Abstract
Biodegradable polymers with high elasticity, low thrombogenicity, and drug loading capacity continue to be pursued for vascular engineering applications, including vascular grafts and stents. A biodegradable elastomeric polyurethane was designed as a candidate material for use as a drug-eluting stent coating, such that it was nonthrombogenic and could provide antiproliferative drug release to inhibit smooth muscle cell proliferation. A phosphorylcholine containing poly(ester urethane) urea (PEUU-PC) was synthesized by grafting aminated phosphorylcholine onto backbone carboxyl groups of a polyurethane (PEUU-COOH) synthesized from a soft segment blend of polycaprolactone and dimethylolpropionic acid, a hard segment of diisocyanatobutane and a putrescine chain extender. Poly(ester urethane) urea (PEUU) from a soft segment of polycaprolactone alone was employed as a control material. All of the synthesized polyurethanes showed high distensibility (>600%) and tensile strengths in the 20–35 MPa range. PEUUPC experienced greater degradation than PEUU or PEUU-COOH in either a saline or lipase enzyme solution. PEUU-PC also exhibited markedly inhibited ovine blood platelet deposition compared with PEUU-COOH and PEUU. Paclitaxel loaded in all of the polymers during solvent casting continued to release for 5 d after a burst release in a 10% ethanol/PBS solution, which was utilized to increase the solubility of the releasate. Rat smooth muscle cell proliferation was significantly inhibited in 1 wk cell culture when releasate from the paclitaxel-loaded films was present. Based on these results, the synthesized PEUU-PC has promising functionality for use as a nonthrombogenic, drug eluting coating on metallic vascular stents and grafts.
INTRODUCTION
The treatment of pathologies related to small diameter vessel atherosclerosis has been revolutionized by the implementation of angioplasty, stent placement, and, most recently, drug eluting stent placement. The latter technology, in particular, has been significant as it has driven down the rate of vessel reocclusion by the controlled release of pharmaceutical agents such as paclitaxel from polymeric coatings on the metal stent surfaces. While achieving markedly improved outcomes over bare metal stents, drug eluting stents have been associated with an increased risk of thrombotic complications at later times in the implant period, presumably due to inadequate endothelialization and thrombogenicity of the polymer coating.1
Biostable polymer coatings, such as polyacrylate (e.g., polyethylene-co-vinyl acetate, poly(n-butyl methacarylate), parylene C), fluoropolymer (e.g., poly(vinylidine fluoride-co-hexafluoropropylene),2 and poly(ethylene carbonate),3 have been used for commercial drug eluting stents, including Cypher (Johnson & Johnson, Inc., and Cordina, Inc.) and Xience V (Abbott, Inc.). Biodegradable polymers, such as polylactide4 and poly(lactide-co-glycolide),5 have attracted interest as reservoir coatings for antiproliferative drugs with the premise that complete degradation of the coating may avoid chronic inflammation and restenosis induced by residual polymer after complete drug release. Some biodegradable polymer coated drug eluting stents, such as BioMatrix (Biosensors Int) and TaxCor (EuroCor GmbH), have been commercialized in the European market and some clinical studies have been reported evaluating drug eluting stents with biostable versus biodegradable coatings.6 However, while stents with biodegradable coatings generally appear to perform similarly to those with nondegradable coatings, there is little evidence that the biodegradable coatings employed to date have achieved marked reduction in thrombotic complication rates.6 This lack of effect may be due to a reliance only on the biodegradation effect to remove the nidus for thrombosis at late time points, instead of employing degradable polymers that are inherently nonthrombogenic throughout the degradation period.
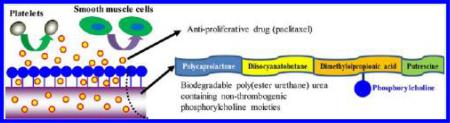
To address the need for a biodegradable, nonthrombogenic polymer, suitable for controlled release and stent coating applications, an elastomeric poly(ester urethane) urea containing nonthrombogenic groups was synthesized and then used as a carrier for the antiproliferative drug paclitaxel. Biomimetic phosphorycholine (PC)-bearing polymers have been widely studied as a good blood compatible surface that can reduce thrombogenicity by the inhibition of platelet deposition and activation.7–10 Furthermore, in previous in vivo studies, phosphorycholine-bearing polymer coated or blended polyurethanes showed promising results as a small diameter vascular graft by reducing initial thrombotic occlusion.11–13 However, those PC-bearing polymers were not integral throughout the primary structural polyurethane material, which may lead to concerns regarding the erosion of the (nondegradable) PC-bearing polymer and loss of the antithrombogenic effectiveness during chronic use. Therefore, in this study, a biodegradable elastomeric polyurethane with pendant PC groups was designed and synthesized for applications where nonthrombogenicity is critical. Specifically, a poly(ester urethane) urea containing carboxyl groups (PEUU-COOH) was synthesized from a soft segment of polycaprolactone and dimethylolpropionic acid, a hard segment diisocyanatobutane and a chain extender putrescine. Aminated phosphorycholine synthesized in the lab was then grafted onto the PEUU-COOH by a condensation reaction to achieve the final product, PC containing PEUU (PEUU-PC). The chemical structure, surface atomic composition, surface hydrophilicity, mechanical properties, and degradation of the polyurethanes were investigated. Ovine blood contact testing was performed to assess acute blood compatibility. Finally, a conventional antiproliferative drug, paclitaxel, was loaded into polyurethane films and the release profile and antiproliferative bioactivity of released drug was evaluated in vitro.
MATERIALS AND METHODS
Materials
Polycaprolactone diol (PCL, Mn = 2000, Sigma) and dimethylolpropionic acid (DMPA, sigma) were dried in a vacuum oven at 60 °C overnight to remove residual water before synthesis. 1,4-Diisocyanatobutane (BDI, Sigma) and putrescine (Sigma) were purified using vacuum distillation before usage. Stannous octotate (Sn(Oct)2, Sigma) was dried using 4 Å molecular sieves. 1,1,1,6,6,6-Hexafluoroisopropanol (HFIP, Oakwood, Inc.), dimethylsulfone (DMSO, Sigma), lipase from Thermomyces lanuginosus (≥100000 U/g, Sigma), dicyclohexylcarbodiimide (DCC, Sigma), cysteamine (Sigma), dimethylolpropionic acid (DMPA, Sigma), benzophenone (Sigma), and paclitaxel (Taxol, LC Laboratories, Inc.) were used as received. 2-Methacryloyloxyethyl phosphorycholine (MPC) was a gift from Prof. Kazuhiko Ishihara at the University of Tokyo. Other chemical agents were purchased from Sigma.
