Abstract
Statins reduce cholesterol synthesis and are widely used for the treatment of hyperlipidemia and ischemic heart disease. Besides their cholesterol-lowering effects, statins also possess broad immunomodulatory and anti-inflammatory properties. Vascular endothelial cells play a critical role in the pathogenesis of inflammatory disease and, alongside leukocytes and antigen presenting cells, represent a key cellular target for statin therapy. Recent studies investigating how these drugs modify endothelial cell function demonstrate that the therapeutic effect of statins can be attributed, in part, to their action on the endothelium. Accordingly statins attenuate endothelial MHC class II expression, increase eNOS and fibrinolytic activity, decrease leukocyte adhesion and transmigration and enhance resistance to local injurious stimuli. Many of these effects are brought about by modulation of small GTPase function and downregulation of pro-inflammatory gene expression.
Introduction
Chronic inflammatory diseases represent a wide range of conditions that include rheumatoid arthritis, multiple sclerosis, atherosclerosis and psoriasis. The causative factors and pathogenesis of these inflammatory-related diseases are diverse but common to all is the fundamental role played by the vascular endothelium in lesion development. How endothelial cells (ECs) function in the promotion, response to and resolution of an inflammatory episode remains a major focus for research, especially as the vasculature presents itself as an attractive pharmacological target.
Over the preceding decade statins (HMG-CoA reductase inhibitors; see Box 1) have emerged as potentially powerful modulators of the inflammatory process and for that reason are increasingly studied for their therapeutic efficacy in a multitude of immune-related diseases. The mechanism by which statins modulate the immune response is complex but is frequently referred to as lipid-independent. More accurately this mechanism should be referred to as LDL-cholesterol independent as the so-called lipid-independent effects primarily involve inhibition of isoprenoid lipid production and subsequent protein prenylation and activity of signaling proteins such as the small GTPases (see Box 2) [1]. It has also been proposed that a reduction in cholesterol synthesis alters the composition of membrane lipid rafts, structures which are integral to the assembly of important cell signalling microdomains [2,3]. Unlike their effect on protein prenylation and lipid raft composition, which are dependent on the inhibition of HMG-CoA reductase, statins have also been shown to bind directly to the leukocyte integrin LFA-1 and so interfere with binding to its principle ligand intercellular adhesion molecule (ICAM)-1 [4]. In most cases, however, the effect of statins can be reversed with mevalonate supplementation, suggesting that HMG-CoA reductase independent effects are not a major mechanism. The cell processes affected by statins, in particular those dependent on small GTPases, are ubiquitous throughout an organism and therefore affect the function of most cell types. Accordingly, their impact on immune function is not restricted to modulation of leukocyte biology (see accompanying review by Zamvil et al) but extends to other cells such as antigen presenting cells (APCs), epithelial cells and ECs [5-7]. This pleiotropic action of statins may explain their effectiveness as immunomodulators when applied to a broad range of inflammatory diseases but uncertainty remains over the relative importance of each effector mechanism in bestowing clinical benefit.
Box 1. Clinical evidence for LDL-cholesterol-independent effect of statins.
Since their introduction in 1987, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase antagonists, otherwise known as statins, have been widely used to reduce low-density lipoprotein (LDL) cholesterol and treat atherosclerosis. Their efficacy in reducing cardiovascular morbidity and mortality is supported by large intervention trials [10]. The observation that the benefits of statins occur early, extend to patients within the normal LDL-cholesterol range and exceed those of other lipid-lowering drugs, despite comparable falls in total cholesterol, suggest that statins have LDL-cholesterol-independent effects. This hypothesis is widely supported by experimental studies demonstrating LDL-cholesterol-independent, immunomodulatory, anti-inflammatory and vasculoprotective actions [5]. Thus, statins downregulate T cell activation and proliferation, block adhesion molecule interactions, inhibit the production of chemokines, matrix metalloproteinases (MMP), attenuate MHC class II-restricted antigen presentation, inhibit activation of NF-κB, reduce leukocyte migration, upregulate EC protective genes and shift the T cell population from a proinflammatory Th1 to a regulatory Th2 phenotype [1,82].
Although the clinical significance of these pleiotropic actions of statins is difficult to establish a recent study has reported that, despite equivalent reductions in serum cholesterol in patients treated with either simvastatin or ezetimibe, an inhibitor of gastrointestinal cholesterol absorption, only simvastatin improved endothelial function [89]. A further confounding factor is the use in many experimental studies of supratherapeutic concentrations of statins, raising the question of clinical relevance. Notwithstanding this, LDL-cholesterol-independent biological effects of statins have attracted considerable interest in recent years and have led to clinical trials aimed at evaluating statin efficacy in autoimmune disease [1].
Box 2. Statin pharmacology and protein prenylation.
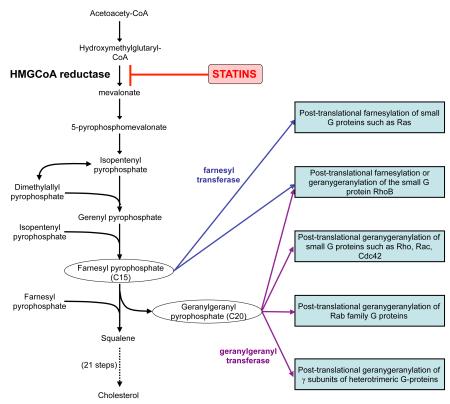
HMG-CoA reductase is the rate-limiting enzyme of the mevalonate pathway, through which cells synthesise cholesterol from acetate moieties. Statins, bind to HMG-CoA reductase at nanomolar concentrations leading to competitive displacement of HMG-CoA. Inhibition of HMG-CoA reductase, and the subsequent reduction in cholesterol synthesis, induces hepatocytes to increase their surface expression of LDL receptors, thereby increasing LDL uptake and reducing plasma LDL cholesterol. Currently available statins include pravastatin, simvastatin and lovastatin, that are naturally occurring (from fungi) and the synthetic statins atorvastatin, fluvastatin and rosuvastatin. These statins possess important intermolecular differences that contribute to their distinct pharmacokinetic properties [90]. For example pravastatin and rosuvastatin are, unlike the other statins, water soluble whilst rosuvastatin and atorvastatin have by far the longest plasma half life [90]. The differences between statins with respect to their ability to bind HMG-CoA reductase and whether they are predominantly lipophilic or hydrophilic may also influence their extrahepatic effects. However, hydrophilic statins exert similar effects on the vasculature to lipophilic statins implying that specific mechanisms may exist for the uptake of the former. Although most extrahepatic effects appear to be mediated through inhibition of mevalonate production [1], statins may modulate the immune response independently of their inhibitory effect on HMG-CoA reductase, through interference with the CD11a (LFA-1)/ICAM-1 interaction [4].
