Abstract
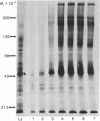
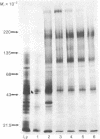
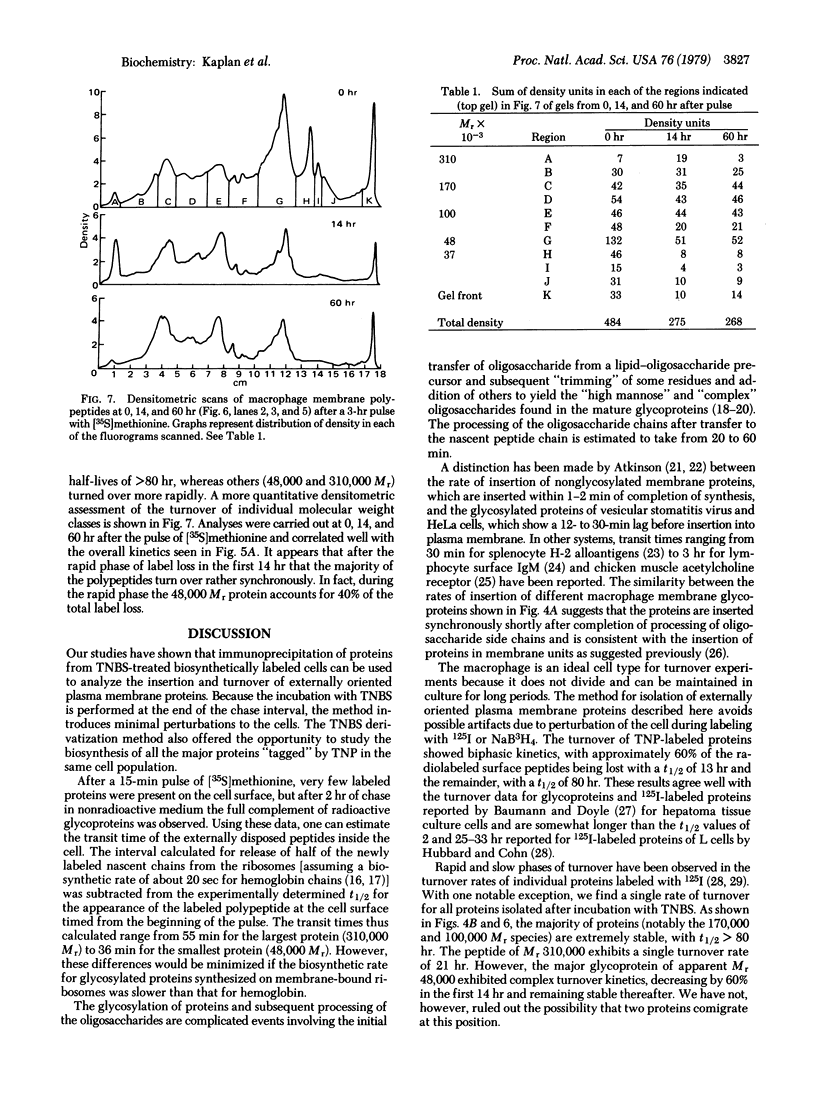
The composition, insertion, and turnover of externally disposed proteins on the macrophage plasma membrane were analyzed. Cells labeled with [35S]methionine were incubated with the nonpermeant reagent trinitrobenzene sulfonic acid to introduce the trinitrophenyl moiety on free amino groups of externally oriented membrane proteins. The cells were then incubated with rabbit anti-dinitrophenyl IgG and the immune complexes formed with the trinitrophenyl-proteins were isolated from detergent lysates of the cells by using fixed Staphylococcus aureus as the immunoadsorbent. Proteins isolated by this method were analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. The interval between the release of newly synthesized proteins from ribosomes and their appearance at the cell surface, where they became accessible to trinitrobenzene sulfonic acid, was studied in pulse-chase experiments. The "transit" time of four major membrane glycoproteins (48,000--310,000 Mr) ranged from 36 to 55 min and their appearance on the cell surface occurred in a relatively synchronous fashion. The turnover of most proteins of molecular weight above 50,000 was very slow (t1/2 greater than 80 hr) and was rather synchronous. Two exceptions were the 310,000 Mr protein, which was lost with a t1/2 = 21 hr, and a major glycoprotein (Mr 48,000), which exhibited more complex kinetics. Although the overall turnover of surface proteins was biphasic in nature, the rapid phase of protein loss was largely due to low molecular weight species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrotti J. J., Garvin J. E. Reaction of human serum albumin and human erythrocytes with tritiated 2,4,6-trinitrobenzenesulfonic acid and tritiated picryl chloride. Biochim Biophys Acta. 1972 Jan 17;255(1):79–90. doi: 10.1016/0005-2736(72)90009-0. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H. Gycoprotein and protein precursors to plasma membranes in vesicular stomatitis virus infected HeLa cells. J Supramol Struct. 1978;8(1):89–109. doi: 10.1002/jss.400080108. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H. Synthesis and assembly of HeLa cell plasma membrane glycoproteins and proteins. J Biol Chem. 1975 Mar 25;250(6):2123–2134. [PubMed] [Google Scholar]
- Baumann H., Doyle D. Turnover of plasma membrane glycoproteins and glycolipids of hepatoma tissue culture cells. J Biol Chem. 1978 Jun 25;253(12):4408–4418. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bonsall R. W., Hunt S. Reactivity of the human erythrocyte membrane to sodium trinitrobenzenesulphonate. Biochim Biophys Acta. 1971 Oct 12;249(1):281–284. doi: 10.1016/0005-2736(71)90105-2. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Gardner J. M., Fambrough D. M. Kinetics of biosynthesis of acetylcholine receptor and subsequent incorporation into plasma membrane of cultured chick skeletal muscle. Cell. 1977 Mar;10(3):365–373. doi: 10.1016/0092-8674(77)90023-x. [DOI] [PubMed] [Google Scholar]