Synthesis of Aminated Phosphorycholine (PC-NH2)
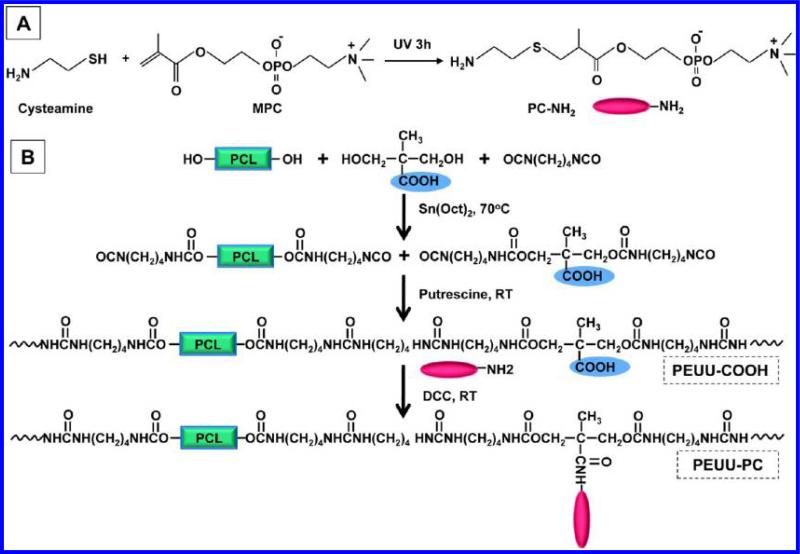
Functional phosphorycholine (PC) molecules with amino groups (PC-NH2) were synthesized under UV irradiation by thiol-ene reaction (Figure 1A).14 The synthesis procedure was as follows: a round-bottom flask equipped with a magnetic stirrer was charged with anhydrous methanol (10 mL) after adding MPC (10 mM final concentration), cysteamine (11 mM final concentration), and benzophenone as a catalyst. After argon injection for 5 min to remove the air, the flask containing the reaction mixture was sealed and placed under a high intensity UV lamp (UVP Model B 100AP, Upland, CA) at a 15 cm gap at room temperature for 3 h. Anhydrous dimethyl ether/chloroform mixed solvent (50/50) was used to precipitate the product and remove the unreacted monomer after excess solvent was evaporated from the reactive bath using a rotary evaporator. The obtained product was dried in a vacuum oven. The chemical structure of PC-NH2 was confirmed by 1H NMR. For PC-NH2 (in CDCl3), the peaks were: δ (ppm) 1.26–1.28 (α-CH3CH), 1.55–1.65 (1.26–1.28 (α-CH3)), 2.22–2.51 (SCH2CH), 2.54–2.64 (CH2CH2S), 2.81–2.91 (CH2NH2), 2.80 (3.01 (CH2NH2)), 3.28–3.43 (CH2N(CH3)3), 3.70–3.83 (CH2N(CH3)3), 4.01–4.15 (OCH2), 4.19–4.41 (CH2PO4CH2, 4H). The PC-NH2 was successfully purified, and monomer peaks at both 5.61–5.75 and 6.02–6.12 ppm (C=C) were not observed on the NMR spectrum.
Figure 1.
Schematic for synthesis of (A) aminated phosphorycholine and (B) poly(ester urethane) ureas containing carboxyl groups (PEUU-COOH) and then phosphorycholine groups (PEUU-PC).
Synthesis of Poly(ester urethane) Urea with Carboxyl Groups (PEUU-COOH)
PEUU-COOH was synthesized from a soft segment of PCL and DMPA blend, a hard segment BDI, and a chain extender putrescine (Figure 1B). PCL and DMPA were dissolved in DMSO in a three-necked flask with argon protection and stirring at 70 °C, followed by BDI and Sn(Oct)2 catalyst (3 drops) addition. The PCL:DMPA molar ratio was 70:30. After 3 h, the prepolymer solution was cooled to room temperature, then putrescine/DMSO solution was added dropwise to the prepolymer solution. The molar ratio of (PCL +DMPA)/BDI/putrescine was 1:2:1 and the final polymer solution concentration was approximately 4%. The reaction continued overnight with stirring at room temperature. The polymer was precipitated in deionized water, rinsed using ethanol, and then dried in a vacuum oven at 60 °C for 3 d. The yield of PEUU-COOH was above 90%. Control PEUU was synthesized from a soft segment PCL alone. PEUU and PEUU-COOH films were fabricated by solvent casting using HFIP, following drying in a vacuum oven at 60 °C for 3 d.
Synthesis of Poly(ester urethane) Urea with Phosphorylcholine Groups (PEUU-PC)
PEUU-PC was obtained by grafting aminated phosphorycholine into PEUU-COOH through a condensation reaction between carboxyl and amino groups (Figure 1B). Specifically, PEUU-COOH was completely dissolved in agitated DMSO solvent at 70 °C and then cooled to room temperature. An excess amount of PC-NH2 was dissolved in DMSO and then added to the PEUU-COOH/DMSO solution, following addition of an excess amount of DCC. The reaction continued at room temperature overnight. For polymer precipitation, the polymer solution was poured into ethylene ether, and then an excess of deionized water was added to precipitate the polymer. The polymer was rinsed 3× using deionized water and then 100% ethanol 2× to completely remove unreacted PC-NH2. The final product was dried in a vacuum oven at 60 °C for 3 d. The PEUU-PC yield was approximately 75%. PEUU-PC films were obtained using solvent casting in HFIP, as described above.
Fabrication of Paclitaxel-Loaded Films
Polyurethane and paclitaxel (5 wt % to polymer) were dissolved in HFIP to obtain a 5% (w/v) solution, and then the mixture was poured into a Teflon dish. After complete solvent evaporation at room temperature, the paclitaxel-loaded film was dried in a vacuum oven at room temperature for 2 d, and then stored in a freezer at –20 °C for further testing. For all samples, the same amount of polymer and drug was used.