The mechanisms by which statins exert their effect are predominantly through inhibition of downstream products of mevalonate metabolism, in particular farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). These isoprenoid lipid intermediates serve as adjuncts in post-translational (iso)prenylation of a variety of important cell signaling proteins [1,5,71]. Prenylation occurs on proteins containing a C-terminal CaaX motif. However, it is not confined to these proteins, but also occurs on members of the Rab family of Ras-related G proteins and, which has been largely overlooked, on the γ-subunit of heterotrimeric G-proteins. In addition to promoting membrane interactions, prenylation plays a role in crucial protein-protein interactions. This suggests that a reduction in the synthesis of these intermediate metabolites, through statin inhibition of HMG-CoA reductase, will have a profound modulating affect on cell signaling pathways. Amongst the group of CaaX motif containing proteins likely to be subject to altered function are members of the small GTPase family of molecular switch proteins that include Rho, Rac and Cdc42. These proteins cycle between an inactive GDP-bound state and an active GTP-bound state. By reducing GTPase prenylation, statins prevent activation of GTP-binding proteins, so disrupting organization of the actin cytoskeleton and complex processes important to the immune response [1,5,71].
The recognition that atherosclerosis has an important immune component [8], and the key role of the endothelium in orchestrating leukocyte recruitment and dictating lesion size, duration and resolution, has led to it becoming an early and major focus of statin research [1,9,10]. Experimental models suggest EC injury is the initiating event in atherogenesis, occuring predominantly at arterial branch points where blood flow is of reduced velocity and has a pro-atherogenic reversing or oscillatory flow pattern. This is in contrast to high shear, unidirectional laminar blood flow which is atheroprotective. Subsequent inflammatory changes in the endothelium favour transendothelial migration of monocytes and T cells into the sub-intimal space, a critical step for the development of atheroma [8,11]. Statin therapy results in a rapid and significant improvement in endothelial function in both hyper- and normocholesterolaemic patients with atherosclerosis [9,12]. The ability of statins to increase eNOS levels and fibrinolysis, to modulate T cell function and to reduce leukocyte adhesion and platelet activation, contribute significantly to their effect on atherogenesis. Moreover, the observed reduction in C-reactive protein in patients treated with statins is testament to the efficacy of their anti-inflammatory actions (reviewed in Jain [10]). How statins impact on vascular function in other inflammatory and autoimmune diseases, however, is less clear but is becoming recognised as being of equal importance [13]. Indeed, mounting evidence indicates that statins improve vascular function in rheumatoid arthritis (RA) [14,15].
Regulation of MHC class II
Whilst vascular ECs cannot be considered professional APCs, they are nonetheless competent at presenting antigen in the context of MHC class II and expressing key costimulatory molecules. In a landmark study by Kwak, statin treatment of human vascular saphenous vein EC (a readily available source of human EC) resulted in the direct inhibition of IFN-γ-induced MHC-II expression and subsequent T-cell activation (Fig. 1) [16]. This response was mediated through modulation of the CIITA transactivator promoter pIV and was reversed by both mevalonate and GGPP, but not squalene, demonstrating the probable involvement of small GTPases in the inhibitory event [17]. These findings have been confirmed in human renal microvascular ECs [18] and more recently in porcine vascular endothelium [19]. In the latter study, animals treated with 3mg/kg/day atorvastatin for 16 days were found to express 25 fold less MHC class II expression compared to control levels. More controversially, an alternate mechanism has also been proposed whereby statins mediate disruption of lipid rafts which are thought to be crucial for normal MHC class II function [20]. Clearly both downstream statin effects may operate together and contribute to the overall failure of EC to successfully present antigen.
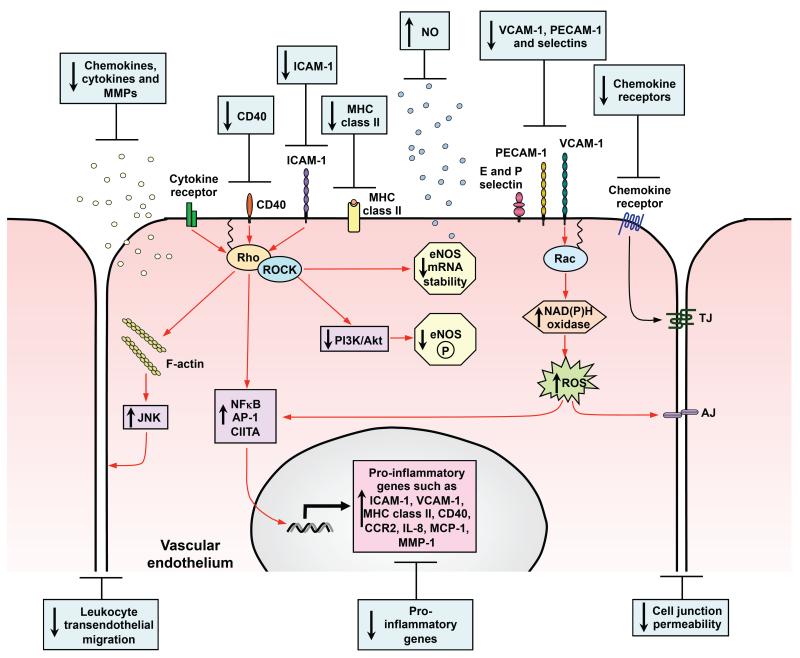
Figure 1. Effects of statins on the pro-inflammatory vascular endothelium.
During inflammation the endothelial cell is activated leading to the induction and upregulation of numerous signaling pathways that promote the inflammatory event (illustrated by red arrows). Statins downregulate a number of these key pathways including those responsible for pro-inflammatory gene induction, support of leukocyte migration and increased cell junction permeability. The negative effect of statins on proinflammatory gene regulation results in reduced expression of MHC class II, CD40, adhesion molecules, chemokines, cytokines, MMPs and chemokine receptors. In addition, statins inhibit pathways that downregulate anti-inflammatory responses which results in increased eNOS mRNA stability and NO production which has anti-inflammatory effects. Red arrows represent those pathways that are blocked by statins primarily through their action on small GTPase prenylation and blue boxes describe the outcome of statin treatment.
