Abstract
Introduction
The survival for patients with locally advanced, unresectable non-small cell lung cancer receiving standard of care concomitant chemoradiation remains disappointingly low. A reduction in both local and distant recurrence is needed to improve patients’ outcome. Performing molecular studies on serially collected tumor specimens may result in a better selection of therapeutic options.
Methods
We conducted a phase II single-institution trial of two cycles of induction chemotherapy with gemcitabine and carboplatin followed by high-dose conformal radiation concomitant with weekly paclitaxel and carboplatin in 39 patients. The trial required a dedicated tumor biopsy before treatment initiation. In addition, tumor biopsies were requested, if safely feasible, before initiation of chemoradiation and 2 months after completion all therapy.
Results
Induction chemotherapy was well tolerated, and 38 patients proceeded with chemoradiation. The mean delivered radiation dose was 70.2 Gy, 23 patients received the full dose of 74 Gy, and 19 patients completed all treatment on schedule without dose reductions or delays. Median overall and progression-free survivals were 22.7 and 14.3 months, respectively. A total of 82 procedures, including 46 transthoracic core needle biopsies, were performed. Thirteen patients had all three serial tumor biopsies. Three of these procedures resulted in complications that required an intervention; all for the treatment of a biopsy-induced pneumothorax.
Conclusions
We conclude that induction gemcitabine/carboplatin followed by concurrent paclitaxel/carboplatin with conformal radiation to 74 Gy is safe and tolerable with promising efficacy. We demonstrated that dedicated and serial tumor collections are safe, feasible, and acceptable for patients with non-small cell lung cancer.
Keywords: Non-small-cell lung cancer, Conformal radiation, Gemcitabine, Carboplatin, Paclitaxel, ERCC1, RRM1
The achievement of long-term disease control through reduction of local recurrence and metastatic spread remains the key goal in the treatment of locally advanced, inoperable non-small cell lung cancer (NSCLC), which accounts for approximately 25% of cases (http://seer.cancer.gov/data/).1 The addition of systemic therapy to radiotherapy was a major step toward this goal, and it has become the standard of care (http://www.nccn.org). Clinical research has addressed the sequencing of both modalities, and concurrent therapy is superior to a sequential approach.2–5 To further improve outcomes, induction chemotherapy to reduce systemic disease burden followed by concurrent therapy for improved local control has been suggested as a promising approach in a retrospective analysis.6 Unfortunately, a prospective randomized phase III trial, designed to formally address this question, was closed early due to poor accrual. Nevertheless, results suggested an improvement in overall survival (OS), although not statistically significant, for patients receiving induction chemotherapy followed by chemoradiation, as opposed to chemoradiation alone.6
The advent of modern radiotherapeutic techniques to safely escalate radiation doses is one approach to reduce local recurrence rates. An initial phase I/II trial conducted by investigators at the University of North Carolina demonstrated that it was safe to increase the radiation dose from 60 to 74 Gy, in conjunction with induction and concurrent paclitaxel/carboplatin therapy.7–9
In this study, we report our result from a prospective phase II trial of induction gemcitabine/carboplatin followed by concurrent radiotherapy to 74 Gy with weekly paclitaxel/carboplatin. The choice of agents and sequencing was based on available data at the time of trial design. Chemoradiation followed by consolidation docetaxel was viewed by many as the “gold standard” with a median OS of 26 months.10 Nevertheless, the administration of consolidation chemotherapy was difficult (78% of patients started consolidation treatment and 75% completed this treatment). Induction chemotherapy had been considered as a reasonable alternate approach, with gemcitabine and platinum providing the longest median OS (18.3 months) compared with paclitaxel/platinum or vinorelbine/platinum.11 In addition to reporting safety, efficacy, and feasibility of this chosen regimen, we also report our experience with dedicated serial tumor biopsies, which were required for molecular investigations before treatment initiation and after induction therapy and 2 months after completion of all therapy if feasible. The predictive utilities of mRNA expression values for the genes ERCC1 and RRM1 have been previously reported.12
