Abstract
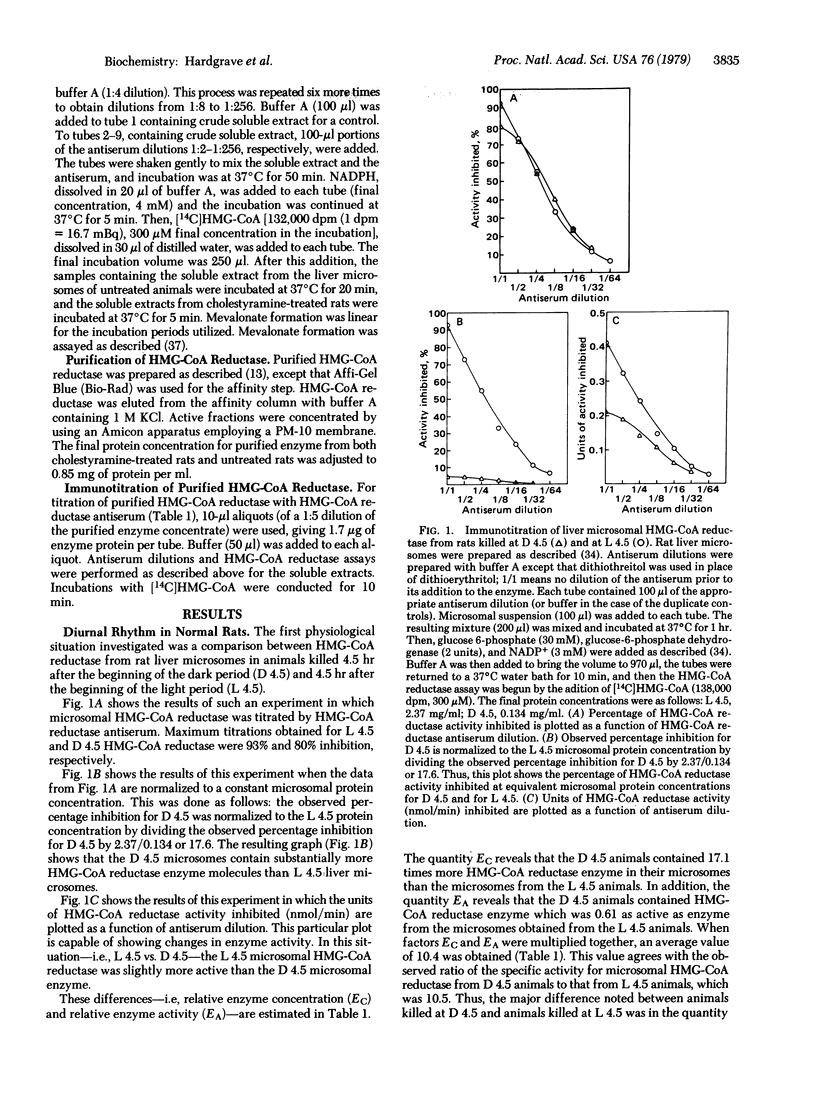
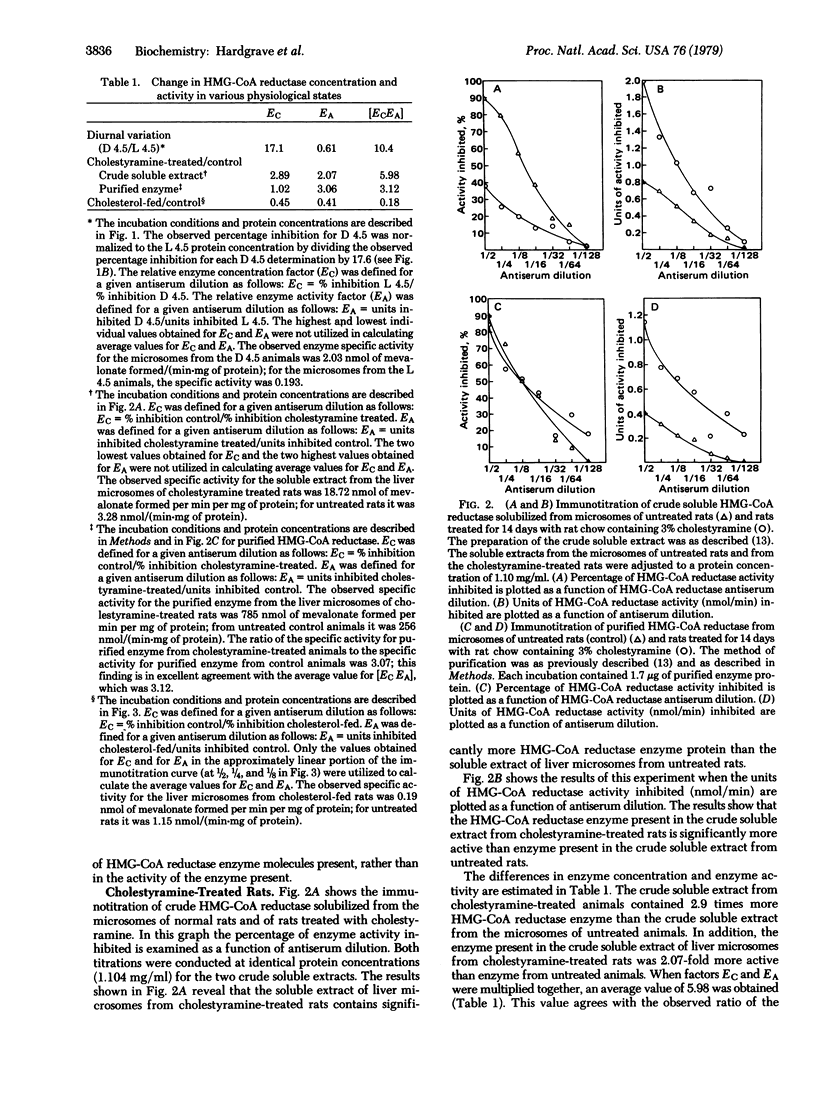
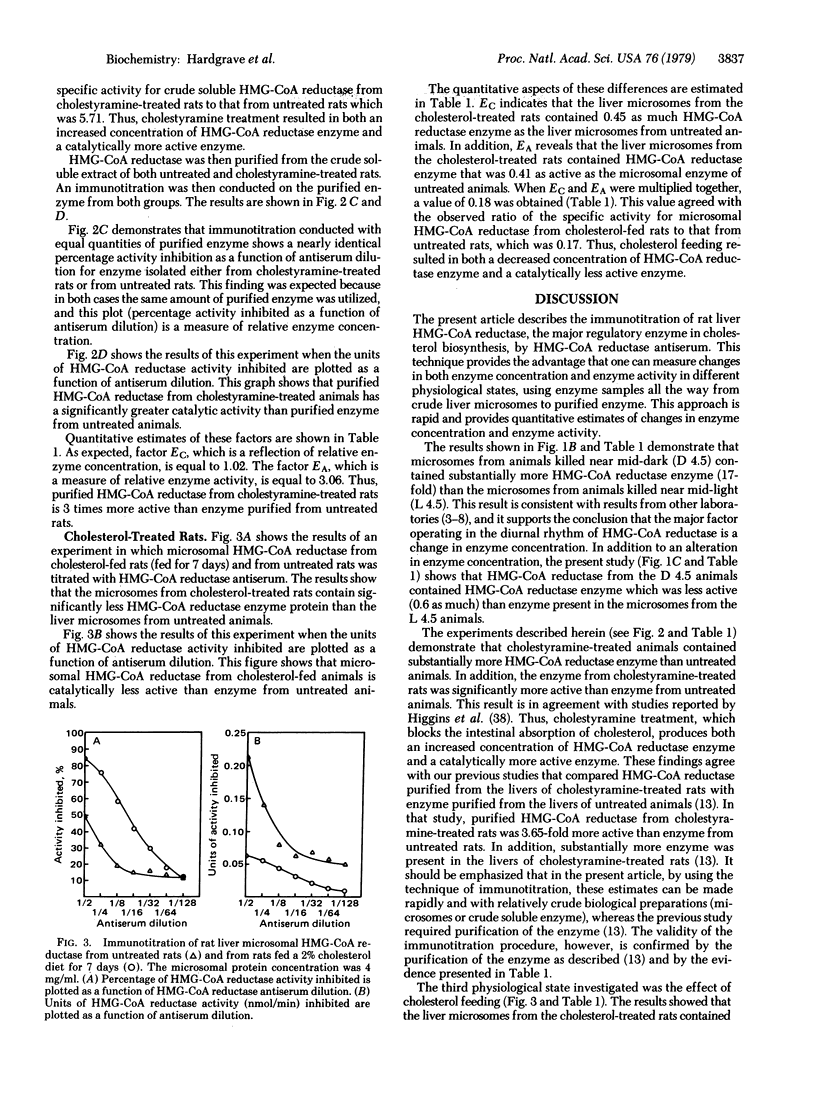
The immunotitration of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase [mevalonate:NADP+ oxidoreductase [CoA-acylating), EC 1.1.1.34], the major regulatory enzyme in cholesterol biosynthesis, by HMG-CoA reductase antiserum is described. This technique provides the advantage that one can rapidly measure relative changes in both enzyme concentration and enzyme activity in different physiological states by using enzyme samples all the way from crude liver microsomes to purified enzyme. Regarding the diurnal rhythm of HMG-CoA reductase, the major difference noted between animals killed near the middle of the dark period (D 4.5) compared to animals killed near the middle of the light period (L 4.5) was in the concentrations of HMG-CoA reductase present, rather than in the activity of the enzyme, with substantially more enzyme found near mid-dark. Cholestyramine treatment resulted in both an increased concentration of HMG-CoA reductase and a catalytically more active enzyme. On the other hand, cholesterol feeding resulted in both a decreased concentration of HMG-CoA reductase and a catalytically less active enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. E., Redd W. L., Tormanen C. D., Hardgrave J. E., Scallen T. J. The quantitative assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase: comparison of a thin-layer chromatographic assay with a rapid chloroform extraction assay. J Lipid Res. 1977 May;18(3):408–413. [PubMed] [Google Scholar]
- Beg Z. H., Allmann D. W., Gibson D. M. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and wth protein fractions of rat liver cytosol. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1362–1369. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr 3-Hydroxy-3-methylglutaryl coenzyme A reductase: regulation of enzymatic activity by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3678–3682. doi: 10.1073/pnas.75.8.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirne O. R., Heller R., Watson J. A. Regulation of 3-hydroxy-3-methylglutaryl coenzyme a reductase in minimal deviation hepatoma 7288C. Immunological measurements in hepatoma tissue culture cells. J Biol Chem. 1977 Feb 10;252(3):950–954. [PubMed] [Google Scholar]
