Abstract
Purpose
This study evaluated the tolerability, pharmacokinetics (PK), and preliminary antitumor activity of EZN-2208, a water-soluble, poly(ethylene) glycol conjugate of SN38.
Methods
Patients with advanced malignancies were enrolled in dose-escalating cohorts (3 + 3 design). EZN-2208 was administered as a 1-hour intravenous infusion given weekly for 3 weeks per each 4-week cycle. Doses ranged from 1 to 12 mg/m2.
Results
Forty-one patients received EZN-2208. All patients had received prior cancer therapy (median = 2, range = 1–11). Twenty patients (49%) had received prior irinotecan, and one patient had received prior topotecan. One patient in the 9-mg/m2 cohort had dose-limiting toxicity (grade 3 febrile neutropenia), and one patient in the 12-mg/m2 cohort had grade 3 neutropenia that resulted in the inability to deliver the third dose of EZN-2208. The most commonly reported drug-related adverse events were nausea (51%), diarrhea (46%), fatigue (41%), alopecia (29%), neutropenia (24%), and vomiting (22%). Administration of EZN-2208 results in prolonged exposure to SN38. Stable disease, sometimes prolonged and associated with tumor shrinkage, was observed as best response.
Conclusions
EZN-2208 has an acceptable safety profile in previously treated patients with advanced malignancies. The recommended phase II dose of EZN-2208 administered according to this schedule was 9 mg/m2.
Keywords: topoisomerase-1 inhibitors, SN-38, polyethylene glycol, Phase 1 clinical trials
INTRODUCTION
SN38 (10-hydroxy-7-ethyl-camptothecin) is a potent topoisomerase I inhibitor and the active moiety of irinotecan (CPT-11), a prodrug, approved for the treatment of patients with colorectal cancer, with known activity against a variety of cancers. SN38 has 100- to 1,000- fold more potent in vitro cytotoxic activity compared with CPT-11 [1]. SN38 itself has not been used as an anticancer drug in humans due to poor solubility in any pharmaceutically acceptable excipient.
EZN-2208 is a water-soluble poly(ethylene) glycol (PEG) conjugate of SN38 [2]. PEGylation of SN38 on the E-ring preserves the active lactone form of SN38 in the prodrug until its release, as a result of hydrolysis under basic conditions, whereupon equilibrium with the inactive carboxy form occurs, whereas with irinotecan, equilibrium occurs with the prodrug as well as with SN38 [2]. EZN-2208 has shown activity in multiple preclinical models of solid tumors and hematologic malignancies, including an in vivo model of CPT-11 resistance [3–6]. EZN-2208 enables parenteral delivery of SN38. Preclinically, the drug was found to result in a longer circulating half-life and higher exposure of the SN38 in tumors compared to irinotecan [2, 3]. SN38 is the unique active metabolite of EZN-2208, in distinction to irinotecan, which has a complex metabolic pathway involving release of bis-piperidine by carboxylesterases and oxidative metabolism by cytochrome P450 (CYP3A) enzymes. The metabolites of irinotecan include 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino]carbonyloxycamptothecin (APC), and 7-ethyl-10-(4-amino-1-piperidino) carbonyloxycamptothecin (NPC), which are detectable in the blood, and may undergo subsequent metabolism to SN38 [7, 8].
SN38 is subsequently converted to a glucuronide derivative. Interactions with drugs metabolized by CYP3A that are present with irinotecan and variation in clearance due to CYP3A variations are unlikely to be present with EZN-2208 [9]. In animal models, EZN-2208 accumulates in tumors, where it releases SN38. The superior antitumor activity of EZN-2208 compared to CPT-11 is attributed to the higher exposure of tumors to SN38 via preferential accumulation of EZN-2208 in the tumor (enhanced permeability and retention [EPR] effect) [3]. Administration of EZN-2208 results in down-modulation of hypoxia-inducible factor-1α (HIF-1α) in the tumor in vivo, resulting in down-regulation of target genes messenger ribonucleic acid (mRNA), and in greater anti-proliferation, anti-apoptotic, DNA fragmentation, and enhanced anti-angiogenic effects compared to irinotecan [4–5] For these reasons, EZN-2208 was expected to provide improved therapeutic index compared to irinotecan.
The primary objectives of this trial were to determine the maximum tolerated dose (MTD) and recommended phase II dose (RP2D) of EZN-2208 given weekly for 3 weeks per 4-week cycle. Secondary objectives included evaluation of safety and tolerability, determination of the pharmacokinetic (PK) profile, and evaluation of preliminary antitumor activity.
PATIENTS AND METHODS
The study protocol was approved by the Institutional Review Board (IRB) for each study center. Written informed consent was obtained from each patient before study-specific procedures were performed.