PATIENTS AND METHODS
Eligibility Criteria
The study was approved by the University of South Florida’s Institutional Review Board (ClinicalTrials.gov no. NCT00226590). Eligible patients had to have histologically confirmed adenocarcinoma, squamous cell carcinoma, or large cell carcinoma stage IIIA or “dry” IIIB according to version 6 AJCC staging criteria. Histological confirmation of N2 nodal involvement was required in patients with mediastinal lymph node enlargement of less than 2 cm on computed tomography (CT) scans of the chest and upper abdomen or suspicious on whole body [18F]-fluorodeoxyglucose positron emission tomography (PET), and it was not mandatory for patients with obvious nodal involvement (2.0 cm or greater). CT with intravenous contrast, PET, magnetic resonance imaging or CT of the brain with intravenous contrast, Eastern Cooperative Oncology Group performance status of 0 or 1, and weight loss of ≤5% in the preceding 3 months were required. No prior systemic chemotherapy or thoracic radiotherapy was permitted. History of a prior or concomitant malignancy in the past 5 years was an exclusion criterion, except for surgically cured basal cell carcinoma of the skin or carcinoma in situ of the cervix. Additionally, no concomitant life threatening or uncontrolled serious medical illness such as cardiac arrhythmia, end stage congestive heart failure, liver disease with significant hepatic insufficiency, or organic brain syndrome were permitted. Laboratory testing had to demonstrate adequate marrow reserve, and hepatic and renal function. The informed consent document explained the therapeutic interventions and the required tumor biopsies (see later).
Therapeutic Protocol
Induction chemotherapy consisted of two cycles of gemcitabine (1000 mg/m2) on days 1 and 8 and carboplatin (area under the curve, 5) on day 1 given at 4-week intervals. Doses were modified if hematologic and hepatic toxicities or sensory neuropathy were encountered. Prophylactic colony-stimulating factors were not allowed. They were permitted in the setting of severe febrile neutropenia or life-threatening infection. Erythropoietin was allowed to maintain hemoglobin levels of 11 g/dl or greater. Carboplatin (area under the curve, 2) and paclitaxel (50 mg/m2) were administered weekly during radiotherapy, after review of weekly blood tests. Chemotherapy was discontinued if hematologic or hepatic toxicity was excessive.
Radiation therapy was to begin in week 9 (day 57 ± 14 days), and two target volumes (TVs) were used. The first (TV1) included the mediastinum from the thoracic inlet to the subcarinal space, ipsilateral hilum, and bilateral mediastinal (N2) nodes. The supraclavicular fossae were not to be treated routinely, but treatment was permissible when high paratracheal nodes were known to be involved by tumor or when the location of the primary tumor in the upper lobe made their avoidance difficult. Contralateral hilar coverage was not necessary unless gross adenopathy (>1 cm) was present. This volume included the primary tumor and all lymph nodes of size 1.0 cm or greater on CT scan, with a margin of 1.5 to 2.0 cm. Volumes were derived from a treatment planning CT scan obtained before initiation of chemotherapy, unless there was progression of intrathoracic disease during induction chemotherapy, in which case the larger TV was to be used. Patients were simulated pre and postchemotherapy, and the union of these planning CTs was used to determine the planned tumor volume. TV2 included only the known tumor volume as defined on CT (primary tumor and lymph nodes >1.0 cm) and any nodal sites proven to be involved by invasive staging, with a margin of 1.0 to 1.5 cm. Fifty Gy were to be delivered to TV1, with a cone down to TV2 for an additional 24 Gy in 1.8 to 2 Gy fractions. Nominal tumor dose was stipulated to be 74 Gy (±5%). All fields were to be treated every week-day for a total treatment duration of 8 weeks. Tissue heterogeneity corrections and posterior cord blocks were not permitted. The protocol-recommended maximal lung volume to receive 2000 cGy of radiation was 37%; however, a precise definition of “lung” was not provided. The maximum permissible doses were 50 Gy to the spinal cord, 40 Gy to the heart (50% of the heart was permitted to receive 50 Gy), and 64 Gy to the esophagus.