Polymer Characterization
Polymer chemical structure was characterized by 1H nuclear magnetic resonance (1H NMR, 300 MHz, Bruker Biospin Co., Billerica, MA) using DMSO-d6 solvent. Polymer surface composition was analyzed by X-ray photoelectron spectroscopy (XPS) using a Surface Science Instruments S-probe spectrometer with a takeoff angle of 55° (performed at NESAC-BIO, Univ. of Washington). The surface composition of a given sample was averaged from two composition spots and one high resolution C 1s analysis. The mean value for three different samples was determined. The water contact angle of the polymer film surface in air was measured using a sessile drop method on a UCA contact angle instrument (UCA optima, AST Products Inc.; n = 12 per polymer). Thermal properties were measured by differential scanning calorimetry (DSC, DSC-60, Shimazu) at a scanning range of –100 to 200 °C at a heating rate of 20 °C/min with a nitrogen flow.
A 2 × 20 mm strip was cut from the polymer film and its mechanical properties were measured on an MTS Tytron 250 MicroForce Testing Workstation at room temperature with a crosshead speed of 25 mm/min according to ASTM D638-98. Four samples were tested for each polymer.
Polymer degradation behavior was evaluated by weight loss after hydrolytic and enzymatic degradation. For hydrolysis, the weighed polymer film (W0) was immersed in 10 mL of PBS at 37 °C. At each time point, the sample was rinsed with deionized water 3× and dried in a vacuum oven at 60 °C for 3 d, followed by weighing (W1). For enzymatic degradation, the weighed polymer film (W0) was placed in 2 mL of 100 U lipase/PBS solution loaded in a 20 mL glass vial, and then the glass vial was immersed in a water bath at 37 °C. The fresh lipase/PBS solution was replaced twice per week. At each time point, the sample was rinsed 3× using deionized water, dried in a vacuum oven at 37 °C for 3 d, and then weighed (W1). The mass remaining was calculated as W1/W0 × 100%. Three samples were used for each polymer at each time point.
Ovine Blood Contact
Whole ovine blood was collected by jugular venipuncture using an 18 gauge 1 1/2″ needle directly into a syringe containing heparin (3.0 U/mL) after discarding the first 3 mL for blood contacting experiments. NIH guidelines for the care and use of laboratory animals were observed, and all animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. Previous reports from our group using this withdrawal technique from acclimated sheep showed that the platelets obtained were highly responsive to agonist stimulation in vitro.15,16 The polymer surfaces were assessed for surface thrombotic deposition in vitro by employing a simple rocking test17 with heparinized ovine blood (heparin 3.0 U/mL) and incubated for 2 h at 37 °C on a hematology mixer (Fisher Scientific, Pittsburgh, PA). After ovine blood contact, surfaces were gently rinsed with PBS and immersed in a 2.5% glutaraldehyde solution for 2 h at 4 °C to fix the surface adherent platelets, and treated for 1 h in 1% (w/v) OsO4. The samples were serially dehydrated with increasing ethanol solutions and then sputter-coated with gold/palladium. Each sample surface was observed by scanning electron microscopy (SEM; JSM-6330F, JEOL USA, Inc., Peabody, MA). Deposited platelets on each surface were quantified by a lactate dehydrogenase (LDH) assay17 with an LDH Cytotoxicity Detection Kit (Clontech Laboratories, Inc. Mountain View, CA).
Paclitaxel Release Profile Measurement
Samples cut from paclitaxel-loaded films were weighed and then immersed in 10 mL of 10% (v/v) ethanol/PBS solution at 37 °C. Release measurements were performed under sink conditions. At each defined time point, the 10 mL releasate solution was collected and 10 mL of fresh 10% ethanol/PBS solution was added. Four separate samples were used for each polymer type. The paclitaxel in the collected releasate was detected at 230 nm using an ultraviolet spectrometer (Perkin-Elmer UV/vis Lambda 40, U.S.A.). A standard curve was obtained from a series of known concentrations of paclitaxel ethanol/PBS solutions.
Rat Vascular Smooth Muscle Cell Growth Inhibition
A series of 6 mm diameter discs were punched from paclitaxel-loaded films using a standard punch. The samples were sterilized under 30 min of UV irradiation, then directly immersed into a well (24-well cell culture plate), which was preseeded with 5 × 103 rat vascular smooth muscle cells (rSMCs) with 2 mL of cell culture medium (DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution). The cell culture medium was exchanged every 3 d. A control group was comprised of cells cultured in wells without the addition of the paclitaxel-loaded disk. A mitochondrial activity assay (MTT; Sigma) was conducted to measure rSMC metabolic activity. For each group, four samples were used in parallel. A live/dead kit (Invitrogen Inc.) was also employed to stain rSMCs at each time point, and fluorescent images were taken using an Olympus fluorescent microscope to visualize relative cell numbers and to detect dead cells. A control group of PEUU without paclitaxel loading was not included in this work because previous reports have shown the ability of this surface to support cell growth without toxicity.18,19
Statistical Analysis
All results are represented as mean ± standard deviation. The data were analyzed by one-way ANOVA, followed by posthoc Neuman-Keuls testing. P < 0.05 was considered to represent a significant difference. Repeated measures ANOVA was used for polymer degradation and drug release comparisons using IBM SPSS Statistics, version 20.