Patients
Eligible patients were ≥ 18 years of age and had a histologically or cytologically confirmed diagnosis of advanced or metastatic solid tumor refractory to standard therapy or had no standard therapy that increased survival. Patients had to have measurable or evaluable disease by RECIST (Response Evaluation Criteria in Solid Tumors) Version 1.0 [10], be willing to be tested for uridine diphosphate glucuronosyl-transferase isoform 1A1 (UGT1A1) genotype, and have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. The patients also had to have: hemoglobin ≥ 9.0 g/dL, absolute neutrophil count ≥ 1,500/µL, platelet count ≥ 100,000/µL, serum creatinine ≤ 1.5× the upper limit of normal (ULN), total bilirubin and partial thromboplastin time within normal limits, and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5× ULN (may have been ≤ 5× ULN if the increase was due to metastatic disease to the liver).
Exclusion criteria were: concurrent serious medical illness that could potentially interfere with protocol compliance; known chronic infectious disease; active diarrhea; chronic enteropathy; known coagulation disorder; pregnancy or lactation; known or clinically suspected central nervous system involvement; requirement for CYP3A4 enzyme-inducing medications, ketoconazole, or inhibitors of platelet function; prior chemotherapy, immunotherapy, investigational agent, or other agents used to treat cancer within 4 weeks before the first dose of EZN-2208; prior wide-field radiotherapy (>25% of bone marrow); lack of recovery of reversible side effects related to administration of agents used to treat the cancer; and any condition that the investigator considered to make the patient unsuitable for study participation.
Drug Administration and Dose-Escalation
EZN-2208 was administered as an intravenous (i.v.) infusion over 60 minutes given weekly for 3 weeks per 4-week cycle. The starting dose (1 mg/m2) of EZN-2208 was calculated based on one-sixth of the MTD observed in beagle dogs. The dose of EZN-2208 for subsequent cohorts was increased according to a modified Fibonacci dose-escalation scheme [11]. Dose escalation was based on first-cycle drug-related toxicities. The definition of dose-limiting toxicity (DLT) is provided in Table 1. Using a 3 + 3 dose-escalation design, up to six patients were treated at each dose level or until the MTD was determined. Patients received EZN-2208 until disease progression, unacceptable toxicity, or withdrawal of consent. Dose adjustments and/or delays were permitted to allow for patient tolerability, but dose re-escalation was not allowed.
Table 1.
Definition of Dose-Limiting Toxicity (DLT)a
| Drug-Related Hematologic Toxicity |
| Febrile neutropenia: Fever (≥38.5°C) with grade 3 or 4 neutropenia |
| Asymptomatic grade 4 neutropenia (ANC <500/µL) of >5 days’ duration |
| Grade 4 thrombocytopenia (platelet count <25,000/µL) |
| Other grade 4 hematologic toxicity, including ↓ in Hb, at investigator’s discretion |
| Drug-Related Nonhematologic Toxicity |
| Grade 3 or 4 nausea and vomiting despite maximum supportive care |
| Other Grade 3 or 4 nonhematologic toxicities with the following exceptions: |
| Grade 3 laboratory abnormalities that were transient (<24 hours), correctible, and not associated with clinical sequelae were not considered DLTs. |
| Toxicity that resulted in a >14-day delay in scheduled administration of EZN-2208 |
| Toxicity clearly related to rapid progressive disease was not considered a DLT. |
Abbreviations: ANC, absolute neutrophil count; Hb = hemoglobin.
DLT was defined as any of the above drug-related toxicities occurring during the first treatment cycle.
Patients homozygous for UGT1A1*28 were evaluated in separate cohorts, starting at two dose levels below the dose level of the cohort that was being enrolled at that time. DLTs occurring in patients with a UGT1A1*28/*28 genotype were not used to establish the MTD in patients not homozygous for UGT1A1*28.
Assessment of Safety
Toxicity assessment was performed weekly. The intensity of toxicities was graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. The investigators assessed AEs as likely or unlikely related to EZN-2208. Laboratory evaluation including a complete blood count with differential and platelets, serum chemistries (bicarbonate, blood urea nitrogen, calcium, chloride, creatine phosphokinase, creatinine, glucose, lactate dehydrogenase, magnesium, phosphate, potassium, sodium, total protein, albumin, alkaline phosphatase, ALT, AST, and total bilirubin), coagulation tests (prothrombin time, partial thromboplastin time [PTT], and international normalized ratio [INR]) were performed weekly. Urinalysis was performed on Day 1 of each cycle. A physical examination was performed on Day 1 of each cycle.