Response, Survival, and Toxicity Assessment
Patients were assessed for response after induction chemotherapy (during week 8 from treatment initiation) and again after chemoradiation (during weeks 25–26 and 8–9 weeks after completion of chemoradiation) using both CT and PET scans. Radiographic response was expressed as a continuous variable by calculating the percentage of change in the sum of all greatest tumor diameters comparing the posttreatment with pretreatment scans ([1 − sum postlesions/sum prelesions] × 100; i.e., a reduction in tumor diameters after therapy has a positive value and an increase a negative value) and also by RECIST as best confirmed response.13 OS was defined as the interval between the date of diagnosis and the date of death. Progression-free survival (PFS) was defined as the interval between the date of first therapy and the date of progression or death. Local PFS was defined as the interval between the date of first therapy and the date of local (in the field of radiation) progression or death. Patients without an event were censored as of the date of last follow-up. Kaplan-Meier estimates were generated to describe the OS, PFS, and local PFS. Toxicity was monitored and documented according to the common terminology criteria for adverse events (CTCAE, version 3.0).
Dedicated Tumor Biopsies for Molecular Studies
The trial mandated a tumor biopsy of an index lesion before treatment initiation. A second and third tumor biopsy were requested if they could be performed safely before chemoradiation (week 8) and 8 weeks after completion of chemoradiation (weeks 25–26). For specimens collected by transthoracic biopsy, a 19-gauge guiding needle was placed under CT guidance. A 20-gauge core biopsy gun device with a 1 to 2 cm throw was then passed coaxially through the guiding needle for up to six passes. Collected samples were immediately frozen in liquid nitrogen and processed as described previously.12
RESULTS
Patient Characteristics
Between November 2003 and March 2006, 43 patients with stage IIIA/IIIB NSCLC were accrued. Four patients withdrew consent before treatment initiation. Two did so after the protocol-mandated tumor biopsy (both desired treatment closer to home), one did not want to delay treatment initiation, and one was uncomfortable with radiotherapy. The clinical characteristics of the 39 patients who initiated the protocol-specified therapy are listed in Table 1.
TABLE 1.
Characteristics of Patients Who Initiated Therapy
| Age (yr) | |
| Minimum | 47 |
| Maximum | 87 |
| Mean | 64.2 |
| Median | 62.4 |
| Race/ethnicity | |
| White (1 Hispanic) | 37 |
| Black | 1 |
| Asian | 1 |
| Sex | |
| Male | 20 |
| Female | 19 |
| Performance status | |
| 0 | 16 |
| 1 | 23 |
| Clinical stage | |
| IIB | 1a |
| IIIA | 20 |
| IIIB | 18 |
| Histopathologyb | |
| Adenocarcinoma | 12 |
| Squamous cell | 12 |
| Large cell | 3 |
| NSCLC (NOS) | 12 |
| Smoking status | |
| Never | 3 |
| Current | 14 |
| Former | 22 |
| Smoking pack-years | |
| Minimum | 0 |
| Maximum | 102 |
| Mean | 42.7 |
| Median | 38.0 |
This patient (T3N0M0 NSCLC) was enrolled with an institutional review board-approved exception.
In four patients, the diagnosis was established using the protocol-required biopsy (2 = NSCLC; 1 = squamous cell carcinoma; and 1 = large cell carcinoma).
NSCLC, non-small cell lung cancer; NOS, not otherwise specified.
Chemotherapy Dosing and Toxicity
Induction chemotherapy was completed on schedule without dose reduction in 28 (72%, 28/39) patients (Table 2). Seven patients had 1-week dose delays, and four had dose reductions or omissions for severe cytopenia or other toxicity. One patient developed severe heart failure and did not receive further therapy. Thirty-eight patients began concurrent chemotherapy and radiation. Nineteen received all weekly doses, and 19 had dose modifications (Table 2).
TABLE 2.
