Abstract
Breast cancer patients often develop bone metastasis evidenced by osteolytic lesions, leading to severe pain and bone fracture. Attenuation of breast cancer metastasis to bone and associated osteolysis by fish oil (FO), rich in EPA and DHA, has been demonstrated previously. However, it was not known whether EPA and DHA differentially or similarly affect breast cancer bone metastasis and associated osteolysis. In vitro culture of parental and luciferase gene encoded MDA-MB-231 human breast cancer cell lines treated with EPA and DHA revealed that DHA inhibits proliferation and invasion of breast cancer cells more potently than EPA. Intra-cardiac injection of parental and luciferase gene encoded MDA-MB-231 cells to athymic NCr nu/nu mice demonstrated that DHA treated mice had significantly less breast cancer cell burden in bone, and also significantly less osteolytic lesions than EPA treated mice. In vivo cell migration assay as measured by luciferase intensity revealed that DHA attenuated cell migration specifically to the bone. Moreover, the DHA treated group showed reduced levels of CD44 and TRAP positive area in bone compared to EPA treated group. Breast cancer cell burden and osteolytic lesions were also examined in intra-tibially breast cancer cell injected mice and found less breast cancer cell growth and associated osteolysis in DHA treated mice as compared to EPA treated mice. Finally, doxorubicin resistant MCF-7 (MCF-7dox) human breast cancer cell line was used to examine if DHA can improve sensitization of MCF-7dox cells to doxorubicin. DHA improved the inhibitory effect of doxorubicin on proliferation and invasion of MCF-7dox cells. Interestingly, drug resistance gene P-gp was also down-regulated in DHA plus doxorubicin treated cells. In conclusion, DHA attenuates breast cancer bone metastasis and associated osteolysis more potently than EPA, possibly by inhibiting migration of breast cancer cell to the bone as well as by inhibiting osteoclastic bone resorption.
Keywords: Breast cancer, bone metastasis, omega-3 fatty acids, docosahexaenoic acid, osteolysis
Introduction
Metastatic bone disease is a major cause of morbidity in breast cancer patients. An estimated 192,370 women in USA were diagnosed with, and 40,170 women died of breast cancer in 2009. Among these, many are likely to develop bone metastasis and pain leading to poor quality of life. The Western diet is rich in ω-6 fatty acids (FA), and saturated fats which are the principal confounding factors of breast cancer. Diets rich in ω-6 FA tend to favor development of obesity and cancer [1–3] and a significant association was established between breast cancer risk and ω-6 FA (i.e. linoleic acid, arachidonic acid) concentrations in breast adipose tissue [4]. On the contrary, ω-3 FA are polyunsaturated essential FA with anti-inflammatory properties which counteract the pro-inflammatory ω-6 FA. Fish oil (FO) diet rich in ω-3 FA have beneficial effects in many diseases including various cancers. Decreased consumption of FO during past decade in Japanese women correlates with high incidence of breast cancer [5]. Two main constituents of FO, eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) suppress breast cancer cell growth in vitro and in animal models [6]. A recent study showed that EPA and DHA shifted the pro-survival and proliferative effect of estrogen to a pro-apoptotic effect in human breast cancer cells [7]. Others have shown that EPA and DHA inhibit proliferation of MCF-7 breast cancer cells [8]. A clinical study showed that EPA and DHA intake were inversely associated with breast cancer risk in postmenopausal women [9]. Clinical and experimental works suggest that ω-3 FA are potentially protective against promotion and progression stages of breast cancers by enhancing apoptosis, and are also found to be very effective during chemotherapy [10]. Though other organs such as lungs, liver and brain are involved, bone remains the prevalent site for breast cancer metastasis [11,12]. Bone metastasis of breast cancer cells is often facilitated due to the permissive nature of the fenestrated bone marrow endothelial lining, called sinusoids [13]. Colonized breast cancer cells in the bone microenvironment cause maturation and activation of osteoclasts to form osteolytic lesions leading to severe pain and bone fracture.
Evidence over the past 20 years has shown that EPA and DHA are beneficial for bone health [14–29]. Further, recent evidence suggests the DHA may have more potent bioactivity in bone than EPA [15,18,30,31]. DHA had more potent anti-inflammatory effects relative to EPA, with marked attenuation of NF-κB activation and TNF-α secretion in macrophages [32–34]. DHA specifically enhanced anti-inflammatory IL-10 secretion and reduced the expression of pro-inflammatory M1 (F4/80+/CD11+) macrophages [32]. In addition, DHA is more potent inhibitor of bone resorbing osteoclast formation than EPA [18,35]. DHA supplementation in rat showed that DHA accumulated in the osteoblast-rich and nerve-abundant periosteum of femur and appears to be a vital constituent of marrow and periosteum of healthy modeling bone [36,37]. A few studies have also suggested preventive effect of DHA against ovariectomy-induced bone loss in rat [15,23,38]. Use of ω-3 FA specifically DHA against cancer is gaining attention. DHA has been shown to reduce tumor incidence by 30% and led to increased BRCA-1 protein expression in rats [39]. DHA also up-regulated syndecan-1 (SDC-1) in human breast cancer cells and induce apoptosis by activating PPARγ [40]. Breast cancer cell proliferation was inhibited by DHA through proteasome-dependent degradation of estrogen receptor-alpha, reduced cyclin-D1 expression as well as inhibited MAPK signaling [41]. DHA have potent anti-angiogenic effects inhibiting production of many important angiogenic mediators such as VEGF, PDGF, PDECGF, COX-2, PGE2, nitric oxide, NFκB, matrix metalloproteinases and β-catenin [42]. DHA is also known to act as a chemo-preventive agent by inducing apoptosis through different mechanisms, such as the up regulation of MAP-kinase-phosphatase-1 (MKP-1) and down regulation of ERK1/2 and p38 MAPKs [43] and externalization of phosphatidylserine and membrane disruption [44]. DHA has been found to act synergistically with chemotherapeutic drugs by modulating the tumor cell response to them. FO diet, rich in EPA and DHA, has been shown to affect primary breast tumor growth and metastasis to bone [45]. However, the role of individual ω-3 FA such as EPA and DHA on breast cancer bone metastasis and associated osteolysis has not been investigated yet. In the present study, we examined the effect of EPA-rich-FO and DHA-rich-FO diets on breast cancer metastasis to bone and associated osteolysis. Finally, we also determined if DHA can improve the sensitivity to drug to doxorubicin resistant MCF-7 breast cancer cells.