Effect on co-stimulatory molecules
To generate an effective response, T cells require antigen presented in the context of MHC class II and recognition of additional co-stimulatory molecules. CD40, a member of the tumor necrosis receptor family, through its interaction with its ligand (CD40L, CD154) plays an important role in potentiating the T-cell receptor mediated signal and driving the differentiation of effector T cells. In addition, engagement of CD40 on APCs, including ECs, induces expression of cell adhesion molecules, MMPs, tissue factor, chemokines, and cytokines (eg TNFα and Th1-differentiating cytokines such as IL-12), all of which promote an inflammatory response. In ECs, statins inhibit cytokine-stimulated expression of CD40 resulting in decreased CD40L-induced EC IL-12 expression (Fig. 1) [21,22]. Surprisingly, Wagner and colleagues reported that the inhibition of CD40 expression in human umbilical vein EC (HUVEC) was not reversed by mevalonate, FPP or GGPP, suggesting the effect was independent of HMG-CoA reductase inhibition and possibly being due to interference with integrin function [21]. In contrast other studies using saphenous vein EC report that statins inhibit basal and cytokine-mediated upregulation of CD40 and that this effect is HMG-CoA reductase dependent [23,24] illustrating possible differences in response between vascular EC subsets. In light of the importance attached to CD40-CD154 interactions in atherogenesis and other inflammatory disorders including RA and multiple sclerosis, modulation of these interactions by statins is likely to contribute significantly to their anti-inflammatory activity.
Endothelial cell-derived chemokines and chemokine receptors
Chemokines and their receptors play a central role in regulating leukocyte traffic. Statin treatment of ECs results in a significant attenuation of the chemokines MCP-1 (CCL2) and IL-8 (CXCL8) [25-27] which are involved in the recruitment of monocytes and neutrophils respectively. In addition, statins downregulate mRNA expression in EC for the chemokines MCP-1, MIP-1α (CCL3) and MIP-1β (CCL4) and the chemokine receptors CCR1, CCR2, CCR4 and CCR5 [27]. These statin effects can be reversed by mevalonate and mimicked by the geranylgeranyl transferase inhibitor GGTI-286 suggesting that inhibition of small GTPase prenylation and subsequent downregulation of transcription factors such as NFκB and AP-1 may be a principal mechanism (see below). Alternatively, statins also inhibit CD40 expression and consequently subsequent CD40-mediated signaling that mediates EC expression of pro-inflammatory IL-6, IL-8 and MCP-1 [24].
Endothelial cell adhesion molecules and leukocyte recruitment
The recruitment of leukocytes by the vascular endothelium is a key multistep event in the evolution of inflammatory lesions including atheroma. EC adhesion molecules serve not only as docking structures but also initiate signalling cascades required for successful leukocyte transmigration (see below). The expression of EC adhesion molecules is significantly modulated by statins with the weight of evidence suggesting an inhibitory effect (Table 1; Fig. 1). Most consistent is a reduction in P-selectin [28-31], whereas E-selectin is reported to be both up- [32-37] and down-regulated [30,31,38,39]. Statins also affect the expression of cell adhesion molecules belonging to the immunoglobulin superfamily. Accordingly, ICAM-1, which is directly involved in sustaining both leukocyte adhesion and diapedesis and is induced by various activators, is inhibited by statins [30,31,40-47]. Some studies, however, report an increase in expression [32,34,35], which is at odds with the ability of statins to attenuate leukocyte recruitment. Another member of the immunoglobulin superfamily that is largely responsible for supporting monocyte migration is vascular cell adhesion molecule (VCAM)-1. As with ICAM-1, the induction of VCAM-1 can be prevented by statin treatment, which is in accord with the observed reduction in mononuclear cell infiltration in vivo [21,30, 33,39,44,46,48]. However, VCAM-1 may itself be induced by statins [32,34,35]. Platelet EC adhesion molecule (PECAM)-1, which is implicated in directing migration through the cell-cell junction and basement membrane, displays either reduced expression [45] or altered cellular distribution [49] (Table 1).
Table 1. Summary of the effects of statins on endothelial cell adhesion molecule expression.
| Endothelial cell | Treatment | Statin | Effect on adhesion molecule | Proposed mechanism | Ref |
|---|---|---|---|---|---|
| Human umbilical vein | TNF-α | Mevastatin | VCAM-1 ↓ E-selectin ↑ |
mRNA decreased through inhibition of NFκB but E-selectin protein increases due to reduced surface cleavage. Effects reversed by mevalonate. |
[33] |
| Lovastatin | E-selectin ↓ | Inhibits Rho mediated TNF stimulated E-selectin expression |
[38] | ||
| Lovastatin | VCAM-1 ↑ ICAM-1 ↑ E-selectin ↑ |
Lovastatin, but not pravastatin, pre-treatment enhanced TNF upregulation of protein and mRNA |
[34] | ||
| Atorvastatin | VCAM-1 ↑ ICAM-1 ↑ E-selectin ↑ |
Statin enhancement reversed by mevalonate and GGPP |
[35] | ||
| Simvastatin, Atorvastatin, Cerivastatin |
ICAM-1 ↓ | Effect reversed by mevalonate | [43] | ||
| Simvastatin | VCAM-1 ↓ ICAM-1 ↓ |
Reduced mRNA and activation of NFκB | [44] | ||
| Cerivastatin, Simvastatin |
VCAM-1 ↓ ICAM-1 ↓ (not with Simvastatin) |
Decreased surface expression yet increased mRNA. Correlates with IκBa degradation. Proposed due to increased shedding. Effect reversed by mevalonate |
[46] | ||
| Lovastatin | PECAM-1 (altered distribution) |
Statin effect on intracellular PECAM reversed by mevalonate and GGPP |
[49] | ||
| TNF-α and IL-1β | Simvastatin | VCAM-1 ↑ ICAM-1 ↑ E-selectin ↑ |
Statin enhancement reversed by mevalonate and GGPP but not squalene. Associated with increase in NFκB activation. |
[32] | |
| TNF-α and IFN-γ | Atorvastatin | VCAM-1 ↓ (mRNA) |
Effect reversed by mevalonate | [21] | |
| TNF-α and LPS | Simvastatin, Fluvastatin, Pravastatin |
VCAM-1 ↑ E-selectin ↑ |
Statin enhancement reversed by mevalonate and GGPP |
[36] | |
| Insulin | Cerivastatin, Fluvastatin, Pravastatin |
ICAM-1 ↓ (no effect with Fluvastatin or Pravastatin) |
Cerivastatin inhibits insulin upregulation of ICAM. Reversed by mevalonate. |
[42] | |
| Gucose | Fluvastatin, Pravastatin |
ICAM-1 ↓ P-selectin ↓ E-selectin ↓ |
Hyperglycaemic induction of CAM’s attenuated with statins through increased NO |
[31] | |
| Antiphospholipid | Fluvastatin | VCAM-1 ↑ E-selectin ↑ |
Statin enhancement reversed by mevalonate and GGPP |
[37] | |
| Human iliac artery | No treatment | Fluvastatin | ICAM-1 ↓ PECAM-1 ↓ |
Downregulation of basal ICAM/PECAM by statins reversed by blocking eNOS |
[45] |
| Human coronary artery | Ox-LDL | Simvastatin, Atorvastatin |
VCAM-1 ↓ ICAM-1 ↓ P-selectin ↓ E-selectin ↓ |
Both message and protein reduced as well as NFκB |
[30] |
| ECV304 human cell line | IFN-γ | Lovastatin | ICAM-1 ↓ | Inhibits IFN but not TNF stimulated ICAM expression (ECV304 cells have since been shown to be of epithelial origin) |
[41] |
| Bovine aorta | LPS | Cerivastatin | ICAM-1 ↓ (mRNA) | Mediated through Rho. Reversed with GGPP | [40] |
| Rat mesenteric vessels | Thrombin | Rosuvastatin | P-selectin ↓ | Statin enhanced release of NO | [29] |
| L-NAME | Simvastatin | P-selectin ↓ | Downregulates adhesion and migration correlating with decreased P-selectin |
[28] | |
| Rat aorta | Hypertension | Fluvastatin | ICAM-1 ↓ | Also decreases superoxide anion | [47] |
| Mouse aorta | ApoE deficiency | Rosuvastatin | VCAM-1 ↓ (mRNA) |
Also decreased MCP-1 and MMP-9 | [48] |
| Mouse brain bEND.3 cell line | TNF-α | Lovastatin | VCAM-1 ↓ E-selectin ↓ ICAM-1 no change |
By inhibiting PI3K/Akt/NFκB pathway | [39] |
The conflicting data described above occurs primarily where HUVEC have been employed (Table 1). However, on close examination there is no clear correlation between the effect on adhesion molecule expression and experimental condition such as type of statin used or cell passage number. Despite the discrepant results concerning the effects of statins on adhesion molecule expression, a common feature of statin treatment of animal models of inflammatory diseases is a reduction in the accumulation of mononuclear cell infiltrates into the target tissue. These observations support the hypothesis that attenuation of adhesion molecule expression may contribute to the observed immunomodulatory effects.