- Bové J., Hegardt F. G. Reversible modulation of rat liver 3-hydroxy 3-methyl glutaryl coenzyme A reductase. Evidence for an enzyme-catalyzed phosphorylation-dephosphorylation system. FEBS Lett. 1978 Jun 15;90(2):198–202. doi: 10.1016/0014-5793(78)80368-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Brunschede G. Y., Goldstein J. L. Inactivation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in vitro. An adenine nucleotide-dependent reaction catalyzed by a factor in human fibroblasts. J Biol Chem. 1975 Apr 10;250(7):2502–2509. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974 Nov 25;249(22):7306–7314. [PubMed] [Google Scholar]
- Dietschy J. M., Brown M. S. Effect of alterations of the specific activity of the intracellular acetyl CoA pool on apparent rates of hepatic cholesterogenesis. J Lipid Res. 1974 Sep;15(5):508–516. [PubMed] [Google Scholar]
- Dugan R. E., Slakey L. L., Briedis A. V., Porter J. W. Factors affecting the diurnal variation in the level of -hydroxy- -methylglutaryl coenzyme A reductase and cholesterol-synthesizing activity in rat liver. Arch Biochem Biophys. 1972 Sep;152(1):21–27. doi: 10.1016/0003-9861(72)90188-9. [DOI] [PubMed] [Google Scholar]
- Edwards P. A., Gould R. G. Dependence of the circadian rhythm of hepatic beta-hydroxy-beta-methylglutaryl coenzyme A on ribonucleic acid synthesis. A possible second site of inhibition by dietary cholesterol. J Biol Chem. 1974 May 10;249(9):2891–2896. [PubMed] [Google Scholar]
- Goodwin C. D., Margolis S. Cyclic AMP-sensitive activation of hepatic sterol synthesis and 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1978 Aug;19(6):747–756. [PubMed] [Google Scholar]
- Goodwin C. D., Margolis S. Specific activation of in vitro cholesterol biosynthesis by preincubation of rat liver homogenates. J Biol Chem. 1973 Nov 10;248(21):7610–7613. [PubMed] [Google Scholar]
- Heller R. A., Shrewsbury M. A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase from rat liver. Its purification, properties, and immunochemical studies. J Biol Chem. 1976 Jun 25;251(12):3815–3822. [PubMed] [Google Scholar]
- Higgins M. J., Brady D., Rudney H. Rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase: a comparison and immunological study of purified solubilized preparations, and alteration of enzyme levels by cholestyramine feeding. Arch Biochem Biophys. 1974 Jul;163(1):271–282. doi: 10.1016/0003-9861(74)90477-9. [DOI] [PubMed] [Google Scholar]