Assessment of Pharmacokinetics
Blood samples for PK characterization were collected before the first infusion; 15 and 60 minutes after the start of the first infusion; 90 minutes and 2, 4, 8, 24, 48, 72, 96, 120, and 168 hours after the end of the first infusion; before the third infusion (Day 15); 15 and 60 minutes after the start of the third infusion; 90 minutes, 2 hours, and 4 hours after the end of the third infusion; and before the infusion on Day 1 of Cycle 2. The plasma concentration of SN38, and glucuronidated SN38 (SN38G) were determined by validated high-performance liquid chromatography (HPLC) using fluorescent detection [3]. To determine PEG-SN38 concentration, the plasma pH was raised to 8.0 ± 0.5 with sodium carbonate buffer and the sample was incubated at 30°C for 24 hours to liberate SN38 via ester bond hydrolysis. The sample was then acidified with citric acid, extracted and analyzed for SN38 using HPLC [3]. The concentration of PEG-SN38 was determined by subtracting free SN38 determined originally from total SN38 measured after hydrolysis.
Data were analyzed using the nonlinear mixed-effect modeling software program Monolix version 31s (http://wfn.software.monolix.org) using a population pharmacokinetic approach. Parameters were estimated by computing the maximum likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization (SAEM) algorithm combined to a MCMC (Markov Chain-Monte Carlo) procedure [12, 13].
A proportional model was used to describe the residual variability, and the between-subject variabilities (BSV, η) were ascribed to an exponential model. Specific tests comparing the log-likelihood, the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were performed to test different hypotheses regarding the final model, covariate(s) effect on pharmacokinetic parameter(s), residual variability model (proportional versus proportional plus additive error model), structure of the variance-covariance matrix for the BSV parameters.
For SN38G, a model was developed to describe SN38G time-courses and derive apparent parameters of free SN38G disposition. The infused SN38 dose and SN38G were converted in molar units, assuming 1 mole SN38 was metabolized to 1 mole SN38G. For SN38 and SN38G, the clearance and volume parameters are thus apparent parameters, ((CL or Q)/Fm and (Vc or VP)/Fm where Fm is the unknown metabolic fraction) because the true bioavailability of SN38 and SN38G from their precursors, PEG-SN38 and SN38 respectively, are not known.
Assessment of UGT1A1 Genotype
A blood sample for the determination of UGT1A1 genotype was collected during pre-study. Samples were analyzed by polymerase chain reaction (PCR) followed by size analysis using capillary electrophoresis to detect four polymorphisms [*36(TA5), *1(TA6), *28(TA7) and *37(TA8)] in the promoter region of UGT1A1. (UGT1A1 GenotypR, Specialty Laboratories, Valencia, CA).
Assessment of Antitumor Activity
Patient response to treatment was evaluated according to RECIST Version 1.0 [10] before treatment and approximately every 6 to 8 weeks thereafter.
Statistical Analysis
Descriptive statistics were provided. Categorical data were summarized by frequency and percentages; continuous data were summarized by mean and standard deviation or median and range, as appropriate.
RESULTS
Patient Characteristics
Between May 2007 and January 2010, 43 patients were enrolled at two study centers in the United States. Two patients discontinued the trial before receiving EZN-2208: one withdrew consent, and one died due to progressive disease (PD). Demographics and baseline characteristics for the 41 patients who received EZN-2208 are summarized in Table 2. Thirty eight patients were white and 3 were Black or African American; 5 patients indicated Hispanic or Latino ethnicity. Twenty patients (49%) had received prior irinotecan, and one had received prior topotecan.
Table 2.
Demographics and Baseline Characteristics for Treated Patients (N = 41)
| Characteristic | No. of Patients |
|---|---|
| Sex | |
| Female | 21 |
| Male | 20 |
| Age, years | |
| Median | 60 |
| Range | 35–85 |
| ECOG performance status | |
| 0 | 17 |
| 1 | 22 |
| 2 | 2 |
| UGT1A1 genotype | |
| *1/*28 | 23 |
| *1/*1 | 16 |
| *28/*28 | 2 |
| Cancer type | |
| Colorectal | 24 |
| Breast | 3 |
| Pancreatic | 3 |
| Esophageal | 2 |
| NSCLC | 2 |
| Anal | 1 |
| Carcinoid | 1 |
| Gallbladder | 1 |
| Gastric | 1 |
| Ovarian | 1 |
| Prostate | 1 |
| Soft tissue sarcoma | 1 |
| Prior cytotoxic chemotherapies | |
| 1 | 8 |
| 2 | 14 |
| ≥3 | 19 |
| Median | 2 |
| Range | 1–11 |
| Prior irinotecan-containing regimen | |
| No | 21 |
| Yes | 20 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NSCLC, non–small-cell lung cancer; UGT1A1, uridine diphosphate glucuronosyl-transferase isoform 1A1.