Endothelial adhesion molecule receptor signalling
In addition to facilitating leukocyte adhesion, cell adhesion molecules also contribute to the overall pro-inflammatory activation of the EC. In this regard engagement of ICAM-1 on the EC surface results in the initiation of multiple signalling cascades one of which leads to reorganisation of the actin cytoskeleton and promotion of lymphocyte diapedesis [50]. Remodelling of the EC cytoskeleton is thought to be a prerequisite for transvascular leukocyte migration as F-actin is involved in both the formation of the endothelial transmigratory docking structure [51,52] and maintenance of cell-cell junction integrity. Critical to the ICAM-1 mediated signalling pathway in ECs responsible for facilitating lymphocyte migration is the upstream activation of Rho GTPase. Thus, as statins inhibit Rho prenylation, the EC ICAM-1-mediated signalling pathway required for successful transvascular lymphocyte migration is also inhibited [53,54]. The fact that this effect can be reversed by ECs expressing Rho with a myristoylation site, which renders the cell insensitive to statin induced loss of isoprenylation [53], provides compelling evidence that EC ICAM-1-mediated signalling via Rho is essential for successful lymphocyte transendothelial migration and is a target for statins.
As with ICAM-1, VCAM-1 engagement has also been reported to generate EC signalling pathways that are requisite for leukocyte, and in particular monocyte, migration. VCAM-1-mediated signalling encompasses calcium dependent activation of the small GTPase Rac1, increased NAD(P)H oxidase activity and subsequent generation of reactive oxygen species (ROS) [55]. Induction of this pathway also results in Rho activation, actin reorganisation and loss of the cell-cell adherens junction protein VE-cadherin [56,57]. Inhibition of these GTPases with statins would therefore reduce paracellular permeability, leukocyte migration and the production of ROS [58] which is also an activator of the pro-inflammatory transcription factor NFκB.
Endothelial cell inflammatory gene regulation
Reduction in the activation of pro-inflammatory transcription factors represents a key mechanism by which statins exert their immunomodulatory effects (Table 2) [10] (Fig. 1). Various statins decrease basal NF-κB DNA binding activity and NF-κB-dependent gene expression [59,60], at least in part, through induction or stabilization of its cytosolic inhibitor IκBα [60]. Likewise, Wagner reported that atorvastatin inhibits cytokine-mediated activation of NF-κB, STAT-1 and subsequent de novo synthesis of interferon regulatory factor-1 (IRF-1), resulting in reduced EC CD40 expression [21]. It has also been suggested that statins may inhibit NF-κB activation independently of the classical IKK-pathway [39,61] (see Table 1). In addition, statins may decrease activity of AP-1, a dimer of c-Jun and either Fos or ATF, which plays an important role in endothelial inflammatory responses, regulating genes responsible for MMPs, cytokines, chemokines, adhesion molecules, iNOS and Fas ligand [60]. In the same study HIF-1α activity, which was diminished by TNF-α in ECs, was enhanced by statins.
Table 2. Summary of the transcriptional effects of statins in vascular endothelial cells.
IFN: interferon, IRF-1; interferon regulatory factor-1, MHC: major histocompatibility complex, NF-kB: nuclear factor kappa B, PMA: phorbol myristate acetate, PPAR: peroxisome proliferator-activated receptor, STAT1: signal transducer of transcription-1, TNF: tumor necrosis factor
| Transcription factor | Endothelial cell | Treatment | Statin | Proposed mechanism | Ref |
|---|---|---|---|---|---|
| CIITA | Human saphenous vein |
IFNγ | Atorvastatin Lovastatin Pravastatin |
Statins inhibit expression of MHC Class II transactivator (CIITA) |
[16] |
| NF-κB | Human aortic Ea.hy926 cell line |
TNFα | Simvastatin Atorvastatin Lovastatin |
Stabilization of cytoplasmic IκBα leading to reduced NF-κB signaling |
[60] |
| Human umbilical vein |
TNFα | Cerivastatin | Reduced NF-κB signaling independent of classic IκB kinase and mediated via inhibition of PI3K/Akt |
[61] | |
| TNFα or Antiphosplipid antibodies |
Fluvastatin | Impaired NF-κB binding to DNA | [59] | ||
| TNFα/IFNγ | Atorvastatin | Inhibition of NF-κB and STAT-1- dependent synthesis of IRF-1. |
[21] | ||
| AP-1 | Human aortic Ea.hy926 cell line |
TNFα | Simvastatin Atorvastatin Lovastatin |
Attenuated cJun expression leading to reduced AP-1 DNA binding |
[60] |
| HIF-1α | Human aortic Ea.hy926 cell line |
TNFα | Simvastatin Atorvastatin Lovastatin |
Statin-induced increase in HIF-1α activity and reversal of EC dysfunction |
[60] |
| KLF2 | Human umbilical vein |
None | Simvastatin Lovastatin Cerivastatin Pravastatin (no effect) |
Increases KLF2 mRNA levels through inhibition of geranygeranylation resulting in upregulation of KLF2 trascriptional targets |
[63] |
| Mevastatin Simvastatin Lovastatin Pravastatin (no effect) |
Statin-induced increase in MEF2 activity leading to induction of KLF2 promoter activity through inhibition of Rho |
[64] | |||
| PPARα | Human umbilical vein |
PMA | Simvastatin Fluvastatin Pravastatin Cerivastatin |
Statin-induced activation of PPARα inhibits EC release of pro- inflammatory mediators |
[66] |
In addition to the inhibitory effect of statins on pro-inflammatory gene expression, it has now emerged that they are also able to activate genes that are protective or anti-inflammatory. Recent attention has focused on the Kruppel-like family of transcription factors; potent regulators of EC gene expression [62]. Kruppel-like factor 2 (KLF2) is statin-inducible in human ECs [63-65] and this is reversed by mevalonate and GGPP [63,64]. Consistent with a role for inhibition of Rho GTPase, treatment with the Rho inhibitor C3 exoenzyme or the geranygeranyl transferase inhibitor GGTI-298 augmented KLF2 expression [63,64]. The effect of statins on KLF2 mRNA expression was transcription-dependent, most likely mediated by increased transactivation of the KLF2 promoter via a myocyte enhancer factor binding site [64]. This novel effect lends further weight to the proposal that ECs represent a key therapeutic target for statins in inflammatory-related diseases. Statins, through inhibition of RhoA, may also induce expression or increase activity of the nuclear receptor ligand-activated transcription factors peroxisome proliferator-activated receptors (PPAR) α and β [66,67]. Moreover, simultaneous treatment with statins and fibrates, another class of lipid-lowering drugs, results in a synergistic effect on PPARα transactivation, suggesting distinct mechanisms of action [67]. Activation of PPARα by statins suppresses NF-κB and AP-1-mediated gene activation resulting in a down-regulation of pro-inflammatory mediators such as VCAM-1 and IL-6 and recent in vivo studies have reported PPARα-dependent anti-inflammatory effects of simvastatin [68].