Delivery and Toxicity of Induction and Concurrent Chemotherapy
| Induction Chemotherapy
| |||||
|---|---|---|---|---|---|
| Dose Reduction (%)
|
Cycle Delay (%)
|
||||
| Cycle 1 | Cycle 2 | Cycle 2 | Cycle 1 | Cycle 2 | Cycle 2 |
| D 8 | D 1 | D 8 | D 8 | D 1 | D 8 |
| 0 (0%) | 3 (7.7%) | 0 (0%) | 1 (2.6%) | 4 (10.2%) | 3 (7.7%) |
| Reasons for Dose Reduction | Reasons for Cycle Delay |
|---|---|
| Thrombocytopenia (n = 1) | Neutropenia (n = 5) |
| Neutropenic fever (n = 1) | Thrombocytopenia (n = 1) |
| Severe Reynaud’s (n = 1)a | Viral syndrome (n = 1) |
| Elevated liver function tests (n = 1) |
| Concurrent chemotherapy
| |
|---|---|
| Discontinuation (n = 7)b | Cycles Skipped (n = 12)c |
| Allergic reaction (n = 3) | Neutropenia (n = 4) |
| Ongoing thrombocytopenia (n = 1) | Thrombocytopenia (n = 5) |
| Severe esophagitis (n = 1)d | Shortness of breath (n = 2) |
| Cardiac failure (n = 1)e | Fever (n = 2) |
| Neutropenia (n = 1) | Dehydration (n = 1) |
| Colon perforation (n = 1)e | Gastroenteritis (n = 1) |
Second induction cycle not delivered.
One patient had more than one reason to discontinue chemotherapy.
Several patients had more than one reason for not receiving chemotherapy.
Chemotherapy discontinued.
Patient taken off study.
Radiation Dosing and Toxicity
Twenty-three patients (61%, 23/38) received the prescribed 7400 cGy. The mean dose was 7024 cGy delivered over 7.5 weeks (Table 3). The main reason (N = 10) for dose reduction was the protocol-recommended V20 dosage. The median V20 was 34% (±7%), and it ranged from 20 to 44%. Dose-volume histograms were used for V20 calculations, and lung was defined as total lung minus gross tumor volume. There were two exceptions: in one patient, lung was defined as total lung minus planned tumor volume, and in the other, lung was defined as total lung including the tumor volume (the V20 in this patient was 44%).
TABLE 3.
Delivery and Toxicity of Radiotherapy
| Minimum | Maximum | Mean | Standard Deviation | |
|---|---|---|---|---|
| Dose (cGy) | 3400 | 7400 | 7024 | 695 |
| Elapsed days | 23 | 67 | 53 | 8 |
| Discontinuation (n = 2) | Dose Reduction (n = 13) |
|---|---|
| Esophagitis after 3400 cGy (n = 1) | Unacceptable V20a (n = 10) |
| Bowel perforation (n = 1, unrelated) | Dosimetric concerns (n = 1) |
| Unknown (n = 1) | |
| Patient refusal (n = 1) | |
| Volume of lung receiving 20 Gy ≥37%a |
Lung volume receiving more than 2000 cGy.
Toxicities requiring treatment discontinuation were encountered in two patients. One patient (70-year-old woman) received only 3400 cGy because of severe esophagitis; she died 2 months later from bowel ischemia. The patient had prior arterial stenting for atherosclerotic disease. The second patient (61-year-old man) succumbed to a bowel perforation requiring right hemicolectomy after 3600 cGy. This event was judged to be unrelated to the patient’s malignancy or therapy received. Treatment was interrupted for 1 week in one patient (65-year-old man) because of dehydration, and the total dose given was 7200 cGy.
Chemoradiation-specific toxicities (grade ≥3) encountered after treatment discontinuation included five patients with pneumonitis, which resolved with steroid therapy in four and led to oxygen dependence in one. Two additional patients developed an ipsilateral hydropneumothorax 14 months after completion of therapy without evidence of disease recurrence, and both died of respiratory failure 6 to 8 weeks after the event. One patient developed an esophageal stricture requiring dilatation 3 months after completion of therapy.
