et al. identified consensus sequences near the 5' splice site and branch points enriched in the ciRNAs and demonstrate that these sequences are sufficient to convert an unstable intron into a stable ciRNA. The sequence motifs of the 5' splice site and branch points of ciRNAs are GU-rich and C-rich, respectively, raising the possibility that base-pairing between these sequences may be involved in the escape of ciRNAs from debranching.
Over the past few years many studies have demonstrated that non-coding RNAs function to directly or indirectly control transcription, splicing, translation, or RNA stability. Many lincRNAs, including Xist, act as guides or scaffolds for chromatin-modifying complexes that change the chromatin structure of target regions and either silence or enhance transcription (Wang and Chang, 2011). sno-lincRNAs, sequester the Fox family splicing regulators to change the alternative splicing output of transcripts (Yin et al., 2012). Furthermore, U1 snRNP has been shown to inhibit premature polyadenylation of pre-mRNAs by binding nascent transcripts independent of its role in splicing (Berg et al., 2012). Finally, miRNAs have been shown to both modulate the translation efficiency and the stability of target mRNAs (Fabian et al., 2010).
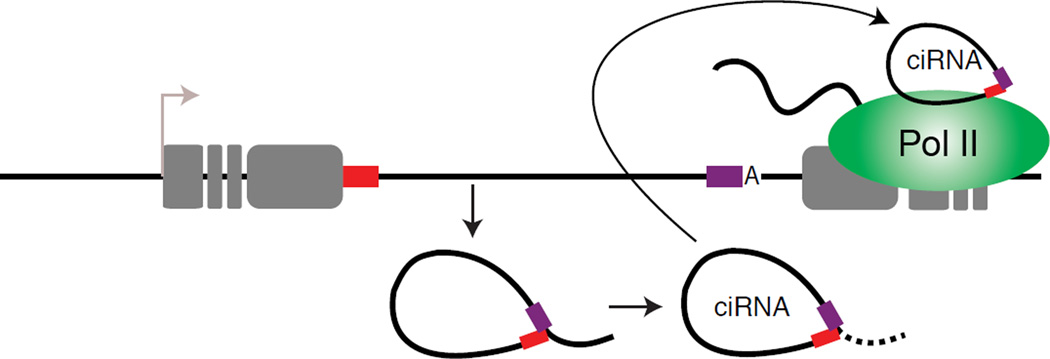
To investigate the potential mechanism(s) by which ciRNAs might function, Zhang et al. first examined the subcellular localization pattern of ciRNAs. Similar to many other lincRNAs, ciRNAs are nuclear retained, associate with chromatin and localize to the sites of host gene transcription, suggesting that they may function by modulating expression of the host gene. To test this, the authors used antisense oligonucleotides (ASO) to knockdown specific ciRNAs and observed decreased levels of the parent mRNAs. Strikingly, this effect is only observed when the ASO is targeted to the ciRNA intron, but not to other non-ciRNA introns in the same gene. They also use UV crosslinking followed by immunoprecipitation to show that at least one ciRNA is associated with elongating RNA Polymerase II (Pol II). Finally, the authors use nuclear run-on analysis to demonstrate that nascent RNA transcription of the parent gene is reduced upon depletion of the ciRNA but not other introns of the host gene. Together these data suggest that ciRNAs are processed co-transcriptionally, remain nearby the host gene, associate with elongating Pol II, and enhance transcription of the host gene. Thus, ciRNAs appear to function through a novel mechanism of cis-regulation of transcription of their host genes(Figure 1).
Figure 1. Stable circular intron RNAs (ciRNAs) enhance host gene transcription.
ciRNAs are generated from lariat introns that escape debranching. Sequences near the 5’ splice site (red box) and branch point (purple box) are minimally sufficient for an intron to escape debranching and become a stable ciRNA. The stable ciRNA binds to elongating RNA Polymerase II and enhances transcription.
The mechanisms of ciRNA biogenesis and ciRNA-mediated transcriptional control, although not elucidated in detail in this study, poses many interesting questions. Do ciRNAs form because they are sub-optimal substrates for the debranching enzyme and are therefore a by product of an inefficient process or does a mechanism exist that ensures the formation of ciRNA from these introns. Does every copy of the ciRNA forming introns generate a stable ciRNA? The most intriguing question is the requirement of a circular intron; is the circularity of ciRNAs required for their ability to enhance transcription? If so, it would be interesting to identify proteins that bind specifically to the circular form of ciRNAs, but not the linear forms of the same introns. While the answers to these questions require much further experimentation, the ciRNAs described by Zhang et al. are yet another exciting addition to the increasingly diverse repertoire of functional non-coding RNAs encoded in eukaryotic genomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Berg MG, Singh LN, Younis I, Liu Q, Pinto AM, Kaida D, Zhang Z, Cho S, Sherrill-Mix S, Wan L, Dreyfuss G. Cell. 2012;150:53–64. doi: 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MF, Sonenberg N, Filipowicz W. Annual Review of Biochemistry. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Gardner EJ, Nizami ZF, Talbot CC, Jr, Gall JG. Genes and Development. 2012;15:2550–2559. doi: 10.1101/gad.202184.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapanov P, Willingham AT, Gingeras TR. Nature Reviews Genetics. 2007;8:413–423. doi: 10.1038/nrg2083. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Chang HY. Annual Review of Biochemistry. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Chang HY. Mol Cell. 2011;48:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz JE, Sharp PA. Science. 2013;340:440–441. doi: 10.1126/science.1238522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin QF, Yang L, Zhang Y, Xiang JF, Wu YW, Carmichael GG, Chen LL. Mol Cell. 2012;48:219–230. doi: 10.1016/j.molcel.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Molecular Cell. 2013 doi: 10.1016/j.molcel.2013.08.017. in press. [DOI] [PubMed] [Google Scholar]