- Doyle D., Baumann H., England B., Friedman E., Hou E., Tweto J. Biogenesis of plasma membrane glycoproteins in hepatoma tissue culture cells. J Biol Chem. 1978 Feb 10;253(3):965–973. [PubMed] [Google Scholar]
- Emerson S. G., Cone R. E. Turnover and shedding of Ia antigens by murine spleen cells in culture. J Immunol. 1979 Mar;122(3):892–899. [PubMed] [Google Scholar]
- Gordesky S. E., Marinetti G. V., Love R. The reaction of chemical probes with the erythrocyte membrane. J Membr Biol. 1975;20(1-2):111–132. doi: 10.1007/BF01870631. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. II. Metabolic fate of iodinated polypeptides of mouse L cells. J Cell Biol. 1975 Feb;64(2):461–479. doi: 10.1083/jcb.64.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T., Hunter T., Munro A. Control of haemoglobin synthesis: rate of translation of the messenger RNA for the alpha and beta chains. J Mol Biol. 1969 Jul 14;43(1):123–133. doi: 10.1016/0022-2836(69)90083-7. [DOI] [PubMed] [Google Scholar]
- KNOPF P. M., LAMFROM H. CHANGES IN THE RIBOSOME DISTRIBUTION DURING INCUBATION OF RABBIT RETICULOCYTES IN VITRO. Biochim Biophys Acta. 1965 Mar 15;95:398–407. doi: 10.1016/0005-2787(65)90186-3. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Roseman M., Litman B. J., Thompson T. E. Transbilayer exchange of phosphatidylethanolamine for phosphatidylcholine and N-acetimidoylphosphatidylethanolamine in single-walled bilayer vesicles. Biochemistry. 1975 Nov 4;14(22):4826–4830. doi: 10.1021/bi00693a008. [DOI] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Tarone G., Comoglio P. M. Plasma membrane proteins exposed on the outer surface of control and Rous sarcoma virus-transformed hamster fibroblasts. Exp Cell Res. 1977 Nov;110(1):143–152. doi: 10.1016/0014-4827(77)90280-4. [DOI] [PubMed] [Google Scholar]
- Tarone G., Prat M., Comoglio P. M. Affinity chromatography purification of erythrocyte membrane proteins after selective labeling with trinitrobenzene sodium sulfonate. Biochim Biophys Acta. 1973 Jun 22;311(2):214–221. doi: 10.1016/0005-2736(73)90268-x. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. The presence of two Fc receptors on mouse macrophages: evidence from a variant cell line and differential trypsin sensitivity. J Exp Med. 1977 Apr 1;145(4):931–945. doi: 10.1084/jem.145.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R., Tarone G., Peroni F., Comoglio P. M. A comparative study of SV40-transformed fibroblast plasma membrane proteins labelled by enzymatic iodination or with trinitrobenzene sulfonate. FEBS Lett. 1974 Oct 1;47(1):107–112. doi: 10.1016/0014-5793(74)80436-9. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Cell surface immunoglobulin. IX. A new method for the study of synthesis, intracellular transport, and exteriorization in murine splenocytes. J Exp Med. 1974 Jun 1;139(6):1599–1620. doi: 10.1084/jem.139.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Synthesis of surface H-2 alloantigens in murine splenocytes. J Immunol. 1975 Aug;115(2):374–381. [PubMed] [Google Scholar]