How statin-mediated modulation of protein prenylation results in altered gene expression remains largely unexplored. Nevertheless, gene activation via signalling pathways incorporating prenylated proteins is well established and includes signalling pathways involving Rho, Ras and Rac as well as some heterotrimeric G-proteins.
eNOS and NO production
It is well recognised that the anti-inflammatory effect of statins on the vascular endothelium can be closely associated with their ability to upregulate endothelial nitric oxide synthase (eNOS) activity and hence nitric oxide (NO) production (for reviews see [69,70]) (Fig.1). At least two distinct mechanisms have been proposed; a rapid activation of eNOS function and, over the longer term, enhancement of mRNA stability. Under inflammatory conditions the Rho-Rho kinase pathway becomes activated, which is a negative regulator of both eNOS activity and mRNA stability, resulting in reduced NO biosynthesis [71,72]. Early studies established that statin treatment reduces prenylation of endothelial Rho and a concomitant increase in eNOS activity which, as one would predict, was reversed by GGPP [71,73]. Statin-mediated post-translational activation of eNOS has been attributed to activation of the PI3K-Akt pathway. Thus, simvastatin treatment of resting HUVEC results in rapid PI3K-dependent phosphorylation of the serine threonine kinase Akt and Akt-dependent phosphorylation and activation of eNOS [74,75]. This may also increase the binding affinity of eNOS for calmodulin, resulting in displacement of the inhibitory binding partner caveolin-1. In addition, statins reduce caveolin-1 levels, decreasing its inhibitory effects on NO synthesis [76]. An alternative pathway has also been proposed whereby statins may enhance eNOS activity through activation of AMP activated protein kinase [45]. Paradoxically, statins have also been shown to inhibit iNOS transcription through inhibition of NF-κB but this has as yet to be confirmed in EC.
The evidence for statins inducing eNOS activity and hence NO production is compelling but the impact on the inflammatory process is less clear. There is evidence, however, that NO prevents leukocyte chemotaxis and downregulates adhesion pathways through an inhibition of adhesion molecule expression which in turn attenuates leukocyte migration. The importance of NO in leukocyte recruitment is supported by the failure of statins to inhibit leukocyte/EC interactions in eNOS deficient mice [29]. In addition to anti-inflammatory effects, statin-induced NO may have added benefits, including inhibition of endothelin-1 and improved endothelial function in atherosclerosis and autoimmune inflammatory diseases such as systemic sclerosis [77] and rheumatoid arthritis [14,15]. Contrary to these findings with in other studies
Vascular permeability
Increased vascular permeability is a ubiquitous feature of inflammation and plays a significant role in pathogenesis. In a recent study statins significantly decreased permeability in an in vitro model of the human blood-brain barrier (BBB) [78], an effect reversed by FPP and GGPP but not squalene. The authors suggest that statin-mediated changes in permeability may be due to a decrease in the endothelial secretion of the chemokines MCP-1 (CCL2) and IP-10 (CXCL10). This observation adds weight to the finding that MCP-1, via its CCR2 receptor, can alter substantially the integrity of the BBB by inducing a redistribution of tight junction proteins [79]. This raises the possibility that statins will also inhibit Rho-Rho kinase mediated vascular barrier opening, a hypothesis supported by the fact that simvastatin reverses VEGF-induced hyperpermeability via inhibition of RhoA-dependent myosin light chain diphosphorylation and cytoskeletal remodelling [80]. The potential clinical benefits are illustrated by the observation that statins prevent hyperpermeability induced by dextrose in vitro and across the BBB of diabetic rats [81].
Vasculoprotective effects of statins
The vascular endothelium is exposed to pro-inflammatory cytokines, complement components, activated leukocytes and oxidized LDL which may contribute to chronic endothelial dysfunction and atherogenesis, through generation of ROS and reduced NO biosynthesis. In the microvasculature a patent blood supply is requisite for the prevention of collateral hypoxic damage and for ultimate resolution of the inflammatory event and so must be preserved. To this end, the endothelium has evolved cytoprotective mechanisms that act to maintain vascular integrity and recent studies have highlighted that these may be enhanced by statins [12,82] (Fig. 2).