The median duration of EZN-2208 treatment was 8.0 weeks (range = 4.0 to 69.1 weeks). The primary reasons for discontinuation of EZN-2208 were PD (n = 32, 78%), withdrawn consent (n = 3, 7%), AE (n = 2, 5%), investigator’s decision (n = 2, 5%), patient noncompliance (n = 1, 2%), and patient did not return to clinic (n = 1, 2%).
Tolerability and Safety
In the 9-mg/m2 group, one patient had dose-limiting, grade 3 febrile neutropenia, during Cycle 1. In the 12-mg/m2 group, one patient had grade 3 neutropenia during Week 2 that resulted in the inability to deliver the third dose of EZN-2208 during Cycle 1. In conjunction with these events, EZN-2208 administered every 3 weeks was found to have an MTD of 10 mg/m2, with a DLT of febrile neutropenia [14]. The dose intensity for patients in the present study was significantly higher than the dose intensity (5.3 mg/m2/week) exceeding the MTD in the every 3 week study [14]. After extensive review of data from both studies by the investigators, it was determined not to be prudent to continue with dose escalation in the present study, but to recommend 9 mg/m2 as the RP2D of EZN-2208 administered weekly for 3 weeks per 4-week cycle, and to reevaluate that dose in all Phase 2 studies in the more homogeneous population of these studies.
The most commonly reported AEs considered likely related to EZN-2208 were nausea (51%), diarrhea (46%), fatigue (41%), alopecia (29%), neutropenia (24%), and vomiting (22%) (Table 3). Two patients (5%), one in the 9-mg/m2 group and one in the 12-mg/m2 group, had neutropenia with a worst toxicity grade of 4 (considered drug related for both patients); the duration of the grade 4 neutropenia, which occurred during Cycle 2 for both patients, was 2 days and 7 days. The most common drug-related AEs with a worst toxicity grade of 3 were neutropenia (10%) and fatigue and peripheral neuropathy (5% each). All other drug-related AEs with a worst toxicity grade of 3 were reported in one patient each. Two patients had a homozygous (*28/*28) UGT1A1 genotype, and both received 2 mg/m2 of EZN-2208. Neutropenia was not noted for either patient.
Table 3.
Treatment-Emergent Drug-Related Adverse Events Reported for >1 Patient
| Dose (mg/m2) |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 41) |
||||||||||||||||||||||||||
| 1 (n = 3) |
2 (n = 3) |
2H* (n = 2) |
3.3 (n =3) |
5 (n = 6) |
7 (n = 8) |
9† (n = 10) |
12 (n = 6) | Any | ||||||||||||||||||
| Adverse Event | All | Gr 3 | All | Gr 3 | All | Gr 3 | All | Gr 3 | All | Gr 3 | All | Gr 3 | All | Gr 3–4 | All | Gr 3–4 | No. | % | ||||||||
| Hematologic/Coagulation | ||||||||||||||||||||||||||
| Neutropenia | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 | 2 | 3 | 3 | 10 | 24 | ||||||||||||
| Anemia | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 5 | 12 | ||||||||||||
| aPTT prolonged | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 7 | |||||||||||||
| Leukopenia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 7 | |||||||||||||
| Gastrointestinal toxicity | ||||||||||||||||||||||||||
| Nausea | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 3 | 0 | 8 | 0 | 3 | 0 | 21 | 51 | ||||||||
| Diarrhea | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 6 | 0 | 5 | 1 | 3 | 0 | 19 | 46 | ||||||||||
| Vomiting | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 9 | 22 | |||||||||||
| Constipation | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 6 | 15 | ||||||||||||
| Dyspepsia | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 7 | ||||||||||||||
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 5 | |||||||||||||||
| OtheNonhematologic toxicity | ||||||||||||||||||||||||||
| Fatigue | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 6 | 2 | 3 | 0 | 17 | 41 | ||||||||
| Alopecia | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 6 | 0 | 2 | 0 | 12 | 29 | ||||||||||||
| Anorexia | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 8 | 20 | ||||||||||
| Dysgeusia | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 7 | |||||||||||||
| Dehydration | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 5 | ||||||||||||||
| Dizziness | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 5 | ||||||||||||||
| Hypokalemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 5 | ||||||||||||||
| Hypomagnesemia | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 5 | |||||||||||||||
| Peripheral neuropathy | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 5 | ||||||||||||||
| Skin hyperpigmentation | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 5 | ||||||||||||||
Abbreviation: aPTT, activated partial thromboplastin time; Gr, grade.