Materials and Methods
Materials
The MDA-MB-231 and doxorubicin registrant MCF-7 cell lines were purchased from American Type Culture Collection (Rockville, MD) and maintained at 37°C in Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and penicillin and streptomycin. The ω–3 FA, DHA and EPA and corn oil (CO) were purchased from Cayman Chemical Company (Ann Arbor, MI). EPA rich FO (EPA-FO) and DHA rich FO (DHA-FO) were obtained from Ocean Canada Ltd (Dartmouth, Nova Scotia). pGL3-control vector (Luciferase reporter plasmid containing firefly luciferase gene) was purchased from Promega (Madison, WI). Antibodies against CD44, P-gp and β-actin were purchased from Santa Cruz (Dallas, TX). TRAP staining reagent was purchased from Sigma (St. Louis, MO)
Generation of MDA-MB-231-Luc cell line
MDA-MB-231-Luc was established by the stable transfection of firefly luciferase gene (PGL3-control vector) using Lipofectamine Plus Reagent (Life Technologies Inc., Grand Island, NY). pcDNA3 vector (Invitrogen Co, Carlsbad, CA) was co-transfected for the selection by G418 (Sigma, St. Louis, MO). Luciferase quantification was performed using the Luciferase Assay System (Promega, Madison, WI).
Cell Proliferation Assay
MDA-MB-231 and MDA-231Luc cells were plated at a density of 2 × 104 cells/100 μl per well in a 96 well plate. Cells were allowed to adhere overnight and replaced with fresh media and FA. After 48 hours of incubation, 20 μl MTS reagent was added to each well, and incubated for 4 hours at 37°C. Absorbance was read at 490nm wavelength.
Invasion Assay
2 × 104 cells in 200 μl serum free DMEM was added to upper chamber and 700 μl of DMEM supplemented with 10% FCS and FA were added to the lower chamber of a 24-well BioCoat Matrigel invasion assay plate (BD Biosciences, Bedford MA). After 48 hours of incubation, cells which had migrated through the pores to the lower side of the membrane were fixed with 10% formalin and stained with 0.1% crystal violet blue and counted.
Animals, experimental diets and feeding
The 3 week old athymic NCr-nu/nu female mice were obtained from NIH animal facilities and used according to the approved protocol by the Animal Care and Use Committee (IACUC). CO, EPA-FO and DHA-FO diets were prepared in our laboratory as previously described [17]. The composition of food is provided in Table 1. The mice were fed a diet containing experimental diets for 4 weeks prior to the intra-cardiac/intra-tibial injection of breast cancer cells.
Table 1.
Composition of AIN-93 semi-purified diets containing corn oil (CO) and EPA-FO and DHA-FO.
| a Diet ingrediants | CO | EPA-FO | DHA-FO |
|---|---|---|---|
| Casein | 14.00 | 14.00 | 14.00 |
| Corn starch | 47.43 | 47.43 | 42.73 |
| Dextronized corn starch | 14.50 | 14.50 | 14.50 |
| Sucrose | 9.00 | 9.00 | 9.00 |
| Cellulose | 5.00 | 5.00 | 5.00 |
| AIN-93 mineral mix | 3.50 | 3.50 | 3.50 |
| AIN-93 vitamin mix | 1.00 | 1.00 | 1.00 |
| L-cysteine | 0.18 | 0.18 | 0.18 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 |
| TBHQ | 0.10 | 0.10 | 0.10 |
| Vitamin E | 0.04 | 0.04 | 0.04 |
| Corn oil | 5.00 | 1.00 | 1.00 |
| b EPA-FO | 0.00 | 4.00 | 0.00 |
| b DHA-FO | 0.00 | 0.00 | 4.00 |
All diet ingredients (expressed as percent total diet) were purchased from MP Biomedicals (Irvine, CA)
EPA-FO (EPA rich fish oil containing 55% EPA and 5% DHA) and DHA-FO (DHA rich fish oil containing 5% EPA and 60% DHA) was supplied by Ocean Nutrion, Nova Scotia, Canada). EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid
Quantitative assessment of tumor burden in visceral organs
After 4 weeks post injection of MDA-MB-231 Luciferase tumor cells, animals were sacrificed and luciferase activity was quantified in different organs as described previously [46].
Generation of osteolytic and visceral metastasis in nude mice animal model-intra-cardiac method
After 4 weeks of dietary treatment, mice were injected with 1 × 105 cells in 100 μl of PBS intra-cardially as described previously [46,47]. The mice were maintained in their respective diets for 4 weeks post injection and performed x-ray as described previously [46,47]. The radiolucent osteolytic areas of bone metastasis were quantified using a computer-assisted MetaMorph analysis program.
Histological analysis of bone lesions
In parallel to radiographic analysis, radiographically affected and unaffected forelimb and hind limb long bones were excised, fixed in 10% formalin in PBS (pH 7.2) for 2 days, then decalcified. Subsequently, they were embedded in paraffin and stained with hematoxylin and eosin (H&E) as previously described [47].
Tartrate resistant acid phosphatase (TRAP) and CD44 staining of bones
TRAP staining of bone sections were performed as described previously [48]. Osteoclasts were visualized by using a Tartrate-resistant-acid-phosphatase (TRAP) kit (Sigma #387A-1KT). CD44 positive cells were visualized using monoclonal antibody against CD44 (Santa Cruz) following the manufacturer’s instructions.
Generation of osteolytic tumors in nude mice animal model-intra-tibial method
After 4 weeks of dietary treatment, an IP injection of Buprenorphine HCL (essentially equivalent to that of morphine on a milligram basis) at 2 mg/kg body weight was given for operative pain. Then, under general anesthesia, the patella skin was washed with a betadyne solution, and dried. A superficial skin incision was made in the skin overlying the patella using scissors. A 30-gauge needle was inserted at the level of the intercondylar notch and into the medullary canal to create an initial core pathway, followed by insertion of a 29-gauge needle to make the final pathway into the bone. Then, 10 μl PBS containing 1 × 106 cells was very slowly injected using a 25 μl Hamilton syringe with a 29-gauge needle to prevent leakage of cells outside the bone. Skin wound was closed using silk threads (4-0, Ethicon). After surgery, neomycin/polymyxin B/bacitracin zinc ophthalmic ointment (Bausch & Lomb Pharmaceuticals) was applied to the wound. The mice were maintained in their respective diets for 4 weeks post injection. X-ray and tissue histology was performed as described above.
Proliferation, invasion and P-gp expression in MCF-7dox cells
Proliferation and invasion assays are done as described above and P-gp expression was analyzed by western blot as described previously [14].
Statistical analysis
Data are presented as mean values ± S.E.M. Differences among the groups were tested by one-way analysis of variance and student’s t-test. Newman-Keuls post hoc test were used followed by ANOVA if multiple correlations were made. A p value < 0.05 was considered statistically significant. The analyses were performed with Graphpad prism (La Jolla, CA).