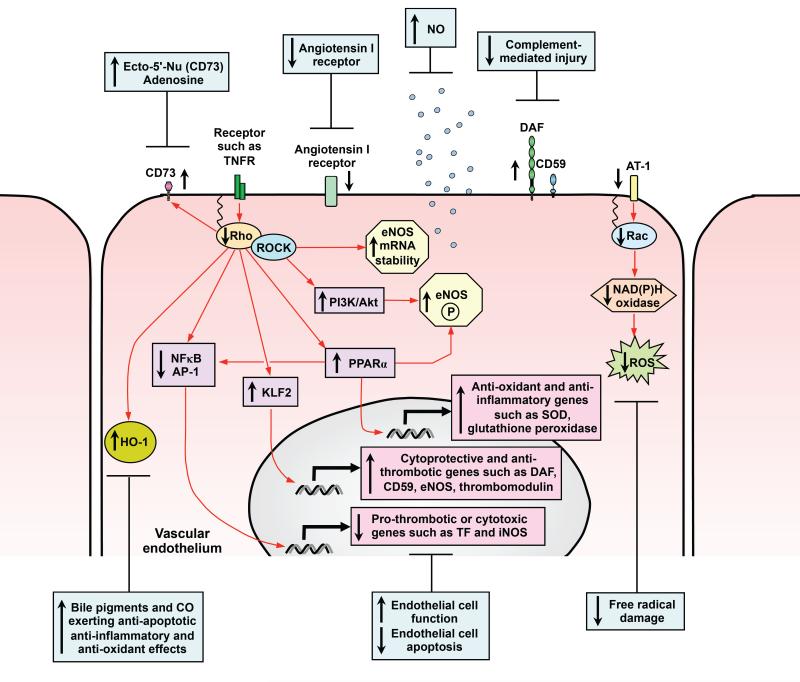
Figure 2. Cytoprotective effects of statins on vascular endothelium.
Defined and potential signalling pathways through which statins may exert vasculoprotective effects on the pro-inflammatory vascular endothelium are shown. Statins induce cytoprotective genes, protect against vascular injury and reverse endothelial cell dysfunction. Activation of PI3K/Akt and increased NO biosynthesis prevent apoptosis, increase vasodilatation and reduce leukocyte and platelet adhesion. Through inhibition of Rac-1-mediated NAD(P)H oxidase activity and reduction in angiotensin AT1-receptor expression, statins ameliorate angiotensin II-induced generation and release of reactive oxygen species ( ROS). Statin-mediated induction of HO-1 has the potential to exert anti-adhesive, anti-oxidant and anti-apoptotic effects. Inhibition of Rho-Rho-associated kinases (ROCK) by statins leading to activation of KLF2 and PPARα may induce eNOS, complement-inhibitory, anti-oxidant and anti-thrombotic genes. RhoA inhibition also enhances ecto-5′-nucleotidase (Ecto-5′-Nu, CD73) cell surface expression and activity, so increasing extracellular adenosine which modulates vascular tone, inhibits platelet activation and leukocyte adhesion.
Through inhibition of Rac-1-mediated NAD(P)H oxidase activity and reduction in angiotensin AT1-receptor expression, statins ameliorate angiotensin II-induced generation and release of ROS [57,83]. Recent attention has focused on the ability of statins to induce expression of the anti-oxidant, anti-inflammatory and anti-apoptotic enzyme hemeoxygenase-1 (HO-1). HO-1 may be induced by statins in ECs and in vivo in a tissue-specific manner [84]. Simvastatin attenuates hypoxia and oxidative stress in the coronary artery wall, preserving endothelial function independently of cholesterol lowering [85]. Moreover, withdrawal of statin therapy induces endothelial dysfunction through activation of gp91 phox-containing NAD(P)H oxidase by Rac-1, resulting in generation of superoxide anions which scavenge NO [86] and forms highly toxic peroxynitrite.
Complement activation has been implicated in atherogenesis, myocardial infarction and post-transplant vasculopathy. Atorvastatin may induce expression of the complement-inhibitory protein decay-accelerating factor (DAF) and protect human EC against complement-mediated injury [87]. DAF upregulation was independent of cholesterol synthesis and was reproduced by a GGTI and reversed by GGPP. This effect of statins may be enhanced by hypoxia leading to additional upregulation of the terminal pathway inhibitor CD59 [88]. The combined effects of DAF, at the level of C3 and C5 convertases, and CD59 inhibiting the terminal attack complex, has the potential to exert anti-inflammatory and vasculoprotective effects on the endothelium. This may contribute to their beneficial effects in ischemic heart disease, during ischemia/reperfusion, RA and systemic lupus erythematosus [12,13].
Conclusions
The LDL-cholesterol-independent immunomodulatory, anti-inflammatory and vasculoprotective effects of statins are currently the subject of intense and justified interest. Recent work has highlighted the potential use of these drugs outside the spectrum of hyperlipidemia and ischemic heart disease and directed focus towards the EC as an important cell target in inflammation in general. New developments in our understanding of the molecular mechanisms underlying the LDL-cholesterol-independent actions of statins on ECs will help direct future research. Thus, the role of the inhibition of isoprenylation and hence small GTPase, and possibly heterotrimeric G-protein, function and the role of transcription factors such as NF-κB and KLF2, may subsequently allow the design of novel targeted therapeutic agents aimed specifically at the vascular endothelium.
Acknowledgements
Dr Greenwood would like to acknowledge the Wellcome Trust and the Multiple Sclerosis Society UK for supporting research related to his review. Work in Dr Mason’s laboratory is funded by the British Heart Foundation and the Arthritis Research Campaign
References
- 1.Greenwood J, et al. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jury EC, Ehrenstein MR. Statins: immunomodulators for autoimmune rheumatic disease? Lupus. 2005;14:192–196. doi: 10.1191/0961203303lu2135oa. [DOI] [PubMed] [Google Scholar]
- 3.Ehrenstein MR, et al. Statins for atherosclerosis - as good as it gets? N. Engl. J. Med. 2005;352:73–75. doi: 10.1056/NEJMe048326. [DOI] [PubMed] [Google Scholar]
- 4.Weitz-Schmidt G, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 5.Liao JK, Laufs U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinlay S. Potential vascular benefits of statins. Am. J. Med. 2005;118(Suppl 12A):62–67. doi: 10.1016/j.amjmed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Beckman JA, Creager MA. The nonlipid effects of statins on endothelial function. Trends Cardiovasc. Med. 2006;16:156–162. doi: 10.1016/j.tcm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 9.Wolfrum S, et al. Endothelium-dependent effects of statins. Arterioscler. Thromb. Vasc. Biol. 2003;23:729–736. doi: 10.1161/01.ATV.0000063385.12476.A7. [DOI] [PubMed] [Google Scholar]
- 10.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 11.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 12.Mason JC. Statins and their role in vascular protection. Clin. Sci. (Lond) 2003;105:251–266. doi: 10.1042/CS20030148. [DOI] [PubMed] [Google Scholar]
- 13.McCarey DW, et al. Do the pleiotropic effects of statins in the vasculature predict a role in inflammatory diseases? Arthritis. Res. Ther. 2005;7:55–61. doi: 10.1186/ar1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Doornum S, et al. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2004;63:1571–1575. doi: 10.1136/ard.2003.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermann F, et al. Simvastatin improves endothelial function in patients with rheumatoid arthritis. J. Am. Coll. Cardiol. 2005;45:461–464. doi: 10.1016/j.jacc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Kwak B, et al. Statins as a newly recognised type of immunomodulator. Nat. Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi MM, et al. Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompatibility complex class II gene activation by HMG-CoA reductase inhibitors. Transplantation. 2001;71:1262–1268. doi: 10.1097/00007890-200105150-00014. [DOI] [PubMed] [Google Scholar]
- 18.Muczynski KA, et al. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: characterization, isolation, and regulation of MHC class II expression. J. Am. Soc. Nephrol. 2003;14:1336–1348. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 19.Geissler I, et al. In vivo suppression of major histocompatibility complex class II expression on porcine vascular endothelial cells by an HMG-CoA reductase inhibitor. Transplantation. 2006;81:922–926. doi: 10.1097/01.tp.0000179154.17329.68. [DOI] [PubMed] [Google Scholar]