Survival and Recurrence Patterns
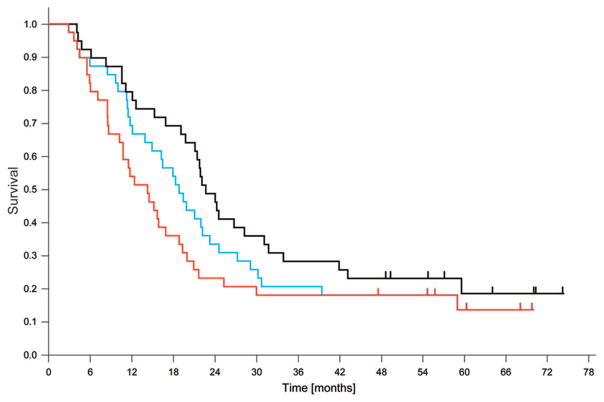
As of the index date for survival analysis, December 2009, six patients were alive without evidence for disease progression, two patients were alive with disease progression, and 31 patients had died. The cause of death was disease progression in 19 patients. Nine patients died of causes unrelated to lung cancer or its treatment, and three patients died of pulmonary causes or cachexia without clear evidence for tumor progression or treatment-related toxicity. The median OS was 22.7 months (95% confidence interval [CI]: 19.7–25.8 months), the median PFS was 14.3 months (95% CI: 9.8–18.8 months), and the median local PFS was 19.4 months (95% CI: 15.0–23.7 months; Figure 1). The 2-year and 5-year OS rates were 49% and 19%, and the PFS rates were 23% and 14%, respectively. Of the 23 patients with disease progression, the sites of first recurrence were outside of the field of radiation in 18 patients; and they were in-field recurrences in seven patients (two had simultaneous in- and out-of-field recurrences; Figure 2). The sites of first out-of-field recurrence were brain in five, multiple lung nodules in four, liver in two, and diverse other or simultaneous multiple sites in seven patients. One additional patient had an in-field recurrence subsequent to a cerebral recurrence, and one patient succumbed to a second lung cancer after a cerebellar recurrence of the first lung cancer.
FIGURE 1.
The median overall survival (OS) for all 39 patients was 22.7 months (black curve), the median progression-free survival (PFS) was 14.3 months (red curve), and the median local PFS was 19.4 months (blue curve). At 2 years, the OS rate was 49%, the PFS rate was 23%, and local PFS rate was 33%. Tick marks indicate censoring events.
FIGURE 2.
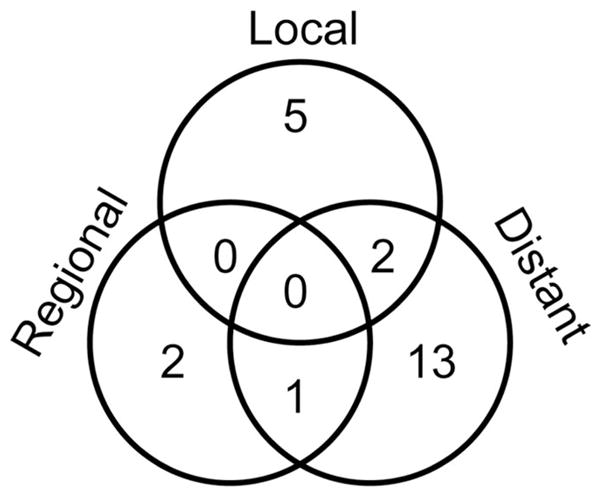
Venn diagram of patterns of first recurrence. The predominant sites of distant recurrences were brain, lung, and liver. Ten patients are deceased, and six are alive without evidence for recurrence.
Experience with Dedicated Tumor Specimen Collection
Dedicated tumor sampling before treatment initiation was a study entry criterion. The trial also requested a second and third tumor biopsy if it could be performed safely after induction chemotherapy and 2 months after completion of chemoradiation. Thus, the first tumor sampling was completed in all 39 patients. In addition, two of the four eligible patients who withdrew consent before treatment initiation had tumor sampling. The procedures used included endoscopy in 15, mediastinoscopy in five (done as part of a standard-of-care lymph nodal evaluation), and transthoracic core needle biopsies (TTNBx) in 21 patients. There were a total of three complications, all a result of TTNBx; two patients had small pneumothoraces that did not require intervention; one patient required chest tube placement, and he recovered completely (the TTNBx in this patient also served to establish the diagnosis).
The second tumor sampling was completed by endoscopy in 11 and by TTNBx in 16 patients. One patient had a small pneumothorax after TTNBx that did not require intervention. The reasons for not collecting tumor in the remaining 12 patients were a reduction in tumor size that precluded safe sampling in nine patients and the use of anticoagulation therapy, severe heart failure, and refusal in one patient, respectively.