RESULTS
Polymer Characterization
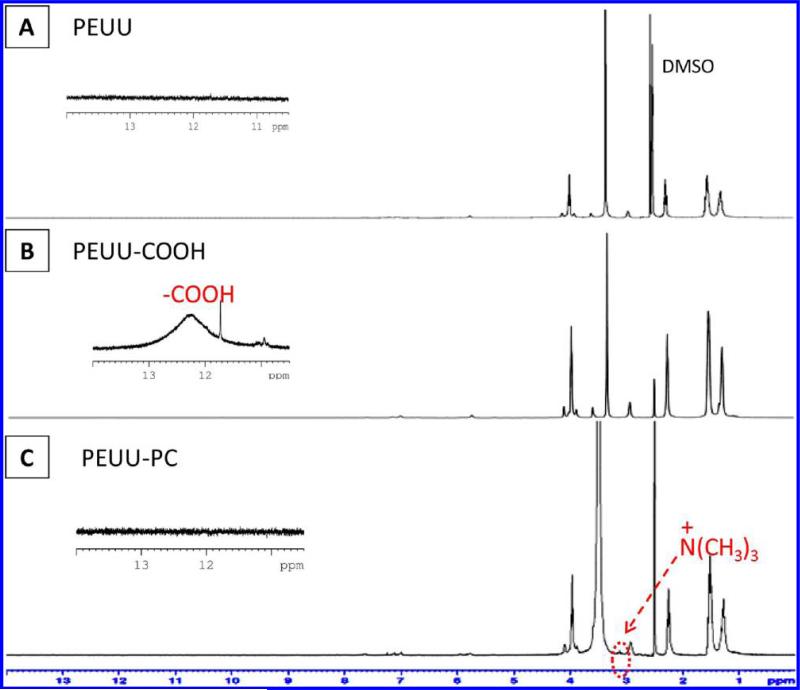
The synthesis of PEUU-PC was confirmed by 1H NMR (Figure 2), and XPS confirmed the surface presence of the PC moieties (Table 1). The introduction of carboxylic groups in PEUU-COOH was verified by a weak and broad peak at δ = 12.24 ppm in Figure 2B, while this peak was not found in either PEUU (Figure 2A) or PEUU-PC (Figure 2C). A specific chemical shift at 3.14 ppm was assigned to –N(CH3)3 of phosphorylcholine in PEUU-PC, while the chemical shift was not observed on the 1H NMR spectra of PEUU and PEUU-COOH (Figure 2). Other specific peaks were assigned to methylene groups. In Table 1, the rise in surface oxygen for the PEUU-COOH surface compared with PEUU was consistent with the presence of a carboxyl group, while the increase in surface nitrogen as well as the detection of phosphorus on the PEUU-PC surface was interpreted to indicate PC grafting onto PEUU-COOH. Polymer surface hydrophilicity as reflected in water contact angle measurements in air (Table 2) showed increased hydrophilicity with PEUU-COOH and further increases for PEUU-PC. The DSC spectra of the polymers showed glass transition temperatures (Tgs) lower than –50 °C (Table 2) and melt temperatures (Tms) attributable to the PCL segments ranging from 34 to 40 °C. There was a decrease in tensile strength for PEUU-COOH and PEUU-PC relative to PEUU (Table 2), while PEUU-PC showed a significantly higher breaking strain than PEUU and PEUU-COOH.
Figure 2.
1H NMR spectra of (A) PEUU, (B) PEUU-COOH, and (C) PEUU-PC. The insets show the specific range for the carboxyl peak from 10 to 13 ppm.
Table 1.
Surface Composition Analysis of Polyurethane Filmsa
| samples | C | O | N | P |
|---|---|---|---|---|
| PEUU | 73.4 ± 3.3 | 21.7 ± 0.7 | 2.0 ± 0.3 | 0.0 ± 0.0 |
| PEUU-COOH | 71.5 ± 4.1 | 21.9 ± 3.8 | 1.6 ± 0.5 | 0.0 ± 0.0 |
| PEUU-PC | 67.1 ± 3.2 | 18.4 ± 4.1 | 4.4 ± 1.4 | 0.2 ± 0.1 |
Atomic percentage determined by X-ray photoelectron spectroscopy (XPS).
Table 2.
Characterization of Polyurethane Filmsa
| samples | tensile strength (MPa) | strain (%) | water contact angle (°) | Tg (°C) | Tm (°C) |
|---|---|---|---|---|---|
| PEUU | 34 ± 3a | 660 ± 85a | 80 ± 2a | –52 | 40 |
| PEUU-COOH | 22 ± 2b | 649 ± 77a | 70 ± 2b | –58 | 34 |
| PEUU-PC | 22 ± 5b | 1250 ± 221b | 53 ± 2c | –51 | 36 |
a, b, and c denote statistically distinct groups for each measured parameter.
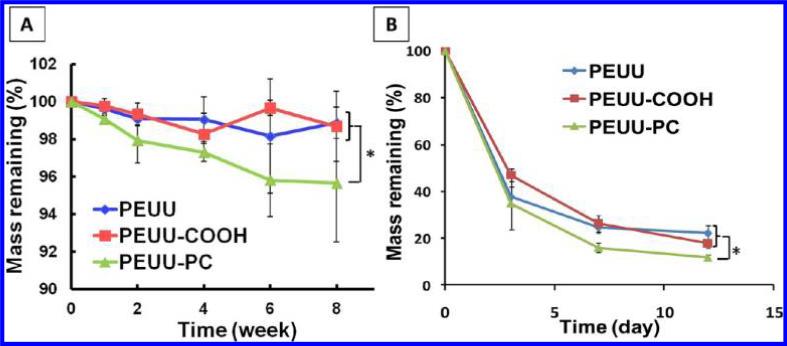
In Vitro Degradation
Polymer degradation was evaluated in both PBS and PBS with lipase at 37 °C (Figure 3). For hydrolytic degradation in PBS (Figure 3A), PEUU and PEUUCOOH showed statistically equivalent behavior without significant mass loss at 8 wks (p > 0.05), while PEUU-PC differed in its behavior from these two polymers and did show mass loss over this period. In lipase solution (Figure 3B), all polymers showed markedly faster degradation than in PBS solution. Within 12 days, PEUU-PC showed more mass loss than both PEUU and PEUU-COOH, which exhibited the same degradation behavior. This result was similar to the trends in degradation behavior observed in PBS.
Figure 3.
Mass remaining for PEUU, PEUU-COOH, and PEUU-PC films in (A) PBS and (B) 100 U/mL lipase in PBS solution at 37 °C.