Results
Effect of EPA and DHA on proliferation and invasion of breast cancer cell
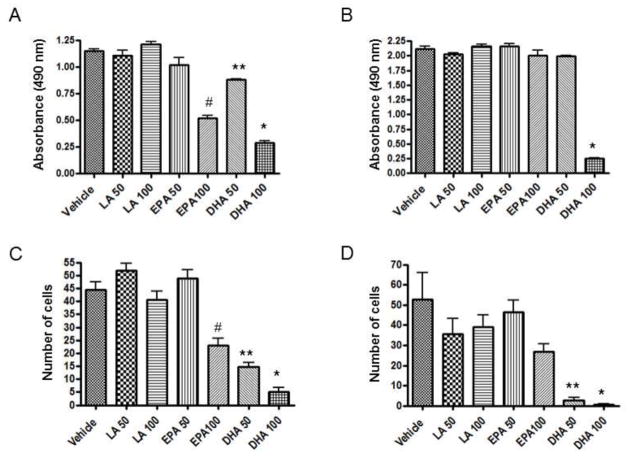
To examine the effect of EPA and DHA on the breast cancer cell load, we performed MTS cell proliferation assay of MDA-MB-231 and MDA-MB-231-Luc cell lines. Our data demonstrated that DHA more potently inhibited the breast cancer cell proliferation than EPA (Fig. 1A&B). The metastasis of the tumor cells requires the local intravasation, which involves passing through the extracellular matrix. Therefore, to examine the effect of EPA and DHA, we performed an invasion assay using a collagen-coated membrane in the culture wells. DHA inhibited the migration of the breast cancer cells through the collagen membrane more potently than EPA (Fig. 1C&D). These results indicate that DHA inhibits both proliferation and invasion of breast cancer cells more potently than EPA.
Fig. 1. Effect of EPA and DHA on proliferation and invasion of breast cancer cell.
For cell proliferation assay MDA-MB-231 (A) and MDA-231Luc (B) cells were plated at a density of 2 × 104 cells/100 μl per well in a 96 well plate. After 24 hours of incubation, cells were replenished with fresh media with various concentrations of fatty acids. Cells were then incubated for an additional 48 hours. At the end of incubation, 20 μl MTS reagent was added to each well, and incubated for 4 hours at 37C. Absorbance was read at 490nm. For invasion assay, 2 × 104 MDA-MB-231 (C) and MDA-231Luc (D) cells in 200 μl serum free DMEM was added to upper chamber and 700 μl of DMEM supplemented with 10% fetal calf serum (FCS) and FA were added to the lower chamber in a 24-well BioCoat Matrigel invasion coated chamber inserts with 8-μm pore size membranes. After 48 hours of incubation, the remaining upper chamber cells were removed and cells which had migrated through the pores to the lower side of the membrane were fixed with 10% formalin and stained with 0.1% crystal violet blue and counted manually. Each value represents the mean ± SEM of two independent triplicate cultures. Number denotes μM concentration of fatty acids. p value <0.05 was considered significant by student’s t test. * p<0.05 vs. EPA 100; # p<0.05 vs. LA 100; ** p<0.05 vs. EPA 50.
Effect of EPA and DHA on breast cancer metastasis to bone after intra-cardiac injection of breast cancer cells
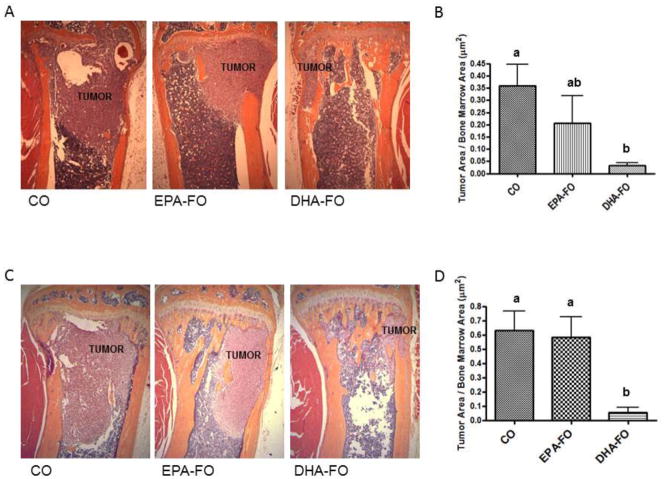
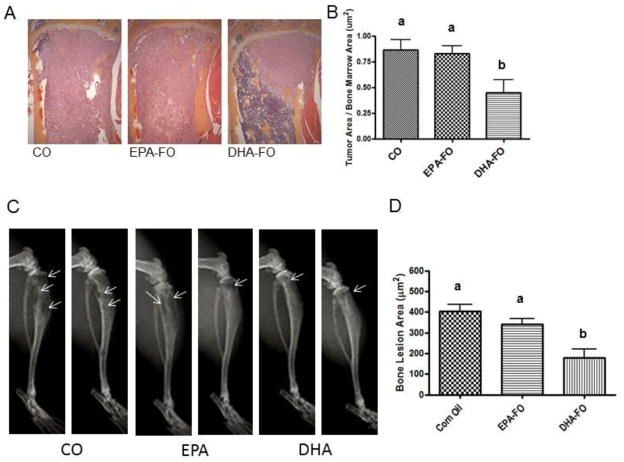
To determine the effect of EPA and DHA on the levels of breast cancer cell metastasis to bone, we performed H&E staining of bone section collected form mice injected with breast cancer cells intra-cardially. Bone samples from DHA-FO treated mice showed the lowest load of breast cancer cell as compared to CO and EPA-FO treated mice (Fig. 2).
Fig. 2. Effect of EPA and DHA on breast cancer metastasis to bone after intra-cardiac injection of breast cancer cells.
The athymic NCr-nu/nu female mice were fed a diet containing CO or EPA-FO or DHA-FO for 4 weeks prior to the intra-cardiac injection of the MDA-MB-231 (A) or MDA-MB-231-Luc (C) cells. The mice were then injected with 1 × 105 cells in 100 μl of PBS intra-cardially. The mice were maintained in their respective diets for 4 weeks post injection. After x-ray, mice were sacrificed and bones were collected and fixed in formalin. After decalcification, paraffin embedded bone sections were prepared and stained for H&E to determine the breast tumor burden in bones. Histomorphometry of tumor burden area was done for MDA-MB-231 (B) or MDA-MB-231-Luc (D). n=5 mice per group. Each value represents the mean ± SEM. Value with different superscripts are significantly different at P<0.05 by Newman Keuls one way ANOVA with multiple comparison test.