- Higgins M., Kawachi T., Rudney H. The mechanism of the diurnal variation of hepatic HMG-CoA reductase activity in the rat. Biochem Biophys Res Commun. 1971 Oct 1;45(1):138–144. doi: 10.1016/0006-291x(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Higgins M., Rudney H. Regulation of rat liver beta-hydroxy-beta-methylglutaryl-CoA reductase activity by cholesterol. Nat New Biol. 1973 Nov 14;246(150):60–61. doi: 10.1038/newbio246060a0. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Lee H. S., Parker R. A., Gibson D. M. Reversible modulation of the activities of both liver microsomal hydroxymethylglutaryl coenzyme A reductase and its inactivating enzyme. Evidence for regulation by phosphorylation-dephosphorylation. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1268–1277. doi: 10.1016/0006-291x(78)91273-1. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Heiniger H. J. Biological activity of some oxygenated sterols. Science. 1978 Aug 11;201(4355):498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of cholesterol synthesis by oxygenated sterols. Lipids. 1978 Oct;13(10):704–707. doi: 10.1007/BF02533749. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem. 1974 Oct 10;249(19):6057–6061. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Shown E. P. Binding of 25-hydroxycholesterol and cholesterol to different cytoplasmic proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2500–2503. doi: 10.1073/pnas.74.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandutsch A. A., Saucier S. E. Prevention of cyclic and triton-induced increases in hydroxymethylglutaryl coenzyme A reductase and sterol synthesis by puromycin. J Biol Chem. 1969 May 10;244(9):2299–2305. [PubMed] [Google Scholar]
- Kirsten E. S., Watson J. A. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in hepatoma tissue culture cells by serum lipoproteins. J Biol Chem. 1974 Oct 10;249(19):6104–6109. [PubMed] [Google Scholar]