Note: Two patients (one in the 9-mg/m2 and one in the 12-mg/m2 cohort) had grade 4 neutropenia; all other patients had grade 3 events.
Patients with homozygous (*28/*28) UGT1A1 genotype.
Three patients, treated at 9 mg/m2, had treatment-emergent grade 3 diarrhea that lasted 1 to 8 days, though none of the episodes were considered dose limiting or reported as serious adverse events. The onset of grade 3 diarrhea was in Cycle 1 (2 patients) and in Cycle 3 (1 patient). These episodes of grade 3 diarrhea in Cycle 1 were considered not to be dose limiting: one patient did not receive optimal antidiarrheal therapy due to noncompliance; the other patient had baseline grade 2 intermittent diarrhea pretreatment and throughout the first course, and experienced grade 3 diarrhea for less than 24 hours. The consensus amongst the investigators was that this event was not treatment related and did not meet the criteria for DLT.
Of the 41 treated patients, 10 (24%) died either within (n = 7) or beyond (n = 3) 30 days of the last dose of EZN-2208. All of these deaths were considered unlikely related to EZN-2208. For these patients, the primary cause of death was disease progression. Three of these patients had grade 5 AEs, all of which were considered unlikely related to EZN-2208: multiorgan failure (n = 1); respiratory failure (n = 1); and cardiogenic shock, hepatic necrosis, increased INR, lung infiltration, prolonged activated PTT (aPTT), pulmonary embolism, and respiratory failure (n = 1).
Most grade 3 and 4 laboratory abnormalities were observed in the higher dose cohorts: 9 and 12 mg/m2 for grade 3 and 4 hematologic laboratory abnormalities, and 5 mg/m2 and higher for grade 3 and 4 chemistry laboratory abnormalities.
Individuals homozygous for UGT1A1*28 are thought to be potentially at increased risk for neutropenia with irinotecan treatment, as the UGT1A1*28 allele is believed to confer reduced UGT1A1-mediated inactivation of SN38 [15–22]. Two patients treated at 2 mg/m2 had a homozygous (*28/*28) UGT1A1 genotype. Neutropenia was not noted for either patient.
Individuals heterozygous for the UGT1A1*28 allele also may be at increased risk for neutropenia [15]. Twenty-three of the 41 treated patients (56%) had a UGT1A1 genotype of *1/*28. Four of these patients were reported to have treatment-emergent clinical AEs of grade 1 to 4 neutropenia considered likely related to EZN-2208; none of these events were associated with fever.
Pharmacokinetics
Data for 40 of 41 treated patients were included in the analysis (results for 1 patient could not be estimated due to lack of data). The PK of plasma EZN-2208 and SN38 were satisfactorily described by a two-compartment open model with linear elimination. PK parameters for EZN-2208, SN38, and SN38G are summarized in Table 4. The mean terminal-phase elimination t1/2 for EZN-2208 and SN38 were 17.1 and 20.7 hours, respectively. For EZN-2208, the terminal (beta) disposition phase was associated with a very high fraction of area-under-the-concentration curve (AUC), and the central volume of distribution was close to the circulating blood volume. This suggests the major part of the parent drug is restrained to the circulation space with little tissue diffusion, whereas, for free SN38, the volume of distribution was very high, indicating significant tissue diffusion of the active metabolite.
Table 4.
Mean (SD) Bayesian Pharmacokinetic Estimates for EZN-2208, SN38,and SN38G
| CL (L/h) |
V1 (L) |
Q1 (L/h) |
V2 (L) |
AUC Fraction α Phase |
AUC Fraction β Phase |
α Half-life (h) |
β Half-lifea (h) |
Elimination Half-lifea (h) |
|
|---|---|---|---|---|---|---|---|---|---|
| EZN-2208 | |||||||||
| Mean | 0.20 | 5.11 | 0.19 | 1.54 | 0.125 | 0.875 | 5.35 | 25.14 | 17.1 |
| SD | 0.063 | 1.94 | 0.20 | 0.56 | 0.062 | 0.062 | 1.95 | 2.20 | 2.79 |
| SN38 | |||||||||
| Mean | 21.1 | 625 | 9.4 | 205 | 0.56 | 0.44 | 14.2 | 73.8 | 20.7 |
| SD | 13.6 | 407 | 19.5 | 323 | 0.34 | 0.34 | 6.71 | 62.9 | 4.42 |
| SN38G | |||||||||
| Mean | 19.7 | 827 | 6.8 | 15017 | 0.69 | 0.31 | 21.9 | 2188 | 34.9 |
| SD | 13.4 | 448 | 3.3 | 12613 | 0.16 | 0.16 | 3.7 | 1523 | 18.2 |
Abbreviations: α, fast distribution phrase; AUC, area-under-the concentration curve; β, slow distribution phase; CL, elimination clearance; Q1, intercompartmental clearance; SD, standard deviation; SN38G = glucuronidated SN38; V1, central distribution volume; V2, peripheral distribution volume.