- 20.Kuipers HF, van den Elsen PJ. Statins and control of MHC2TA gene transcription. Nat. Med. 2005;11:365–366. doi: 10.1038/nm0405-365. [DOI] [PubMed] [Google Scholar]
- 21.Wagner AH, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase-independent inhibition of CD40 expression by atorvastatin in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2002;22:1784–1789. doi: 10.1161/01.atv.0000037098.20829.31. [DOI] [PubMed] [Google Scholar]
- 22.Lin R, et al. Lovastatin reduces apoptosis and downregulates the CD40 expression induced by TNF-α in cerebral vascular endothelial cells. Curr. Neurovasc. Res. 2006;3:41–47. doi: 10.2174/156720206775541796. [DOI] [PubMed] [Google Scholar]
- 23.Schonbeck U, et al. Oxidized low-density lipoprotein augments and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors limit CD40 and CD40L expression in human vascular cells. Circulation. 2002;106:2888–2893. doi: 10.1161/01.cir.0000043029.52803.7b. [DOI] [PubMed] [Google Scholar]
- 24.Mulhaupt F, et al. Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc. Res. 2003;59:755–766. doi: 10.1016/s0008-6363(03)00515-7. [DOI] [PubMed] [Google Scholar]
- 25.Romano M, et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Invest. 2000;80:1095–1100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- 26.Zineh I, et al. Modulatory effects of atorvastatin on endothelial cell-derived chemokines, cytokines, and angiogenic factors. Pharmacotherapy. 2006;26:333–340. doi: 10.1592/phco.26.3.333. [DOI] [PubMed] [Google Scholar]
- 27.Veillard NR, et al. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis. 2006;188:51–58. doi: 10.1016/j.atherosclerosis.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Pruefer D, et al. Simvastatin inhibits leukocyte-endothelial cell interactions and protects against inflammatory processes in normocholesterolemic rats. Arterioscler. Thromb. Vasc. Biol. 1999;19:2894–2900. doi: 10.1161/01.atv.19.12.2894. [DOI] [PubMed] [Google Scholar]
- 29.Stalker TJ, et al. A new HMG-CoA reductase inhibitor, rosuvastatin, exerts anti-inflammatory effects on the microvascular endothelium: the role of mevalonic acid. Br. J. Pharmacol. 2001;133:406–412. doi: 10.1038/sj.bjp.0704070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li D, et al. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J. Pharmacol. Exp. Ther. 2002;302:601–605. doi: 10.1124/jpet.102.034959. [DOI] [PubMed] [Google Scholar]
- 31.Omi H, et al. Statins inhibit high glucose-mediated neutrophil-endothelial cell adhesion through decreasing surface expression of endothelial adhesion molecules by stimulating production of endothelial nitric oxide. Microvasc. Res. 2003;65:118–124. doi: 10.1016/s0026-2862(02)00033-x. [DOI] [PubMed] [Google Scholar]
- 32.Sadeghi MM, et al. Simvastatin modulates cytokine-mediated endothelial cell adhesion molecule induction: involvement of an inhibitory G protein. J. Immunol. 2000;165:2712–2718. doi: 10.4049/jimmunol.165.5.2712. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen LM, et al. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem. J. 2001;360:363–370. doi: 10.1042/0264-6021:3600363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt A, et al. Lovastatin-stimulated superinduction of E-selectin, ICAM-1 and VCAM-1 in TNF-alpha activated human vascular endothelial cells. Atherosclerosis. 2002;164:57–64. doi: 10.1016/s0021-9150(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 35.Bernot D, et al. Effect of atorvastatin on adhesive phenotype of human endothelial cells activated by tumor necrosis factor alpha. J. Cardiovasc. Pharmacol. 2003;41:316–24. doi: 10.1097/00005344-200302000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Dimitrova Y, et al. Effects of statins on adhesion molecule expression in endothelial cells. J. Thromb. Haemost. 2003;1:2290–2299. doi: 10.1046/j.1538-7836.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 37.Dunoyer-Geindre S, et al. Fluvastatin increases the expression of adhesion molecules, monocyte chemoattractant protein-1 and tissue factor in HUVEC stimulated by patient IgG fractions containing antiphospholipid antibodies. Thromb. Haemost. 2005;93:339–345. doi: 10.1160/TH04-05-0297. [DOI] [PubMed] [Google Scholar]
- 38.Nubel T, et al. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18:140–142. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 39.Prasad R, et al. Inhibition of phosphoinositide 3 kinase-Akt (protein kinase B)-nuclear factor-kappa B pathway by lovastatin limits endothelial-monocyte cell interaction. J. Neurochem. 2005;94:204–214. doi: 10.1111/j.1471-4159.2005.03182.x. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi S, et al. Cerivastatin suppresses lipopolysaccharide-induced ICAM-1 expression through inhibition of Rho GTPase in BAEC. Biochem.Biophys. Res. Commun. 2000;269:97–102. doi: 10.1006/bbrc.2000.2238. [DOI] [PubMed] [Google Scholar]
- 41.Chung HK, et al. Lee Statin inhibits interferon-gamma-induced expression of intercellular adhesion molecule-1 (ICAM-1) in vascular endothelial and smooth muscle cells. Exp. Mol. Med. 2002;34:451–61. doi: 10.1038/emm.2002.63. [DOI] [PubMed] [Google Scholar]
- 42.Okouchi M, et al. Cerivastatin ameliorates high insulin-enhanced neutrophilendothelial cell adhesion and endothelial intercellular adhesion molecule-1 expression by inhibiting mitogen-activated protein kinase activation. J. Diabetes Complications. 2003;17:380–386. doi: 10.1016/s1056-8727(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 43.Rezaie-Majd A, et al. Simvastatin reduces the expression of adhesion molecules in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 2003;23:397–403. doi: 10.1161/01.ATV.0000059384.34874.F0. [DOI] [PubMed] [Google Scholar]
- 44.Zapolska-Downar D, et al. Simvastatin modulates TNFalpha-induced adhesion molecules expression in human endothelial cells. Life Sci. 2004;75:1287–1302. doi: 10.1016/j.lfs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Xenos ES, et al. Nitric oxide mediates the effect of fluvastatin on intercellular adhesion molecule-1 and platelet endothelial cell adhesion molecule-1 expression on human endothelial cells. Ann. Vasc. Surg. 2005;19:386–392. doi: 10.1007/s10016-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 46.Landsberger M, et al. Cerivastatin reduces cytokine-induced surface expression of ICAM-1 via increased shedding in human endothelial cells. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.02.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Katoh M, et al. Fluvastatin inhibits O2- and ICAM-1 levels in a rat model with aortic remodeling induced by pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H655–660. doi: 10.1152/ajpheart.2001.281.2.H655. [DOI] [PubMed] [Google Scholar]
- 48.Li W, et al. Rosuvastatin attenuates monocyte-endothelial cell interactions and vascular free radical production in hypercholesterolemic mice. J. Pharmacol. Exp. Ther. 2005;313:557–562. doi: 10.1124/jpet.104.080002. [DOI] [PubMed] [Google Scholar]