At the time of the third tumor sampling, 18 of 38 patients who had started chemoradiation could not be biopsied because of a substantial size reduction, two were deceased, one refused, and three had biopsies withheld due to concurrent medical issues. Samples were obtained by bronchoscopy in three, by TTNBx in nine, and by ultrasound-guided needle biopsy of sites of progression in two patients (liver and supraclavicular lymph node). Two patients required chest tube placement after TTNBx, and both had a complete recovery.
Three serial biopsies for tumor sampling were done in 13 patients and two in 15 patients (no. 1 and no. 2 in 14, no. 1 and no. 3 in one patient). Thirteen patients had only the first biopsy (including the two patients who withdrew consent). Thus, a total of 82 procedures were done resulting in three complications that required an intervention, i.e., chest tube placement for treatment of a pneumothorax as a result of a CT-guided TTNBx.
DISCUSSION
Although bimodality concurrent chemoradiation has been established as superior to a sequential approach, the addition of chemotherapy before or after concurrent treatment remains controversial. Based on a multiinstitutional phase II trial conducted by the Southwest Oncology Group, there was considerable enthusiasm for continued chemotherapy after completion of chemoradiation.10 In addition, a multiinstitutional phase II trial randomized patients to induction carboplatin/paclitaxel (CbP) followed by 63 Gy of radiotherapy alone or radiation and concurrent CbP. In a third arm, patients received concurrent CbP and radiation followed by consolidation CbP. Survival was longest (16.3 months median OS) in the latter arm; however, these patients also had the greatest toxicity.14 A recent randomized phase III trial conducted by the Hoosier Oncology Group and US Oncology did not show a survival benefit if consolidation chemotherapy was given after chemoradiation.15
A single-institution retrospective analysis compared 265 successive patients who were treated with induction chemotherapy followed by chemoradiation or chemoradiation alone.6 Patients who received induction chemotherapy had better OS (median, 22.8 versus 16.8 months; 5-year rate, 25% versus 12%; p < 0.001) and distant metastasis-free survival (5-year rate, 42% versus 23%; p = 0.021). Locoregional control was not significantly different between the two groups.
In 2007, Vokes et al.16 published results of a randomized phase III trial of concurrent 66 Gy radiation and CbP with or without two cycles of induction CbP. The median OS was 14 months with and 12 months without induction therapy, which was not statistically different. The authors concluded that the addition of induction chemotherapy to concurrent chemoradiation added toxicity and provided no statistically significant survival benefit over concurrent chemoradiation alone. Nevertheless, the median survival in both groups was low, and the authors recommended that concomitant weekly CbP should be reexamined.
A European randomized phase III trial used an alternate chemotherapy regimen.17 It consisted of cisplatin (60 mg/m2), gemcitabine (1000 mg/m2, days 1 and 8), and vinorelbine (25 mg/m2, days 1 and 8) given as induction or consolidation therapy for two cycles; and concomitant chemotherapy was cisplatin (60 mg/m2) and vinorelbine (15 mg/m2) given on days 1 and 22 and gemcitabine (200 mg/m2) given on days 8 and 29 together with 66 Gy of radiation. The trial accrued poorly and was terminated early with 49 patients enrolled. Median OS was 23.9 months in the induction arm compared with 17.0 months in the consolidation arm.
The escalation of radiation doses is being pursued as an alternate approach to improving patients’ outcome. In a phase I/II radiation, dose-escalation trial of induction CbP followed by concurrent radiation and CbP, 74 Gy of radiotherapy was established as the maximal tolerated dose.7–9 The investigators enrolled a total of 62 patients and reported a median OS of 24 months. In a subsequent trial with a modified induction regimen but the identical concurrent chemotherapy, these investigators escaladed the radiation dose to 90 Gy, which resulted in two instances of bronchial stenosis and two fatal hemoptysis.18
Using the established safe dose of 74 Gy, Socinski et al.19 conducted a randomized phase II trial of induction chemotherapy followed by concurrent chemoradiation with two different chemotherapy regimens. One group received CbP in 3-weekly cycles during the induction phase with weekly dosing during radiation. The other group received carboplatin/gemcitabine induction followed by biweekly single-agent gemcitabine (35 mg/m2) during radiation. Because of an unacceptable rate of grade 4/5 pulmonary toxicity (4/26 patients), the gemcitabine-containing arm was closed early. Analysis of the 3D-conformal treatment plans showed that the V20 (volume of lung receiving 20 Gy) exceeded 40% in two of three plans (the V20 was unavailable in the fourth patient). The median OS was 24.3 months for patients receiving the paclitaxel-containing regimen.