Effect of EPA and DHA on osteolysis due to breast cancer metastasis to bone after intra-cardiac injection of breast cancer cells
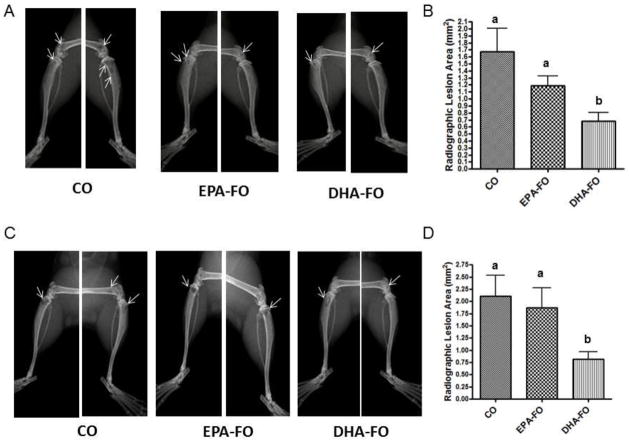
To determine the effect of EPA and DHA on breast cancer bone metastasis associated osteolysis, we measured the osteolytic lesions by x-ray. DHA-FO treated mice showed the lowest number of osteolytic lesions as compared to CO and EPA-FO treated mice (Fig. 3).
Fig. 3. Effect of EPA and DHA on osteolysis due to breast cancer metastasis to bone after intra-cardiac injection of breast cancer cells.
The athymic NCr-nu/nu female mice were fed a diet containing CO or EPA-FO or DHA-FO for 4 weeks prior to the intra-cardiac injection of the MDA-MB-231 (A) or MDA-MB-231-Luc (C) cells. The mice were then injected with 1 × 105 cells in 100 μl of PBS intra-cardially. The mice were maintained in their respective diets for 4 weeks post injection. Deeply anesthetized animals were exposed to X-ray using a Faxitron radiographic inspection unit. Osteolytic lesions are shown in x-ray (A and C). The radiolucent osteolytic areas of bone metastasis were marked and quantified for MDA-MB-231 (B) or MDA-MB-231-Luc (D) injected mice using a computer-assisted MetaMorph analysis program. n=5 mice per group. Each value represents the mean ± SEM. Value with different superscripts are significantly different at P<0.05 by Newman Keuls one way ANOVA with multiple comparison test.
Effect of EPA and DHA on breast cancer cell load in different organs after intra-cardiac injection of breast cancer cells
To determine the effect of EPA and DHA on the metastasis of breast cancer cell to the different organs, we determined the luciferase activity of different organs from the mice injected with MDA-MB-231-Luc inra-cardially. Bone samples from DHA treated mice showed the lowest level of luciferase expression as compared to CO and EPA-FO treated mice (Fig. 4). However, breast cancer metastasis to the other organs was not significantly different among the groups. These results indicate that DHA inhibits breast cancer metastasis specifically to bone more potently than EPA.
Fig. 4. Effect of EPA and DHA on breast cancer cell load in different organs after intra-cardiac injection of breast cancer cells.
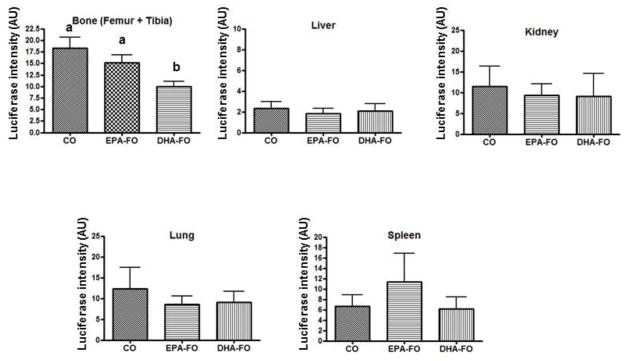
The athymic NCr-nu/nu female mice were fed a diet containing CO or EPA-FO or DHA-FO for 4 weeks prior to the intra-cardiac injection of MDA-MB-231-Luc cells. The mice were then injected with 1 × 105 cells in 100 μl of PBS intra-cardially. The mice were maintained in their respective diets for 4 weeks post injection. After x-ray, mice were sacrificed and whole fresh organs were dissected and immersed in 750 μl cold reporter lysis buffer. Tissues were homogenized on ice, and centrifuged for 15 minutes at 15,000 rpm at 4°C. Supernatant was collected and protein concentration was assessed. Supernatants were then analyzed for luciferase activity using a Turner Designs Luminometer TD-20/20. Results were expressed in luciferase activity/mg of organ protein. n=5 mice per group. Each value represents the mean ± SEM. Value with different superscripts are significantly different at P<0.05 by Newman Keuls one way ANOVA with multiple comparison test.
Effect of EPA and DHA on the expression of CD44 and TRAP in bone after intra-cardiac injection of breast cancer cells
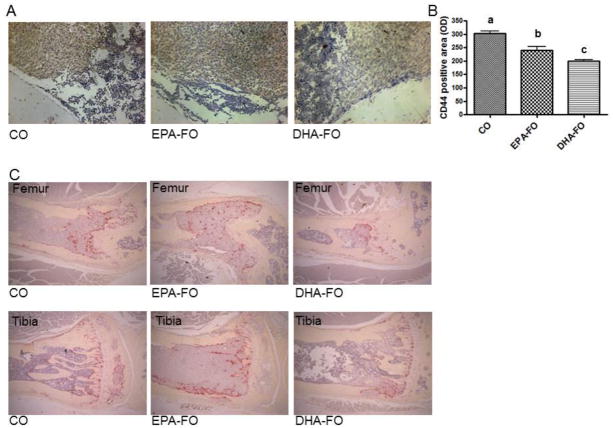
We examined the effect of EPA and DHA on the expression of CD44 on the bone sections by immunostaining. Both EPA-FO and DHA-FO significantly reduced the CD44 protein expression in bone as compared to CO groups (Fig. 5A). However, DHA-FO reduced the CD44 expression in bone more potently than EPA-FO (Fig. 5A&B). To determine the effect of EPA and DHA on the osteoclasts levels on the breast cancer metastasized bone, we performed TRAP staining of bone sections. Interestingly, a reduction of number of TRAP positive osteoclasts is observed in DHA-FO treated mice as compared to CO and EPA-FO treated mice (Fig. 5C). However, there was not much difference between CO and EPA-FO treated groups (Fig. 5C).
Fig. 5. Effect of EPA and DHA on the expression of CD44 and TRAP in bone after intra-cardiac injection of breast cancer cells.
The athymic NCr-nu/nu female mice were fed a diet containing CO or EPA-FO or DHA-FO for 4 weeks prior to the intra-cardiac injection of MDA-MB-231-Luc cells. The mice were then injected with 1 × 105 cells in 100 μl of PBS intra-cardially. The mice were maintained in their respective diets for 4 weeks post injection. After x-ray, mice were sacrificed and bones were collected and fixed. After decalcification bone sections were stained for CD44 (A) and TRAP (C). Histomorphometry of CD44 positive area is shown in bar diagrams (B). n=5 mice per group. Each value represents the mean ± SEM. Value with different superscripts are significantly different at P<0.05 by Newman Keuls one way ANOVA with multiple comparison test.