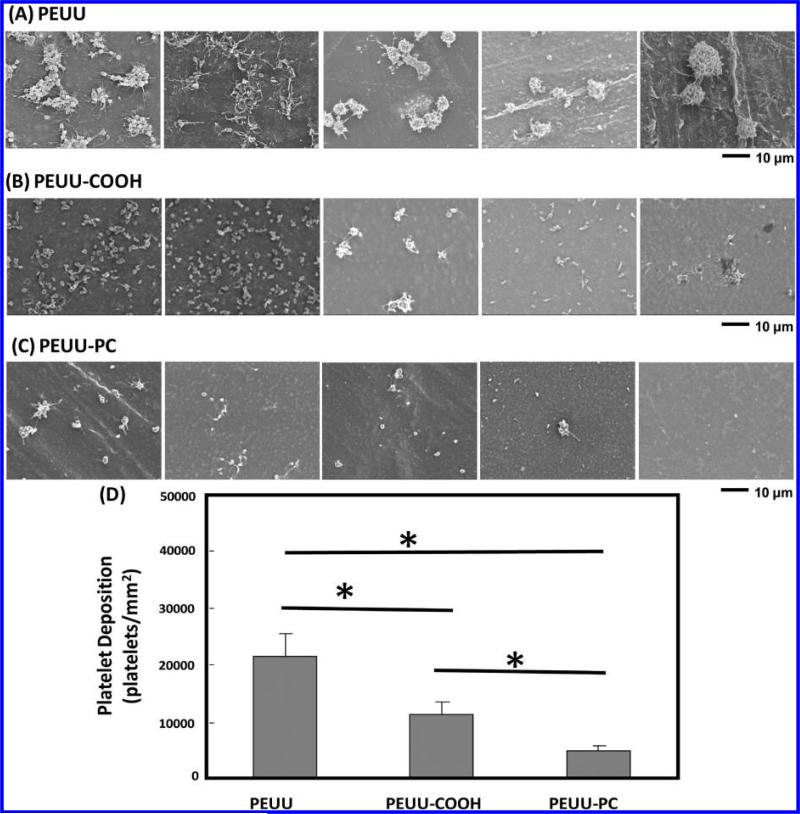
In Vitro Ovine Platelet Deposition
Scanning electron micrographs of polymer film surfaces following ovine blood contact qualitatively demonstrated reduced ovine platelet deposition on polyurethanes with carboxyl groups and further reductions in the presence of PC groups (Figure 4). After 2 h of blood contact, a large number of platelet aggregates were observed on PEUU surfaces with pseudopodia extensions (Figure 4A). On PEUU-COOH surfaces, many individual deposited platelets were visible, with markedly fewer aggregates and pseudopodia extension present, but at a lower level (Figure 4B). For PEUU-PC, only sparse deposition was observed of individual platelets, with some of these platelets displaying pseudopodia extension (Figure 4C). Platelet deposition quantified using the LDH assay (Figure 4D) confirmed the visual results, with PEUU-PC exhibiting significantly lower platelet deposition than PEUU-COOH and PEUU.
Figure 4.
Ovine blood platelet deposition on (A) PEUU, (B) PEUU-COOH, and (C) PEUU-PC was observed under scanning electronic microscopy after 2 h of blood contact at 37 °C. (D) Platelet deposition on polymer films as quantified by LDH assay.
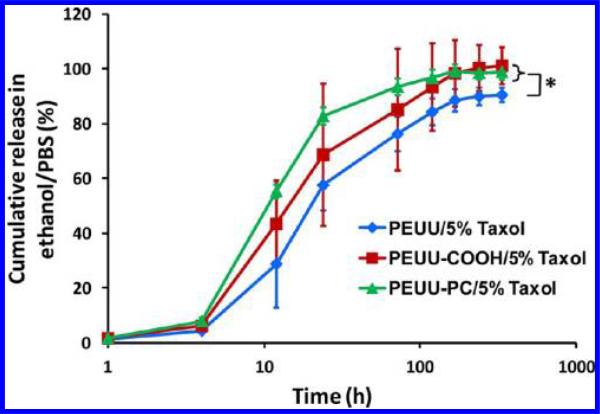
In Vitro Paclitaxel Release
A 60–85% burst release of paclitaxel took place in a 10% ethanol/PBS solution at 37 °C for all polymer films within 24 h (Figure 5). The release profiles then exhibited a slower release for 5 days. For the whole release measurement period, the Taxol release rates of PEUU-COOH and PEUU-PC were significantly higher than in PEUU, while no significant difference was observed between PEUU-COOH and PEUU-PC.
Figure 5.
Paclitaxel release profiles of PEUU, PEUU-COOH, and PEUU-PC films containing 5% paclitaxel were each measured in 10% ethanol/PBS solution at 37 °C.
rSMC Growth Inhibition
The bioactivity of released paclitaxel from the polymer films was evaluated by placing drug-loaded films into cell culture wells preseeded with rSMC. In Figure 6A, without paclitaxel treatment, rSMCs on TCPS showed obvious cellular proliferation and had reached confluence by day 7. However, when any of the three polymers containing paclitaxel were present in the TCPS culture wells, rSMC numbers did not appear to increase under fluorescence microscopy. Live/dead cell staining indicated no apparent cellular death in any of the wells with paclitaxel-releasing polymer. Day 7 micrographs in the presence of paclitaxel were similar to those observed at day 4 (data not shown). Furthermore, metabolic measurements of rSMCs without paclitaxel release showed an increase in this parameter at 4 and 7 days, in significant contrast to those wells with rSMCs with paclitaxel-releasing polymers (Figure 6B) where metabolic index values increased at day 4 but did not increase further at day 7.
Figure 6.
Bioactivity of released paclitaxel was evaluated by (A) rSMC observation following live/dead staining and (B) metabolic index using the MTT assay after placing drug-loaded polyurethane films in rSMC preseeded wells. TCPS without paclitaxel treatment was utilized as a control.
DISCUSSION
Nonthrombogenicity and the prevention of intimal hyperplasia are two critical features desired in polymer-coated, drug-eluting, vascular stents. Elastomeric polyurethanes have served as coating materials for vascular expandable stents due to their high distensibility, processability, and biocompatibility.20–25 Nonthrombogenic moieties, such as PC,26,27 sulfobetain,28–30 sulfate,31,32 and poly(ethylene glycol),33 have been physically and chemically combined with polyurethanes broadly intended for cardiovascular application in an effort to improve their blood compatibility. Among these, PC has been most frequently studied. However, only a few reports have sought to introduce potentially nonthrombogenic moieties directly into the polyurethane structure for blood compatibility improvement. Specifically, glycerophosphorycholine, as a chain extender, was introduced into a nonbiodegradable polyurethane backbone containing polytetramethylene as a soft segment and methylene diphenylene diisocyanate as a hard segment to reduce neutrophil adhesion.34 A biodegradable polyurethane from hexamethylene diisocyanate and a diol of polylactide generated by ring-opening polymerization from glycerophosphorycholine exhibited reduced blood platelet deposition.35 Phosphorylcholine end-capped amphiphilic bio-degradable polyurethanes were synthesized from l-lysine ethyl ester diisocyanate, poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid), 1,4-butanediol, and 4-hydroxy butyl phosphorylcholine for improved biocompatibility and micelle formation for potential drug delivery and bioimaging applications.36 In the current study, a biodegradable poly(ester urethane) urea was introduced by grafting aminated phosphorycholine onto carboxyl groups containing biodegradable poly(ester urethane) urea and the thrombogenicity and potential for antiproliferative drug release was evaluated.