The results of our study with a median OS of 22.7 months in 39 patients compare favorably with these previously published results. As our trial had a nonrandomized single-institution design, a potential bias toward beneficial outcome may exist; although our eligibility criteria are consistent with those used in most trials for patients with locally advanced NSCLC. The chemotherapy selected for our trial combined a gemcitabine-based induction with a taxane-based concomitant regimen and high-dose conformal radiation to 74 Gy. Our data suggest that this approach is safe, feasible, and comparable in toxicity to the above reference non-gemcitabine regimens. Nevertheless, disease progression within and outside the radiation fields remains a problem. Radiotherapy has advanced substantially since our trial was designed, including the use of 4D treatment planning, treating only PET-positive disease, daily image guidance with planned tumor volume reduction, and intensity-modulated radiation therapy. These technologies offer the potential to simultaneously increase the dose to tumor while reducing normal tissue toxicity. In addition, better selection of systemic therapy based on molecular characteristics of the tumor may lead to a reduction of distant recurrences. We demonstrated in our trial that dedicated tumor collection before and during therapy is safe, feasible, and acceptable for patients with NSCLC. Future trials that seek to incorporate molecular investigations can use our rates of serial tumor collections as a guide for selecting adequate patient volumes for correlative studies.
Acknowledgments
Supported by the National Cancer Institute grant no. R01-CA102726.
Footnotes
Disclosure: The author declare no conflicts of interest.
References
- 1.Cancer Facts & Figures 2009. Am Cancer Soc. 2009 [Google Scholar]
- 2.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 3.Zatloukal PV, Petruzelka L, Zemanova M, et al. Concurrent versus sequential radiotherapy with vinorelbine plus cisplatin (V-P) in locally advanced non-small-cell lung cancer. A randomized phase II study. Proc Am Soc Clin Oncol. 2002;21:290a. [Google Scholar]
- 4.Curran WJ, Scott CB, Langer CJ. Long-term benefit is observed in a phase III comparison of sequential versus concurrent chemoradiation for patients with unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol. 2003;22:621a. [Google Scholar]
- 5.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 6.Huang EH, Liao Z, Cox JD, et al. Comparison of outcomes for patients with unresectable, locally advanced non-small-cell lung cancer treated with induction chemotherapy followed by concurrent chemoradiation versus concurrent chemotherapy alone. Int J Radiat Oncol Biol Phys. 2007;68:779–785. doi: 10.1016/j.ijrobp.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Socinski MA, Rosenman JG, Schell MJ, et al. Induction carboplatin/paclitaxel followed by concurrent carboplatin/paclitaxel and dose-escalating conformal thoracic radiation therapy in unresectable stage IIIA/B non-small cell lung carcinoma. Cancer. 2000;89:534–542. [PubMed] [Google Scholar]
- 8.Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B non-small cell lung carcinoma. Cancer. 2001;92:1213–1223. doi: 10.1002/1097-0142(20010901)92:5<1213::aid-cncr1440>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Rosenman JG, Halle JS, Socinski MA, et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2002;54:348–356. doi: 10.1016/s0360-3016(02)02958-9. [DOI] [PubMed] [Google Scholar]
- 10.Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non–small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–2010. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 11.Vokes EE, Herndon JE, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: Cancer and Leukemia Group B study 9431. J Clin Oncol. 2002;20:4191–4198. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Bepler G, Kusmartseva I, Sharma S, et al. RRM1-modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small cell lung cancer. J Clin Oncol. 2006;24:4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 15.Hanna N, Neubauer M, Yiannoutsos C, et al. Hoosier Oncology Group US Oncology Group phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 16.Vokes EE, Herndon JE, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 17.Berghmans T, Van Houtte P, Paesmans M, et al. A phase III randomised study comparing concomitant radiochemotherapy as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer. 2009;64:187–193. doi: 10.1016/j.lungcan.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004;22:4341–4350. doi: 10.1200/JCO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]