Effect of EPA and DHA on breast cancer cell load and associated osteolysis after intra-tibial injection of breast cancer cell
To determine if direct injection of breast cancer cells into the bone can induce osteolysis and whether EPA and DHA have any effect on the proliferation and breast cancer-associated osteolysis, we injected breast cancer cells intra-tibially. DHA-FO group significantly inhibited the proliferation of breast cancer cells in bone as compared to EPA-FO and CO treatment groups (Fig. 6A&B). However, there was no difference between CO and EPA treated mice. Similarly, DHA-FO group significantly inhibited osteolysis of breast cancer injected bone as compared to CO and EPA-FO treated groups (Fig. 6C&D). EPA treated mice showed slightly less osteolysis as compared to CO group, however, not significant.
Fig. 6. Effect of EPA and DHA on breast cancer cell load and associated osteolysis after intra-tibial injection of breast cancer cell.
The athymic NCr-nu/nu mice were fed a diet containing CO or EPA-FO or DHA-FO for 4 weeks prior to the intra-tibial injection of the MDA-MB-231 cells. 10 μl PBS containing 1 × 106 cells was injected intra-tibially. The mice were maintained in their respective diets for 4 weeks post injection. After x-ray, mice were sacrificed and bones were collected and fixed in formalin. (B) Osteolytic lesions are shown in x-ray. After decalcification, the bone sections were stained for H&E to determine the tumor burden in bone. Histomorphometry of tumor burden (B) and osteolytic lesions area (D) is shown in bar diagrams. n=5 mice per group. Each value represents the mean ± SEM. Value with different superscripts are significantly different at P<0.05 by Newman Keuls one way ANOVA with multiple comparison test.
Effect of DHA on proliferation, invasion and P-gp expression on doxorubicin resistant MCF-7 breast cancer cell
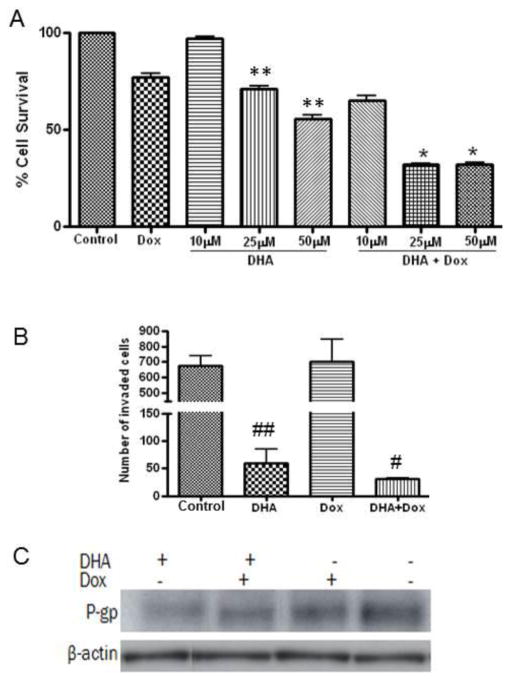
To determine if DHA can improve sensitization of doxorubicin resistant breast cancer cell to doxorubicin, we performed in vitro culture of MCF-7dox in the presence of doxorubicin and DHA alone and in combination. Interestingly, DHA treatment alone appeared to be significant in decreasing proliferation of MCF-7-dox cells as compared to control and doxorubicin alone (Fig. 7A). As expected, doxorubicin alone did not inhibit proliferation of MCF-7dox cell sufficiently. However, doxorubicin could dramatically inhibit the proliferation of MCF-7dox cell in the presence of DHA (Fig. 7A). We also performed the invasion study to see if DHA can suppress the invasion of MCF-7dox cell. Doxorubicin alone did not inhibit the invasion of MCF-7dox cell. Similar to the anti-proliferative effect, treatment with DHA alone appeared to be significant in inhibiting the invasion of MCF-7-dox cells as compared to control and doxorubicin alone (Fig. 7B). Interestingly, doxorubicin in the presence of DHA further enhanced the inhibitory effect of DHA on invasion. One of the major causes for cancer cells to resist current chemotherapy is attributed to the over-expression of P-glycoprotein (P-gp), resulting in insufficient drug delivery to the tumor sites [49]. Therefore, we determined the levels of P-gp in MCF-7dox cells treated with DHA and doxorubicin alone or in combination. Interestingly, DHA alone or in combination with doxorubicin decreased the level of P-gp in MCF-7dox cell (Fig. 7C).
Fig. 7. Effect of DHA on proliferation, invasion and P-gp expression on doxorubicin resistant MCF-7 breast cancer cell.
For cell proliferation assay MCF-7dox cells were plated in a 96 well plate. After 24 hours of incubation, cells were replenished with fresh media with different concentrations of DHA and doxorubicin (2μM) alone or in combination. Cells were then incubated for an additional 48 hours. At the end of incubation, 20 μl MTS reagent was added to each well, and incubated for 4 hours at 37C. Absorbance was read at 490nm. For invasion assay, MCF-7dox cells in 200 μl serum free DMEM was added to upper chamber and 700 μl of DMEM supplemented with 10% fetal calf serum (FCS) and DHA (50 μM) and doxorubicin (2 μM) alone or in combination added to the lower chamber in a 24-well BioCoat Matrigel invasion coated chamber inserts with 8-μm pore size membranes. After 48 hours of incubation, the remaining upper chamber cells were removed and cells which had migrated through the pores to the lower side of the membrane were fixed with 10% formalin and stained with 0.1% crystal violet blue and counted manually. P-gp protein levels were analyzed in MCF-7dox cells treated with 50 μM of DHA and 2 μM of doxorubicin alone or in combination for 48 hours by western blot. Each value represents the mean ± SEM of two independent triplicate cultures. * p<0.05 vs. DHA or doxorubicin alone; ** p<0.05 vs. control or Doxorubicin alone; # p<0.05 vs. doxorubicin alone; ## p<0.05 vs. control or Doxorubicin alone.
Discussion
Breast cancer cell metastasis to bone and related osteolysis is one of the major causes of morbidity and mortality in breast cancer patients [11]. In breast cancer patients, osteolytic lesions frequently occur in the load-bearing bones with increased susceptibility to pathological fracture. It has been demonstrated that EPA and DHA ω-3 FA rich FO prevents breast cancer cell metastasis to bone [45]. However, it was not known whether EPA or DHA or both are the bioactive component that is exerting this inhibition of breast cancer cell metastasis to bone. In this study, we demonstrated for the first time that the DHA not the EPA attenuates the breast cancer metastasis to bone by inhibiting proliferation and invasion of breast cancer cells, as well as osteolysis by inhibiting breast cancer stimulated bone resorbing osteoclast formation.