All polymers were in a rubbery state at room temperature due to their low Tgs (< –50 °C), which would be physically compatible with a coating application to expandable vascular stents. The noted decrease of Tg, Tm, and tensile strength of PEUU-COOH might be attributable to a decreased molecule weight compared with PEUU, although this is only speculative because molecular weight was not readily measured.37 The potential interactions between long PC side groups, such as ionic and hydrogen bonding and chain entanglement, might make PEUU-PC show a higher strain with a similar tensile strength when compared with PEUU-COOH.
The hydrophilicity of poly(ester urethane)ureas was markedly increased after carboxyl group introduction and was highest after PC moiety grafting. Compared with carboxyl groups, PC groups exposed on polymer film surfaces would have higher hydrophilicity, to putatively reduce protein adsorption. Also, with this increase in polymer hydrophilicity, these polymers could be expected to undergo accelerated hydrolytic degradation. Degradation studies showed faster degradation for PEUU-PC relative to PEUU and PEUU-COOH over 8 wks in aqueous or enzymatic environments. Previous studies, where poly(ether ester urethane)urea hydro-philicity was increased by raising the ether to ester ratio also showed increased degradation.38
The hydrolytic enzyme lipase has previously been utilized to accelerate the degradation of polymers containing ester, carbonate, urethane, urea, and amide groups.39–41 The use of enzyme-containing buffers, thus, allows the investigation of slower degrading polymers in an accelerated time frame. Our previous reports have confirmed that polyurethane containing polycaprolactone segments degraded much faster in enzyme solution than in PBS solution.42 Furthermore, it is generally possible to control the degradation behavior in both PBS and in the accelerant enzyme solution. For instance, introducing more hydrophilicity into the polymer backbone with PEG segments acts to speed degradation,38 or switching esters to carbonates generally slows degradation.19 For specific enzymatic sensitivity, it is possible to introduce peptide sequences into the backbone that are responsive to a particular enzyme.43
PC group grafting was associated with reduced acute ovine blood platelet deposition in vitro, as was expected based on the literature regarding the nonthrombogenic behavior of these moieties. PC-based polymers have been reported to exhibit a reduced cellular affinity resulting from high free water fraction on the surface due to the zwitterionic nature of MPC, resistance to protein adsorption, limited plasma protein activation, and lateral mobility of molecules.44,45 Besides nonthrombogenicity, PC copolymers have also been associated with reduced inflammatory response46 and negligible cytotoxicity.47 The data in this study showed PC grafting further reduced platelet deposition relative to the carboxylic-enriched surface of PEUU-COOH, although the latter was also associated with reduced thrombogenicity compared to PEUU. The PEUU-COOH effect was consistent with previous reports on potential nonthrombogenic effects associated with carboxylated surfaces.48 As a secondary effect to its inhibition of cell adhesion, the PEUU-PC may be expected to have a negative effect on intimal hyperplasia, even without the controlled release of an antiproliferative agent. In vivo assessment in a rat model of a small diameter blood vessel fabricated from a PEUU and phospholipid polymer blend exhibited thin neo-intimal formation at 8 wks, whereas for a control vessel fabricated from PEUU alone, hyperplastic occlusion was commonly observed.12
All of the polyurethanes studied exhibited similar paclitaxel release profiles with a 60–80% burst release followed by a 5 day slow release in ethanol/PBS solution, although PEUU-COOH and PEUU-PC showed significantly greater release over the study period, presumably due to their higher hydrophilicity. Because paclitaxel is a highly hydrophobic small molecule, ethanol was mixed into the PBS collection fluid to improve paclitaxel solubility and accelerate its release. Thus, actual paclitaxel release times in an aqueous physiological system may be much longer than 5 days. Furthermore, the burst release observed was attributed to the low Tgs (< –50 °C) of the polyurethanes, which resulted from the PCL of the soft segment. The paclitaxel release kinetics for the polyurethanes were similar to that for neat PCL.49,50 Finally, rSMC inhibition results confirmed paclitaxel maintained its bioactivity after polymer loading and solvent contact, suggesting feasibility for future application in solvent-based device coating. In future studies, alternative antiproliferative drugs, such as everolimus, zotarolimus, and sirolimus, which have also been used in commercialized drug eluting stents, would be candidate antiproliferatives to evaluate. It is notable that rSMC size at day 7 with paclitaxel treatment appeared to be bigger than that at day 1. This effect is attributed to rSMC spreading at later time points, particularly with paclitaxel treatment providing relatively more available surface area due to inhibited proliferation.
The biodegradable PEUU-COOH, which was an intermediate product and control material in this study, has the potential to be applied to a variety of biomedical material applications. The active carboxyl groups in the polyurethane could be conjugated with bioactive molecules containing amino or hydroxyl groups, including peptides, growth factors, drugs, and even fluorescent agents. Physical bonding with such molecules might also be achieved. Also synthesized in this study was a functionalized phosphorycholine (PC) molecule containing a 1:1 PC/amino group ratio. The synthesis utilized a photo-initiator (benzophenone), although photoinitiation might not be necessary for this reaction.14 With photoinitiator use, one could also prepare amino-functional macromolecules (or polymers), which have repeating PC moieties, and the chain length of PC macromers could be controlled by manipulating the initial monomer feed ratio via a thiol-ene radical photopolymerization reaction.51
Some limitations in this report are worth mentioning. First, the amount of PC in PEUU-PC was limited by the number of free carboxyl groups in the PEUU-COOH. This limit follows from the side reaction between the carboxyl group and diisocyanate in dimethylolpropionic acid (DMPA) although diisocyanate is primarily reacted with the two hydroxyl groups in DMPA. When DMPA reached 50 molar % in the soft segments, it was not possible to synthesize high molecular polyurethanes (data not shown). Second, the cytocompatibility of the new polyurethanes was not directly assessed although there is extensive experience with PEUU in a variety of in vivo settings and a variety of PC containing polymers have lacked apparent toxicity in previous in vitro and in vivo studies.52–54 Experiments evaluating the inhibition of SMC proliferation showed a lack of cytotoxicity from solutions contacted with the polymers. Third, this degradable nonthrombogenic polyurethane/paclitaxel system would ideally be evaluated as a coating on a vascular device such as a stent in future in vivo studies and could be compared with other candidate nonthrombogenic, drug-eluting coatings. A chronic animal model for stent placement would allow a more relevant assessment of the thrombogenicity of the stent coating and the antiproliferative releasate activity.