β half-life corresponds to the terminal slope for the plasma concentration decay. Elimination half-life describes the elimination of the drug from the organism, defined as V1*log(2)/CL.
Antitumor Activity
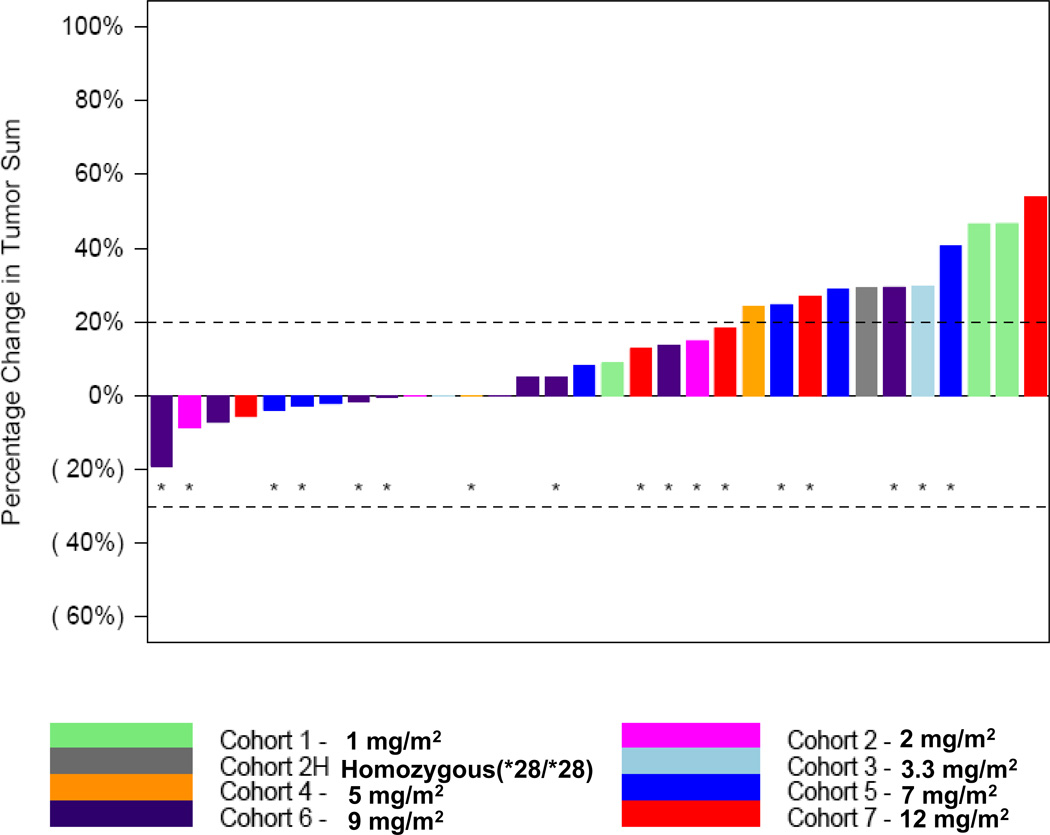
The best overall response (Figure 1) was stable disease (SD) for 19 patients (46%) and PD for 20 patients (49%). Response was not evaluable for 2 patients (5%). Of the 19 patients who had SD, 12 patients had colorectal cancer (CRC); 2 patients each had breast and esophageal cancers; and 1 patient each had non-small-cell lung, gallbladder, and pancreatic cancersdian duration of SD for these 19 patients was 107 days (range = 50+ to 421 days). Nine patients (47%), all with CRC, had received prior irinotecan. The median number of prior cytotoxic therapies for the 19 patients with a best overall response of SD was 2 (range = 1 to 7).
Fig. 1. Maximum change in the sum of target lesions from baseline.
*=Patient received prior irinotecan.
DISCUSSION
This phase I, multicenter, open-label, non-randomized, dose-escalation study evaluated EZN-2208 administered as a 1-hour i.v. infusion given weekly for 3 weeks per each 4-week cycle to patients with advanced malignancies. Forty-one patients with a variety of cancers received EZN-2208. Most patients (98%) had metastatic disease. These patients had been previously treated and had progressed after receiving many standard and investigational agents. All patients had received prior chemotherapy, and 46% had received 3 or more prior chemotherapies.