Osteolysis does not occur due to direct effect of breast cancer cells metastasized to the bone. Rather stimulation of osteoclast maturation and their activation cause formation of bone lesions [11,12]. The colonized breast cancer cells produce TNF-α and IL-6, which along with PTHrP and IL-11 stimulate the bone marrow stromal cells and residing osteoblasts to synthesize more RANKL to activate osteoclasts [12,50] which ultimately results in the formation of osteolytic lesions. Enhanced proliferation of breast cancer cells in the bone was found to be associated with enhanced osteoclast formation in our study. We previously showed that DHA not EPA potently inhibited osteoclastogenesis by suppressing TNF-α and NF-κB signaling [18]. In this study, we found that DHA-FO treated mice exhibited significantly less burden of breast cancer cells as well as osteoclasts as compared to EPA-FO and ω-6 FA rich CO groups. These data together with in vitro cell culture data indicate that DHA attenuates breast cancer bone metastasis and related osteolysis by inhibiting proliferation and migration of breast cancer cells to the bone as well as by reducing osteoclast activity in the bone.
Recent reports demonstrate a positive role of CD44 in the progression of metastasis [51,52]. Many cancer cells including breast cancer cells hyperexpress the cell surface adhesion protein CD44 [53,54]. Nakamura et al. has demonstrated that metastasized breast cancer cells expressing CD44 are present on the bone surface that faces the bone resorbing osteoclasts [55]. CD44 acts as the main glycoprotein receptor for the disaccharide hyaluronan, a major component of the luminal surface of the bone marrow capillary endothelium. Thus, metastatic breast cancer cells expressing high levels of CD44 may be efficiently recruited to bone marrow. Similarly, collagen I, a constituent of bone matrix, serves as a ligand for CD44, indicating efficient recruitment of breast cancer cells to the bone. In a recent study, Mandal et al showed that both EPA and DHA inhibit CD44 mRNA and protein levels in MDA-MB-231 cells when cultured in the presence of EPA and DHA [45]. Further, they demonstrated that breast cancer tumor xenografts from FO treated mice had reduced levels of CD44 mRNA and protein when mice were injected with MDA-MB-231 cells in their mammary fat pad [45]. Our results show that bones from DHA-FO treated mice had a reduced expression of CD44 protein compared to the EPA-FO treated mice when injected with MDA-MB-231 breast cancer cells intra-cardially, thus, providing a mechanism for the attenuation of breast cancer cell migration/invasion by DHA. Furthermore, for the first time, we demonstrate that DHA prevents the formation of osteolytic lesions in the bone more potently than EPA when breast cancer cells are injected either intra-cardially or intra-tibially. Moreover, CD44 has been shown to bind and retain the protein MMP-9, which is present on the surface of breast cancer cells [56]. MMP-9 cleaves collagen I in the bone matrix and may contribute to induce osteolysis found in bone, resulting from breast cancer metastasis. MDA-MB-231 cells also induced an increase in the expression of MMP-9 by migrating osteoclasts [57]. We previously showed that DHA inhibited osteoclastogenesis more potently than EPA by suppressing the expression of MMP-9 [18]. Others have shown that DHA inhibits MMP-9 expression in breast cancer cells via heme oxygenase-1 [58]. Therefore, DHA-mediated prevention of osteolysis in breast cancer metastasized bone is likely to be associated with the modulation of MMP-9 expression in bone microenvironment, thereby attenuating bone resorbing osteoclast formation in bone. In our study, reduced expression of CD44 and TRAP in the DHA-FO treated bone revealed that DHA may prevent breast cancer bone metastasis associated osteolysis by attenuating invasion and bone resorbing osteoclast formation.
Recently, a number of studies show that ω-3 FA, especially DHA, can act as an excellent adjuvant to enhance the effect of various drugs [59–61]. It has been reported that tumor cells can be made more sensitive to chemotherapy than non-tumor cells when membrane lipids are enriched with DHA [62]. In a recent phase II clinical trial, Bouqnoux et al. demonstrated that DHA during chemotherapy was devoid of adverse side effects and can improve the outcome of chemotherapy when highly incorporated [62]. DHA has the potential to specifically chemosensitize tumors. Doxorubicin chemosensitization of breast cancer cell lines by DHA was reported to be cell-line selective, affecting MDA-MB-231 and MCF-7dox (a doxorubicin-resistant cell line) but not the parental MCF-7 cell line [63]. Chemosensitization through FA appear as a new promising adjuvant therapeutic paradigm, since ω-3 FA are physiological molecules found in food and are nontoxic in vivo. We performed further studies to determine if DHA can improve the drug sensitivity of drug resistant breast cancer cells, doxorubicin resistant MCF-7 (MCF-7dox). One of the major causes for cancer cells to resist current chemotherapy is attributed to the over-expression of P-glycoprotein (P-gp), resulting in insufficient drug delivery to the tumor sites [49]. Our study showed that DHA efficiently improved the sensitivity to doxorubicin to MCF-7dox breast cancer cells by suppressing the expression of P-gp in breast cancer cells. However, further in vivo preclinical studies are needed to establish this effect.
Conclusion
Breast cancer cells metastasis to bone is one of the most catastrophic complications for the morbidity and mortality of breast cancer patients. Our results provide preliminary evidence suggesting that the use of DHA supplements alone or in combination with anti-cancer drugs could be an important therapy for this devastating disease. However, further studies are required to determine details behind the mechanisms of how DHA exerts its effect against breast cancer cell progression and metastasis to bone. Our results identify a novel function of a bioactive FO component, DHA as a promising dietary drug to prevent/treat breast cancer progression and bone metastasis and related osteolysis.