- 49.Wei H, et al. Statin-inhibited endothelial permeability could be associated with its effect on PECAM-1 in endothelial cells. FEBS Lett. 2005;579:1272–1278. doi: 10.1016/j.febslet.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Greenwood J, et al. Lymphocyte migration into the central nervous system: implication of ICAM-1 signalling at the blood-brain barrier. Vascul. Pharmacol. 2002;38:315–322. doi: 10.1016/s1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 51.Barreiro O, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenwood J, et al. Lovastatin inhibits brain endothelial Rho-dependent lymphocyte migration and attenuates experimental autoimmune encephalomyelitis. FASEB J. 2003;17:905–907. doi: 10.1096/fj.02-1014fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gegg ME, et al. Suppression of autoimmune retinal disease by lovastatin does not require Th2 cytokine induction. J. Immunol. 2005;174:2327–2335. doi: 10.4049/jimmunol.174.4.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cook-Mills JM, et al. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem. J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Wetering S, et al. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J. Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 57.van Wetering S, et al. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am. J. Physiol. Cell Physiol. 2003;285:C343–352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 58.Wagner AH, et al. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler. Thromb. Vasc. Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- 59.Meroni PL, et al. Statins prevent endothelial cell activation induced by antiphospholipid (anti-beta2-glycoprotein I) antibodies: effect on the proadhesive and proinflammatory phenotype. Arthritis Rheum. 2001;44:2870–2878. doi: 10.1002/1529-0131(200112)44:12<2870::aid-art475>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 60.Dichtl W, et al. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:58–63. doi: 10.1161/01.atv.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- 61.Holschermann H, et al. Statins prevent NF-kappaB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis. 2006;185:240–245. doi: 10.1016/j.atherosclerosis.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki T, et al. Vascular implications of the kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25:1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. 2005. [DOI] [PubMed] [Google Scholar]
- 63.Parmar KM, et al. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 64.Sen-Banerjee S, et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 65.van Thienen JV, et al. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc. Res. 2006 doi: 10.1016/j.cardiores.2006.07.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Inoue I, et al. Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory effect: reduction of mRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPARalpha) in primary endothelial cells. Life Sci. 2000;67:863–876. doi: 10.1016/s0024-3205(00)00680-9. [DOI] [PubMed] [Google Scholar]
- 67.Martin G, et al. Statin-induced inhibition of the Rho-signaling pathway activates PPARα and induces HDL apoA-I. J. Clin. Invest. 2001;107:1423–1432. doi: 10.1172/JCI10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paumelle R, et al. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ. Res. 2006;98:361–369. doi: 10.1161/01.RES.0000202706.70992.95. [DOI] [PubMed] [Google Scholar]
- 69.Laufs U. Beyond lipid-lowering: effects of statins on endothelial nitric oxide. Eur. J. Clin. Pharmacol. 2003;58:719–731. doi: 10.1007/s00228-002-0556-0. [DOI] [PubMed] [Google Scholar]
- 70.Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ. Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao JK. Isoprenoids as mediators of the biological effects of statins. J. Clin. Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ming XF, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol. Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landsberger M, et al. Blockade of geranylgeranylation by rosuvastatin upregulates eNOS expression in human venous endothelial cells. Biochem. Biophys. Res. Commun. 2005;336:1005–1009. doi: 10.1016/j.bbrc.2005.08.225. [DOI] [PubMed] [Google Scholar]
- 74.Kureishi Y, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat. Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urbich C, et al. Double-edged role of statins in angiogenesis signaling. Circ. Res. 2002;90:737–744. doi: 10.1161/01.res.0000014081.30867.f8. [DOI] [PubMed] [Google Scholar]
- 76.Pelat M, et al. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480–2486. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 77.Furukawa S, et al. Protective effect of pravastatin on vascular endothelium in patients with systemic sclerosis: a pilot study. Ann. Rheum. Dis. 2006;65:1118–1120. doi: 10.1136/ard.2005.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ifergan I, et al. Statins reduce human blood-brain barrier permeability and restrict leukocyte migration: relevance to multiple sclerosis. Ann. Neurol. 2006;60:45–55. doi: 10.1002/ana.20875. [DOI] [PubMed] [Google Scholar]
- 79.Stamatovic SM, et al. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J. Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 80.Zeng L, et al. HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. FASEB J. 2005;19:1845–1847. doi: 10.1096/fj.05-4240fje. [DOI] [PubMed] [Google Scholar]
- 81.Mooradian AD, et al. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes. 2005;54:2977–2982. doi: 10.2337/diabetes.54.10.2977. [DOI] [PubMed] [Google Scholar]
- 82.Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am. J. Cardiol. 2005;96:24F–33F. doi: 10.1016/j.amjcard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wassmann S, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2002;22:300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 84.Hsu M, et al. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem. Biophys. Res. Commun. 2006;343:738–744. doi: 10.1016/j.bbrc.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 85.Wilson SH, et al. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–418. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- 86.Vecchione C, Brandes RP. Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ. Res. 2002;91:173–179. doi: 10.1161/01.res.0000028004.76218.b8. [DOI] [PubMed] [Google Scholar]
- 87.Mason JC, et al. Statin-induced expression of decay-accelerating factor protects vascular endothelium against complement-mediated injury. Circ. Res. 2002;91:696–703. doi: 10.1161/01.res.0000038151.57577.19. [DOI] [PubMed] [Google Scholar]
- 88.Kinderlerer AR, et al. Statin-Induced Expression of CD59 on vascular endothelium in hypoxia. A potential mechanism for the anti-inflammatory actions of statins in rheumatoid arthritis. Arthritis Res. Ther. 2006;8:R130–141. doi: 10.1186/ar2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Landmesser U, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–2363. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 90.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109:50–57. doi: 10.1161/01.CIR.0000131519.15067.1f. [DOI] [PubMed] [Google Scholar]