CONCLUSIONS
A biodegradable, nonthrombogenic, elastomeric polyurethane, PEUU-PC, was successfully synthesized in this study by forming a polyurethane with repeating carboxylate groups along its backbone, and condensing aminated PC groups at these points. Acute platelet deposition on PEUU-PC was markedly reduced, while the controlled release of the antiproliferative drug paclitaxel occurred from a PEUU-PC matrix for 5 d after a burst release in an ethanol/PBS solution. The results indicate that this polyurethane could potentially serve as a nonthrombogenic, drug-eluting coating on metallic vascular stents and grafts.
ACKNOWLEDGMENTS
We acknowledge the financial support from the Engineering Research Center: Revolutionizing Metallic Biomaterials, Grant No. 0812348, National Science Foundation. We appreciate the expertise provided by Dr. Lara Gamble at the University of Washington in performing the surface analysis and Prof. Kazuhiko Ishihara at the University of Tokyo for providing MPC monomer.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Abizaid A, Costa JR., Jr. Circ.: Cardiovasc. Interventions. 2010;3:384–393. doi: 10.1161/CIRCINTERVENTIONS.109.891192. [DOI] [PubMed] [Google Scholar]
- 2.Carter AJ, Brodeur A, Collingwood R, Ross S, Gibson L, Wang CA, Haller S, Coleman L, Virmani R. Catheter. Cardiovasc. Interventions. 2006;68(1):97–103. doi: 10.1002/ccd.20769. [DOI] [PubMed] [Google Scholar]
- 3.Bege N, Steinmüller SO, Kalinowski M, Reul R, Klaus S, Petersen H, Curdy C, Janek J, Kissel T. Eur. J. Pharm. Biopharm. 2011;80(3):562–570. doi: 10.1016/j.ejpb.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez-Chico JL, Jüni P, García-García HM, Regar E, Nüesch E, Borgia F, van der Giessen WJ, Davies S, van Geuns RJ, Secco GG, Meis S, Windecker S, Serruys PW, di Mario C. Am. Heart J. 2011;162(5):922–931. doi: 10.1016/j.ahj.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Jabara R, Chronos N, Conway D, Molema W, Robinson K. JACC Cardiovasc. Interventions. 2008;1(1):81–87. doi: 10.1016/j.jcin.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Granillo A, Rubilar B, Rodriguez-Granillo G, Rodriguez AE. World J. Cardiol. 2011;3(3):84–92. doi: 10.4330/wjc.v3.i3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayward JA, Chapman D. Biomaterials. 1984;5:135–142. doi: 10.1016/0142-9612(84)90047-4. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara K, Aragaki R, Ueda T, Watanabe A, Nakabayashi N. J. Biomed. Mater. Res. 1990;24:1069–1077. doi: 10.1002/jbm.820240809. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki Y, Mikami A, Kurita K, Yui N, Ishihara K, Nakabayashi N. J. Biomed. Mater. Res. 1997;36:508–515. doi: 10.1002/(sici)1097-4636(19970915)36:4<508::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Nakaya T, Li YJ. Prog. Polym. Sci. 1999;24:143–181. [Google Scholar]
- 11.Yoneyama T, Sugihara K, Ishihara K, Iwasaki Y, Nakabayashi N. Biomaterials. 2002;23:1455–1459. doi: 10.1016/s0142-9612(01)00268-x. [DOI] [PubMed] [Google Scholar]
- 12.Hong Y, Ye SH, Nieponice A, Soletti L, Vorp DA, Wagner WR. Biomaterials. 2009;30:2457–2467. doi: 10.1016/j.biomaterials.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soletti L, Nieponice A, Hong Y, Ye SH, Stankus JJ, Wagner WR, Vorp DA. J. Biomed. Mater. Res., Part A. 2011;96(2):436–448. doi: 10.1002/jbm.a.32997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyle CE. Angew. Chem., Int. Ed. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CA, Jr., Wearden PD, Kocyildirim E, Maul TM, Woolley JR, Ye SH, Strickler EM, Borovetz HS, Wagner WR. Artif. Organs. 2011;35(6):602–613. doi: 10.1111/j.1525-1594.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson CA, Jr., Shankarraman V, Wearden PD, Kocyildirim E, Maul TM, Marks JD, Richardson JS, Gellman BN, Borovetz HS, Dasse KA, Wagner WR. ASAIO J. 2011;57(6):516–521. doi: 10.1097/MAT.0b013e31822e2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye SH, Johnson CA, Jr., Woolley JR, Snyder TA, Gamble LJ, Wagner WR. J. Biomed. Mater. Res., Part A. 2009;91(1):18–28. doi: 10.1002/jbm.a.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan J, Sacks MS, Beckman EJ, Wagner WR. J. Biomed. Mater. Res. 2002;61(3):493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 19.Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR. Biomaterials. 2010;31(15):4249–4258. doi: 10.1016/j.biomaterials.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trigwell S, De S, Sharma R, Mazumder MK, Mehta JL. J. Biomed. Mater. Res., Part B. 2006;76(2):241–250. doi: 10.1002/jbm.b.30359. [DOI] [PubMed] [Google Scholar]
- 21.Kondyurin AV, Maitz MF, Romanova VA, Begishev VP, Kondyurina IV, Guenzel R. J. Biomater. Sci., Polym. Ed. 2004;15(2):145–159. doi: 10.1163/156856204322793548. [DOI] [PubMed] [Google Scholar]