Acknowledgments
We acknowledge Emily Molina for her kind review of our manuscript for grammatical corrections. We thank Ocean Nutrition Canada for free supply of FO-EPA and FO-DHA for this study. This study was supported by DOD, BC096459, Elsa U. Pardee Foundation 131884/44096 and NIH AG034233 grants.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2004;3:19. doi: 10.1186/1475-2891-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–77. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66:1169–79. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 4.Bagga D, Anders KH, Wang HJ, Glaspy JA. Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer. 2002;42:180–5. doi: 10.1207/S15327914NC422_5. [DOI] [PubMed] [Google Scholar]
- 5.Lands WE, Hamazaki T, Yamazaki K, Okuyama H, Sakai K, Goto Y, Hubbard VS. Changing dietary patterns. Am J Clin Nutr. 1990;51:991–3. doi: 10.1093/ajcn/51.6.991. [DOI] [PubMed] [Google Scholar]
- 6.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2005;92:187–95. doi: 10.1007/s10549-005-2415-z. [DOI] [PubMed] [Google Scholar]
- 7.Cao W, Ma Z, Rasenick MM, Yeh S, Yu J. N-3 poly-unsaturated fatty acids shift estrogen signaling to inhibit human breast cancer cell growth. PLoS One. 2012;7:e52838. doi: 10.1371/journal.pone.0052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, Lanzino M, De Amicis F, Sisci D, Mauro L, Aquila S, Catalano S, Bonofiglio D, Ando S. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARgamma activation in MCF-7 breast cancer cells. J Cell Physiol. 2013;228:1314–22. doi: 10.1002/jcp.24288. [DOI] [PubMed] [Google Scholar]
- 9.Sczaniecka AK, Brasky TM, Lampe JW, Patterson RE, White E. Dietary intake of specific fatty acids and breast cancer risk among postmenopausal women in the VITAL cohort. Nutr Cancer. 2012;64:1131–42. doi: 10.1080/01635581.2012.718033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biondo PD, Brindley DN, Sawyer MB, Field CJ. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J Nutr Biochem. 2008;19:787–96. doi: 10.1016/j.jnutbio.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 13.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–56. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 14.Rahman MM, Bhattacharya A, Banu J, Kang JX, Fernandes G. Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis. J Cell Mol Med. 2009;13:1833–44. doi: 10.1111/j.1582-4934.2008.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulsen RC, Firth EC, Rogers CW, Moughan PJ, Kruger MC. Specific effects of gamma-linolenic, eicosapentaenoic, and docosahexaenoic ethyl esters on bone post-ovariectomy in rats. Calcif Tissue Int. 2007;81:459–71. doi: 10.1007/s00223-007-9080-7. [DOI] [PubMed] [Google Scholar]
- 16.Kruger MC, Coetzee M, Haag M, Weiler H. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res. 2010;49:438–49. doi: 10.1016/j.plipres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya A, Rahman M, Sun D, Fernandes G. Effect of fish oil on bone mineral density in aging C57BL/6 female mice. J Nutr Biochem. 2007;18:372–9. doi: 10.1016/j.jnutbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Rahman MM, Bhattacharya A, Fernandes G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J Cell Physiol. 2008;214:201–9. doi: 10.1002/jcp.21188. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya A, Rahman M, Banu J, Lawrence RA, McGuff HS, Garrett IR, Fischbach M, Fernandes G. Inhibition of osteoporosis in autoimmune disease prone MRL/Mpj-Fas(lpr) mice by N-3 fatty acids. J Am Coll Nutr. 2005;24:200–9. doi: 10.1080/07315724.2005.10719466. [DOI] [PubMed] [Google Scholar]
- 20.Weiss LA, Barrett-Connor E, von Muhlen D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am J Clin Nutr. 2005;81:934–8. doi: 10.1093/ajcn/81.4.934. [DOI] [PubMed] [Google Scholar]
- 21.Kruger G, Huber MC, Bonifer C. The −3.9 kb DNaseI hypersensitive site of the chicken lysozyme locus harbours an enhancer with unusual chromatin reorganizing activity. Gene. 1999;236:63–77. doi: 10.1016/s0378-1119(99)00271-1. [DOI] [PubMed] [Google Scholar]
- 22.Watkins BA, Shen CL, Allen KG, Seifert MF. Dietary (n-3) and (n-6) polyunsaturates and acetylsalicylic acid alter ex vivo PGE2 biosynthesis, tissue IGF-I levels, and bone morphometry in chicks. J Bone Miner Res. 1996;11:1321–32. doi: 10.1002/jbmr.5650110917. [DOI] [PubMed] [Google Scholar]
- 23.Watkins BA, Li Y, Seifert MF. Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: actions on bone mineral and serum biomarkers in ovariectomized rats. J Nutr Biochem. 2006;17:282–9. doi: 10.1016/j.jnutbio.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Maggio M, Artoni A, Lauretani F, Borghi L, Nouvenne A, Valenti G, Ceda GP. The impact of omega-3 fatty acids on osteoporosis. Curr Pharm Des. 2009;15:4157–64. doi: 10.2174/138161209789909728. [DOI] [PubMed] [Google Scholar]
- 25.Moselhy SS, Al-Malki AL, Kumosani TA, Jalal JA. Modulatory effect of cod liver oil on bone mineralization in overiectomized female Sprague Dawley rats. Toxicol Ind Health. 2012;28:387–92. doi: 10.1177/0748233711412428. [DOI] [PubMed] [Google Scholar]
- 26.Kokkinos PP, Shaye R, Alam BS, Alam SQ. Dietary lipids, prostaglandin E2 levels, and tooth movement in alveolar bone of rats. Calcif Tissue Int. 1993;53:333–7. doi: 10.1007/BF01351839. [DOI] [PubMed] [Google Scholar]
- 27.Nawata K, Yamauchi M, Takaoka S, Yamaguchi T, Sugimoto T. Association of n-3 Polyunsaturated Fatty Acid Intake with Bone Mineral Density in Postmenopausal Women. Calcif Tissue Int. 2013 doi: 10.1007/s00223-013-9743-5. [DOI] [PubMed] [Google Scholar]
- 28.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL. Protective effects of fish intake and interactive effects of long-chain polyunsaturated fatty acid intakes on hip bone mineral density in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2011;93:1142–51. doi: 10.3945/ajcn.110.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lage S, Bueno M, Andrade F, Prieto JA, Delgado C, Legarda M, Sanjurjo P, Aldamiz-Echevarria LJ. Fatty acid profile in patients with phenylketonuria and its relationship with bone mineral density. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9189-0. [DOI] [PubMed] [Google Scholar]
- 30.Kruger MC, Schollum LM. Is docosahexaenoic acid more effective than eicosapentaenoic acid for increasing calcium bioavailability? Prostaglandins Leukot Essent Fatty Acids. 2005;73:327–34. doi: 10.1016/j.plefa.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Watkins BA, Li Y, Allen KG, Hoffmann WE, Seifert MF. Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J Nutr. 2000;130:2274–84. doi: 10.1093/jn/130.9.2274. [DOI] [PubMed] [Google Scholar]