The DLT of EZN-2208 consisted of neutropenia and its complications, including febrile neutropenia and the inability to deliver the intended doses of EZN-2208.
Irinotecan has significant dose-limiting side effects, including both acute and delayed severe diarrhea, as well as neutropenia [15, 16]. The side effects of irinotecan are thought to be due to SN38 as well as due to irinotecan and its metabolites. The severe diarrhea associated with irinotecan has been attributed primarily to regeneration of SN38 from excreted SN38G by intraluminal intestinal bacterial glucuronidases [7, 17–22]. The bis-piperidine moiety, which is found in irinotecan but absent from SN38, also has been thought to be partially responsible for acute diarrhea [23].
Life-threatening diarrhea is observed in up to 25% of cancer patients receiving irinotecan [16]. Importantly, no grade 4 diarrhea was reported in the present trial. In this study, treatment-emergent diarrhea considered likely related to EZN-2208 was reported for 19 patients (46%). For most of these patients, the worst toxicity grade of diarrhea was 1 (n = 10) or 2 (n = 8).
The results of the trial reported here are similar to data reported for another phase I trial in which EZN-2208 was administered once every 3 weeks [14]. In that study, EZN-2208 also was generally well tolerated in patients with advanced malignancies. No grade 4 diarrhea was reported. The DLT was febrile neutropenia. The MTD and RP2D were determined to be 10 mg/m2 for EZN-2208 administered without G-CSF and 16.5 mg/m2 for EZN-2208 administered with first-cycle G-CSF.
In summary, EZN-2208 has an acceptable safety profile in previously treated patients with advanced malignancies. The DLTs for EZN-2208 administered as a weekly 1-hour i.v. infusion for 3 weeks of each 4-week cycle consisted of grade 3 febrile neutropenia and grade 3 neutropenia resulting in the inability to deliver the third dose of EZN-2208 during Cycle 1. These findings are in contrast to the occurrence of dose-limiting diarrhea in patients treated with irinotecan. The RP2D of EZN-2208 administered according to this treatment schedule was 9 mg/m2 with evaluation of that dose being performed in Phase II in more homogeneous populations. Administration of EZN-2208 results in prolonged exposure to SN38. Stable disease, sometimes prolonged and associated with tumor shrinkage, was observed as best response. Phase II studies evaluating EZN-2208 in patients with colorectal cancer and breast cancer are ongoing, as well as a Phase I study in pediatric patients.
Acknowledgment
The authors thank Arlene Reiss (consultant medical writer for Enzon) for her assistance in the manuscript preparation.
Funding: Enzon Pharmaceuticals Inc.
Research Funding: Muralidhar Beeram, Kyriakos Papadopoulos, Amita Patnaik, Anthony Tolcher
Footnotes
AUTHORS’ CONTRIBUTIONS
Conception and design: Muralidhar Beeram, Aby Buchbinder, Amita Patnaik, Anthony Tolcher
Provision of patients: Muralidhar Beeram, Tanios Bekaii-Saab, Amita Patnaik, Sanaa Tahiri, Anthony Tolcher
Data collection and assembly: Muralidhar Beeram, Tanios Bekaii-Saab, Aby Buchbinder, Kyriakos Papadopoulos, Amita Patnaik, Larry Schaaf, Sanaa Tahiri
Data analysis and interpretation: Muralidhar Beeram, Tanios Bekaii-Saab, Aby Buchbinder, François Lokiec, Amita Patnaik, Saik Urien, Keyvan Rezaï, Anthony Tolcher
Manuscript writing and final approval: All authors
POTENTIAL CONFLICTS OF INTEREST
Employment and Stock Ownership: Aby Buchbinder, Enzon
Advisory Role: Anthony Tolcher (Uncompensated)
Honoraria: François Lokiec, Keyvan Rezaï, Saïk Urien
Presented in part at the EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, October 23, 2008, Geneva, Switzerland, and the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics, November 18, 2009, Boston, MA.