- 22.Holmes DR, Camrud AR, Jorgenson MA, Edwards WD, Schwartz RS. J. Am. Coll. Cardiol. 1994;24(2):525–531. doi: 10.1016/0735-1097(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 23.Mazumder MM, De S, Trigwell S, Ali N, Mazumder MK, Mehta JL. J. Biomater. Sci., Polym. Ed. 2003;14(12):1351–1362. doi: 10.1163/156856203322599699. [DOI] [PubMed] [Google Scholar]
- 24.Lambert TL, Dev V, Rechavia E, Forrester JS, Litvack F, Eigler NL. Circulation. 1994;90(2):1003–1011. doi: 10.1161/01.cir.90.2.1003. [DOI] [PubMed] [Google Scholar]
- 25.Horita N, Tomita H, Takamuro M, Fuse S, Tsutsumi H. Catheter. Cardiovasc. Interventions. 2006;68(5):727–734. doi: 10.1002/ccd.20788. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Tan D, Li J, Tan H, Fu Q. Biofouling. 2011;27(8):919–930. doi: 10.1080/08927014.2011.615926. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K, Ohuchi K, Hoshi H, Morimoto N, Iwasaki Y, Takatani S. J. Artif. Organs. 2005;8(4):237–244. doi: 10.1007/s10047-005-0308-x. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Lin S, Shen J. Colloids Surf., B. 2008;66(1):90–95. doi: 10.1016/j.colsurfb.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Yuan J, Chen L, Jiang X, Shen J, Lin S. Colloids Surf., B. 2004;39(1−2):87–94. doi: 10.1016/j.colsurfb.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Chen S, Yu Q, Zhang Z, Bernards M, Jiang S. Biomacromolecules. 2007;8(1):122–127. doi: 10.1021/bm060739m. [DOI] [PubMed] [Google Scholar]
- 31.Francolini I, Crisante F, Martinelli A, D'Ilario L, Piozzi A. Acta Biomater. 2012;8(2):549–558. doi: 10.1016/j.actbio.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Silver JH, Hart AP, Williams EC, Cooper SL, Charef S, Labarre D, Jozefowicz M. Biomaterials. 1992;13(6):339–344. doi: 10.1016/0142-9612(92)90037-o. [DOI] [PubMed] [Google Scholar]
- 33.Orban JM, Chapman TM, Wagner WR, Jankowski RJ. Polym. Sci., Part A: Polym. Chem. 1999;37:3441–3448. [Google Scholar]
- 34.Yung LY, Cooper SL. Biomaterials. 1998;19:31–40. doi: 10.1016/s0142-9612(97)00220-2. [DOI] [PubMed] [Google Scholar]
- 35.Cao J, Chen NC, Chen YW, Luo XL. Int. J. Mol. Sci. 2010;11:1870–1877. doi: 10.3390/ijms11041870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ZG, Wan PJ, Ding MM, Yi X, Li JH, Fu Q, Tan H. J. Polym. Sci., Part A: Polym. Chem. 2011;49:2033–2042. [Google Scholar]
- 37.Melinte V, Buruiana T, Mihai A, Buruiana EC. High Perform. Polym. 2011;23(3):238–247. [Google Scholar]
- 38.Guan J, Sacks MS, Beckman EJ, Wagner WR. Biomaterials. 2004;25:85–96. doi: 10.1016/s0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 39.Ashton JH, Mertz JA, Harper JL, Slepian MJ, Mills JL, McGrath DV, Vande Geest JP. Acta Biomater. 2011;7(1):287–294. doi: 10.1016/j.actbio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Z, Hong Y, Nelson DM, Pichamuthu JE, Leeson CE, Wagner WR. Biomacromolecules. 2011;12(9):3265–3274. doi: 10.1021/bm2007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Kuijer R, Bulstra SK, Grijpma DW, Feijen J. Biomaterials. 2006;27(9):1741–1748. doi: 10.1016/j.biomaterials.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Nelson DM, Baraniak PR, Ma Z, Guan J, Mason NS, Wagner WR. Pharm. Res. 2011;28(6):1282–1293. doi: 10.1007/s11095-011-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan J, Wagner WR. Biomacromolecules. 2005;6(5):2833–2842. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakabayashi N, Williams DF. Biomaterials. 2003;24:2431–2435. doi: 10.1016/s0142-9612(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 45.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. J. Biomed. Mater. Res. 1998;39:323–330. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 46.Iwasaki Y, Sawada S, Ishihara K, Khang G, Lee HB. Biomaterials. 2002;23:3897–3903. doi: 10.1016/s0142-9612(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 47.Salvage JP, Rose SF, Phillips GJ, Hanlon GW, Lloyd AW, Ma IY, Armes SP, Billingham NC, Lewis AL. J. Controlled Release. 2005;104:259–270. doi: 10.1016/j.jconrel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Poussard L, Burel F, Couvercelle JP, Merhi Y, Tabrizian M, Bunel C. Biomaterials. 2004;25(17):3473–3483. doi: 10.1016/j.biomaterials.2003.10.069. [DOI] [PubMed] [Google Scholar]
- 49.Lao LL, Venkatraman SS, Peppas NA. Eur. J. Pharm. Biopharm. 2008;70(3):796–803. doi: 10.1016/j.ejpb.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 50.Lao LL, Venkatraman SS, Peppas NA. J. Biomed. Mater. Res., Part A. 2009;90(4):1054–1065. doi: 10.1002/jbm.a.32171. [DOI] [PubMed] [Google Scholar]
- 51.Hoyle CE. J. Polym. Sci. 2004;42:5301–5338. [Google Scholar]
- 52.Watanabe J, Ishihara K. Biomacromolecules. 2005;6(3):1797–1802. doi: 10.1021/bm050138f. [DOI] [PubMed] [Google Scholar]
- 53.Ye SH, Watanabe J, Takai M, Iwasaki Y, Ishihara K. Biomaterials. 2006;27(9):1955–1962. doi: 10.1016/j.biomaterials.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Kimura M, Takai M, Ishihara K. J. Biomater. Sci., Polym. Ed. 2007;18(5):623–640. doi: 10.1163/156856207780852541. [DOI] [PubMed] [Google Scholar]