- Kleinsek D. A., Ranganathan S., Porter J. W. Purification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1431–1435. doi: 10.1073/pnas.74.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S., Venkatesan S., Reeves B. E. On the mechanism for the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, of cholesterol 7alpha-hydroxylase and of acyl-coenzyme A:cholesterol acyltransferase by free cholesterol. Biochim Biophys Acta. 1978 Jul 25;530(1):99–111. doi: 10.1016/0005-2760(78)90130-3. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S. The influence of cholesterol on the activity, on the isothermic kinetics and on the temperature-induced kinetics of 3-hydroxy-3-methylglutaryl coenzyme a reductase. Biochim Biophys Acta. 1977 Oct 24;489(1):126–142. doi: 10.1016/0005-2760(77)90239-9. [DOI] [PubMed] [Google Scholar]
- Nordstrom J. L., Rodwell V. W., Mitschelen J. J. Interconversion of active and inactive forms of rat liver hydroxymethylglutaryl-CoA reductase. J Biol Chem. 1977 Dec 25;252(24):8924–8934. [PubMed] [Google Scholar]
- Philippot J. R., Cooper A. G., Wallach D. F. Regulation of cholesterol biosynthesis by normal and leukemic (L2C) guinea pig lymphocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):956–960. doi: 10.1073/pnas.74.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Rodwell V. W. Diurnal variation and cholesterol regulation of hepatic HMG-CoA reductase activity. Biochem Biophys Res Commun. 1969 Nov 20;37(5):867–872. doi: 10.1016/0006-291x(69)90972-3. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Gaylor J. L. Investigation of regulation of microsomal hydroxymethylglutaryl coenzyme A reductase and methyl sterol oxidase of cholesterol biosynthesis. J Biol Chem. 1977 Aug 25;252(16):5852–5858. [PubMed] [Google Scholar]
- Srikantaiah M. V., Tormanen C. D., Redd W. L., Hardgrave J. E., Scallen T. J. Purification of 3-hydroxy-3-methylglutaryl coenzyme A reductase by affinity chromatography on blue dextran/Sepharose 4B. Comparison of enzyme purified from normal rats and from rats treated with cholestyramine. J Biol Chem. 1977 Sep 10;252(17):6145–6150. [PubMed] [Google Scholar]
- Tormanen C. D., Srikantaiah M. V., Hardgrave J. E., Scallen T. J. Evidence for the presence of a natural inactivating factor of 3-hydroxy-3-methylglutaryl coenzyme A reductase in soluble extracts from rat liver microsomes. J Biol Chem. 1977 Mar 10;252(5):1561–1565. [PubMed] [Google Scholar]
- Ursini F., Valente M., Ferri L., Gregolin C. Inactivation of soluble 3-hydroxy-3-methylglutaryl CoA reductase by ATP. FEBS Lett. 1977 Oct 1;82(1):97–101. doi: 10.1016/0014-5793(77)80894-6. [DOI] [PubMed] [Google Scholar]