REFERENCES
- 1.Chabot GG. Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet. 1977;33:245–259. doi: 10.2165/00003088-199733040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Zhao H, Rubio B, Sapra P, et al. Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem. 2008;19:849–859. doi: 10.1021/bc700333s. [DOI] [PubMed] [Google Scholar]
- 3.Sapra P, Zhao H, Mehlig M, et al. Novel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-1–refractory model. Clin Cancer Res. 2008;14:1888–1896. doi: 10.1158/1078-0432.CCR-07-4456. [DOI] [PubMed] [Google Scholar]
- 4.Sapra P, Kraft P, Mehlig M, et al. Marked therapeutic efficacy of a novel polyethylene glycol-SN38 conjugate, EZN-208, in xenograft models of B-cell non-Hodgkin’s lymphoma. Haematologica. 2009;94:1456–1459. doi: 10.3324/haematol.2009.008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastorino F, Loi M, Sapra P, et al. Tumor regression and curability of preclinical neuroblastoma models by PEGylated SN38 (EZN-208), a novel topoisomerase I inhibitor. Clin Cancer Res. 2010;16:4809–4821. doi: 10.1158/1078-0432.CCR-10-1354. [DOI] [PubMed] [Google Scholar]
- 6.Sapra P, Kraft P, Pastorino F, et al. Potent and sustained inhibition of HIF-α and downstream genes by a polyethyleneglycol-SN38 conjugate, EZN-208, results in anti-angiogenic effects. Angiogenesis. 2011;14:245–253. doi: 10.1007/s10456-011-9209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparreboom A, de Jonge MJA, de Bruijn P, et al. Irinotecan (CPT-1) metabolism and disposition in cancer patients. Clin Cancer Res. 1998;4:2747–2754. [PubMed] [Google Scholar]
- 8.Rivory LP, Haaz MC, Canal P, Lokiec F, Armand JP, Robert J. Pharmacokinetic interrelationships of irinotecan (CPT-1) and its three major plasma metabolites in patients enrolled in phase I/II trials. Clin Cancer Res. 1997;3:1261–1266. [PubMed] [Google Scholar]
- 9.Mathijssen RHJ, de Jong FA, van Schaik RHN, et al. Prediction of irinotecan pharmacokinetics by use of cytochrome P450 3A4 phenotyping probes. J. Natl Cancer Inst. 2004;96:1585–1592. doi: 10.1093/jnci/djh298. [DOI] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Omura GA. Modified Fibonacci search. J Clin Oncol. 2003;21:3177. doi: 10.1200/JCO.2003.99.058. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Analysis. 2005;49:1020–1038. [Google Scholar]
- 13.Kuhn E, Lavielle M. Coupling a stochastic approximation version of EM with a MCMC procedure. ESAIM P&S. 2004;8:115–131. [Google Scholar]
- 14.Kurzrock R, Wheler J, Hong DS, et al. Phase 1, first-in-human, dose-escalation study of EZN-2208, a novel anticancer agent, in patients with advanced malignancies. [Accessed 16 Jan 2012];Mol Cancer Ther. 2009 8:C216. (Abstract) ( http://enzon.com/docs/development_presentations). [Google Scholar]
- 15.Camptosar® (irinotecan hydrochloride injection) package insert. NY: Pfizer; 2010. Aug, (( http://media.pfizer.com/files/products/uspi_camptosar.pdf) [Google Scholar]
- 16.Hu ZY, Yu Q, Zhao YS. Dose-dependent association between UGT1A1*28 polymorphism and irinotecan-induced diarrhoea: a meta-analysis. Eur J Cancer. 2010;46:1856–1965. doi: 10.1016/j.ejca.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 17.de Jong FA, Kehrer DFS, Mathijssen RHJ, et al. Prophylaxis of irinotecan-induced diarrhea with neomycin and potential role for UGT1A1*28 genotype screening: a double-blind, randomized, placebo-controlled study. Oncologist. 2006;11:944–954. doi: 10.1634/theoncologist.11-8-944. [DOI] [PubMed] [Google Scholar]
- 18.Dodds HM, Tobin PJ, Stewart CF, et al. The importance of tumor glucuronidase in the activation of irinotecan in a mouse xenograft model. J Pharmacol Exp Ther. 2002;303:649–655. doi: 10.1124/jpet.102.039040. [DOI] [PubMed] [Google Scholar]
- 19.Iyer L, King CD, Whitington PF, et al. Genetic predisposition to the metabolism of irinotecan. Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sai K, Kaniwa N, Ozawa S, Sawada J. A new metabolite of irinotecan in which formation is mediated by human hepatic cytochrome P-450 3A4. Drug Metab Dispos. 2001;29:1505–1513. [PubMed] [Google Scholar]
- 21.Takasuna K, Hagiwara T, Hirohashi M, et al. Involvement of β-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride in rats. Cancer Res. 1996;56:3752–3757. [PubMed] [Google Scholar]
- 22.Toffoli G, Cecchin E, Gasparini G, et al. Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Onco. 2010;l28:866–871. doi: 10.1200/JCO.2009.23.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodds HM, Hanrahan J, Rivory LR. The inhibition of acetylcholinesterase by irinotecan and related camptothecins: key structural properties and experimental variables. Anticancer Drug Des. 2001;16:239–246. [PubMed] [Google Scholar]