- 32.Oliver E, McGillicuddy FC, Harford KA, Reynolds CM, Phillips CM, Ferguson JF, Roche HM. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between LC n-3 PUFA. J Nutr Biochem. 2012;23:1192–200. doi: 10.1016/j.jnutbio.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Rahman M, Kundu JK, Shin JW, Na HK, Surh YJ. Docosahexaenoic acid inhibits UVB-induced activation of NF-kappaB and expression of COX-2 and NOX-4 in HR-1 hairless mouse skin by blocking MSK1 signaling. PLoS One. 2011;6:e28065. doi: 10.1371/journal.pone.0028065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez-Micaelo N, Gonzalez-Abuin N, Terra X, Richart C, Ardevol A, Pinent M, Blay M. Omega-3 docosahexaenoic acid and procyanidins inhibit cyclo-oxygenase activity and attenuate NF-kappaB activation through a p105/p50 regulatory mechanism in macrophage inflammation. Biochem J. 2012;441:653–63. doi: 10.1042/BJ20110967. [DOI] [PubMed] [Google Scholar]
- 35.Yuan J, Akiyama M, Nakahama K, Sato T, Uematsu H, Morita I. The effects of polyunsaturated fatty acids and their metabolites on osteoclastogenesis in vitro. Prostaglandins Other Lipid Mediat. 2010;92:85–90. doi: 10.1016/j.prostaglandins.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Seifert MF, Lim SY, Salem N, Jr, Watkins BA. Bone mineral content is positively correlated to n-3 fatty acids in the femur of growing rats. Br J Nutr. 2013;104:674–85. doi: 10.1017/S0007114510001133. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Seifert MF, Lim SY, Salem N, Jr, Watkins BA. Bone mineral content is positively correlated to n-3 fatty acids in the femur of growing rats. Br J Nutr. 2010;104:674–85. doi: 10.1017/S0007114510001133. [DOI] [PubMed] [Google Scholar]
- 38.Poulsen RC, Moughan PJ, Kruger MC. Docosahexaenoic acid and 17 beta-estradiol co-treatment is more effective than 17 beta-estradiol alone in maintaining bone post-ovariectomy. Exp Biol Med (Maywood) 2008;233:592–602. doi: 10.3181/0709-RM-259. [DOI] [PubMed] [Google Scholar]
- 39.Jourdan ML, Maheo K, Barascu A, Goupille C, De Latour MP, Bougnoux P, Rio PG. Increased BRCA1 protein in mammary tumours of rats fed marine omega-3 fatty acids. Oncol Rep. 2007;17:713–9. [PubMed] [Google Scholar]
- 40.Sun H, Berquin IM, Owens RT, O’Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–9. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu IF, Hasio AC, Hu MC, Yang FM, Su HM. Docosahexaenoic acid induces proteasome-dependent degradation of estrogen receptor alpha and inhibits the downstream signaling target in MCF-7 breast cancer cells. J Nutr Biochem. 2010;21:512–7. doi: 10.1016/j.jnutbio.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, Berry D, Steward W, Dennison A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45:2077–86. doi: 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 43.Serini S, Trombino S, Oliva F, Piccioni E, Monego G, Resci F, Boninsegna A, Picci N, Ranelletti FO, Calviello G. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis. 2008;13:1172–83. doi: 10.1007/s10495-008-0246-1. [DOI] [PubMed] [Google Scholar]
- 44.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–79. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 45.Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun. 2011;402:602–7. doi: 10.1016/j.bbrc.2010.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiraga T, Williams PJ, Mundy GR, Yoneda T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res. 2001;61:4418–24. [PubMed] [Google Scholar]
- 47.Zhang JH, Wang J, Tang J, Barnett B, Dickson J, Hahsimoto N, Williams P, Ma W, Zheng W, Yoneda T, Pageau S, Chen J. Bone sialoprotein promotes bone metastasis of a non-bone-seeking clone of human breast cancer cells. Anticancer Res. 2004;24:1361–8. [PubMed] [Google Scholar]
- 48.Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39:1107–15. doi: 10.1016/j.bone.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Y, Bu L, Yan H, Jia W. 20S-protopanaxadiol inhibits P-glycoprotein in multidrug resistant cancer cells. Planta Med. 2009;75:1124–8. doi: 10.1055/s-0029-1185477. [DOI] [PubMed] [Google Scholar]
- 50.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–9. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouhtit A, Abd Elmageed ZY, Abdraboh ME, Lioe TF, Raj MH. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol. 2007;171:2033–9. doi: 10.2353/ajpath.2007.070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002;62:2281–6. [PubMed] [Google Scholar]
- 53.Gotte M, Yip GW. Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective. Cancer Res. 2006;66:10233–7. doi: 10.1158/0008-5472.CAN-06-1464. [DOI] [PubMed] [Google Scholar]
- 54.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Jr, Badve S, Nakshatri H. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura H, Hiraga T, Ninomiya T, Hosoya A, Fujisaki N, Yoneda T, Ozawa H. Involvement of cell-cell and cell-matrix interactions in bone destruction induced by metastatic MDA-MB-231 human breast cancer cells in nude mice. J Bone Miner Metab. 2008;26:642–7. doi: 10.1007/s00774-008-0857-1. [DOI] [PubMed] [Google Scholar]
- 56.Hill A, McFarlane S, Johnston PG, Waugh DJ. The emerging role of CD44 in regulating skeletal micrometastasis. Cancer Lett. 2006;237:1–9. doi: 10.1016/j.canlet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Tumber A, Morgan HM, Meikle MC, Hill PA. Human breast-cancer cells stimulate the fusion, migration and resorptive activity of osteoclasts in bone explants. Int J Cancer. 2001;91:665–72. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1101>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 58.Chen HW, Chao CY, Lin LL, Lu CY, Liu KL, Lii CK, Li CC. Inhibition of matrix metalloproteinase-9 expression by docosahexaenoic acid mediated by heme oxygenase 1 in 12-O-tetradecanoylphorbol-13-acetate-induced MCF-7 human breast cancer cells. Arch Toxicol. 2013;87:857–69. doi: 10.1007/s00204-012-1003-3. [DOI] [PubMed] [Google Scholar]
- 59.Yamagami T, Porada CD, Pardini RS, Zanjani ED, Almeida-Porada G. Docosahexaenoic acid induces dose dependent cell death in an early undifferentiated subtype of acute myeloid leukemia cell line. Cancer Biol Ther. 2009;8:331–7. doi: 10.4161/cbt.8.4.7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menendez JA, Lupu R, Colomer R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur J Cancer Prev. 2005;14:263–70. doi: 10.1097/00008469-200506000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Baumgartner M, Sturlan S, Roth E, Wessner B, Bachleitner-Hofmann T. Enhancement of arsenic trioxide-mediated apoptosis using docosahexaenoic acid in arsenic trioxide-resistant solid tumor cells. Int J Cancer. 2004;112:707–12. doi: 10.1002/ijc.20462. [DOI] [PubMed] [Google Scholar]
- 62.Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101:1978–85. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maheo K, Vibet S, Steghens JP, Dartigeas C, Lehman M, Bougnoux P, Gore J. Differential sensitization of cancer cells to doxorubicin by DHA: a role for lipoperoxidation. Free Radic Biol Med. 2005;39:742–51. doi: 10.1016/j.freeradbiomed.2005.04.023. [DOI] [PubMed] [Google Scholar]