Abstract
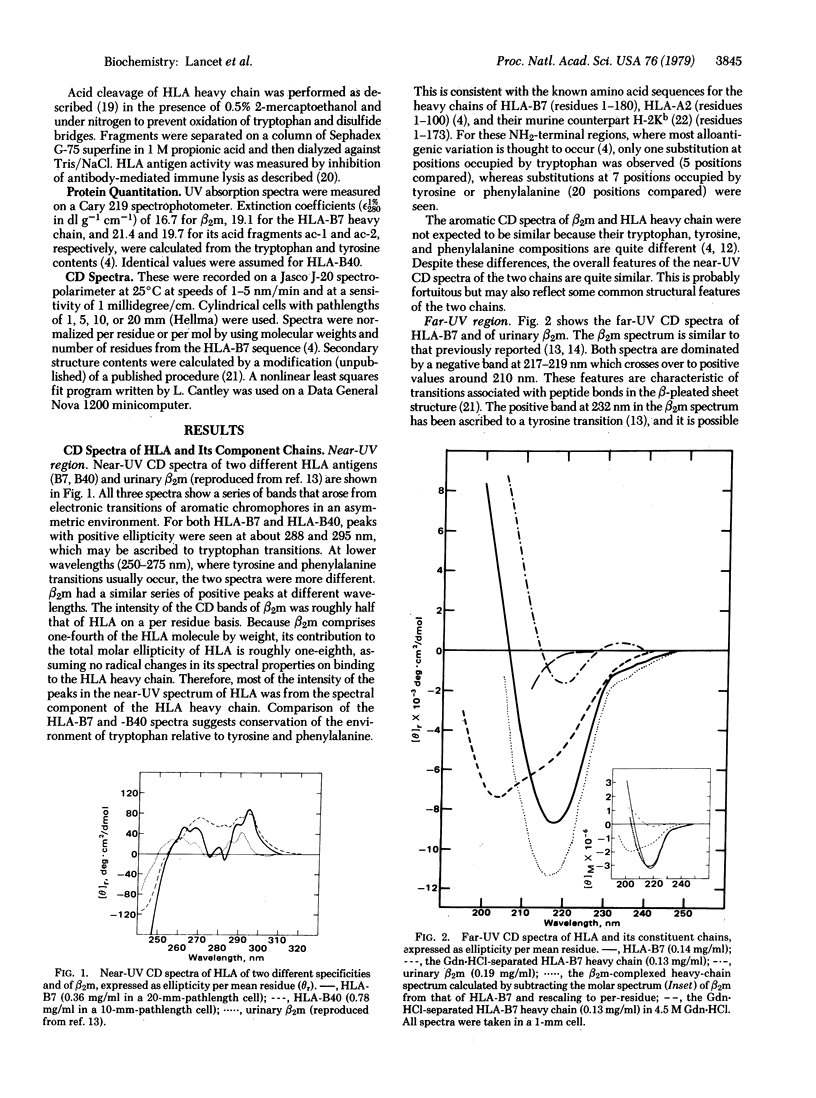
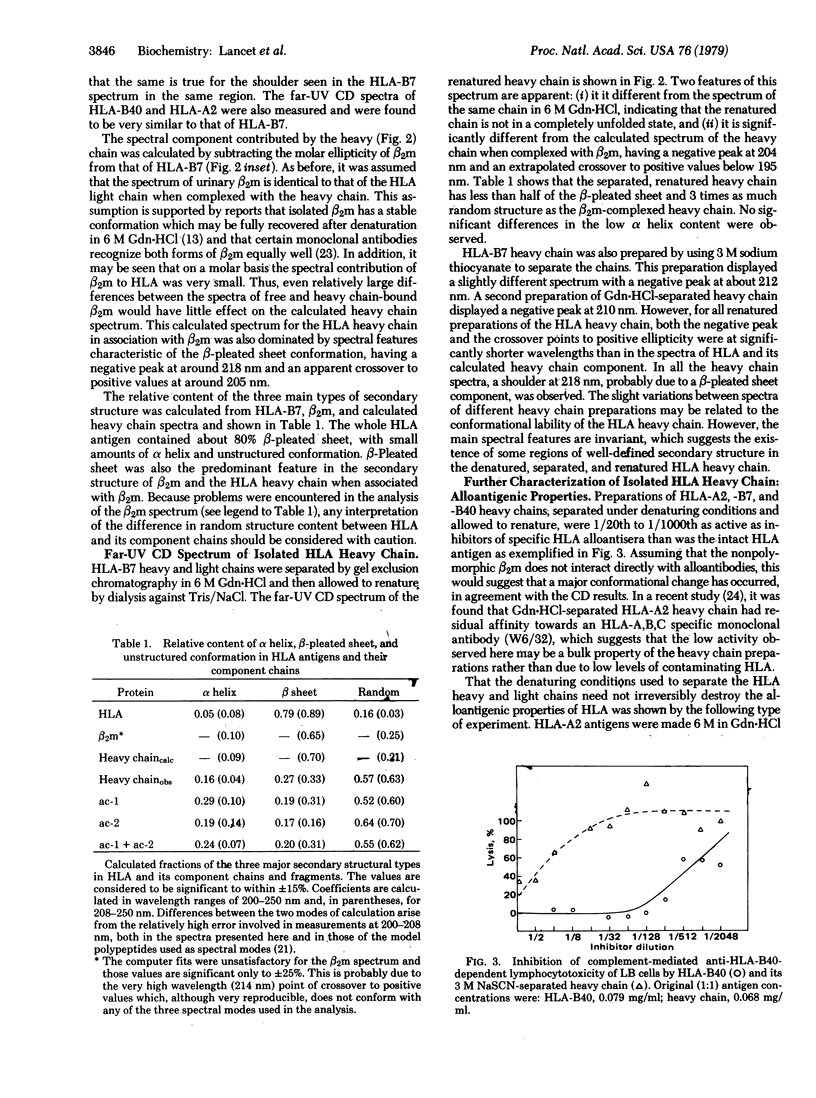
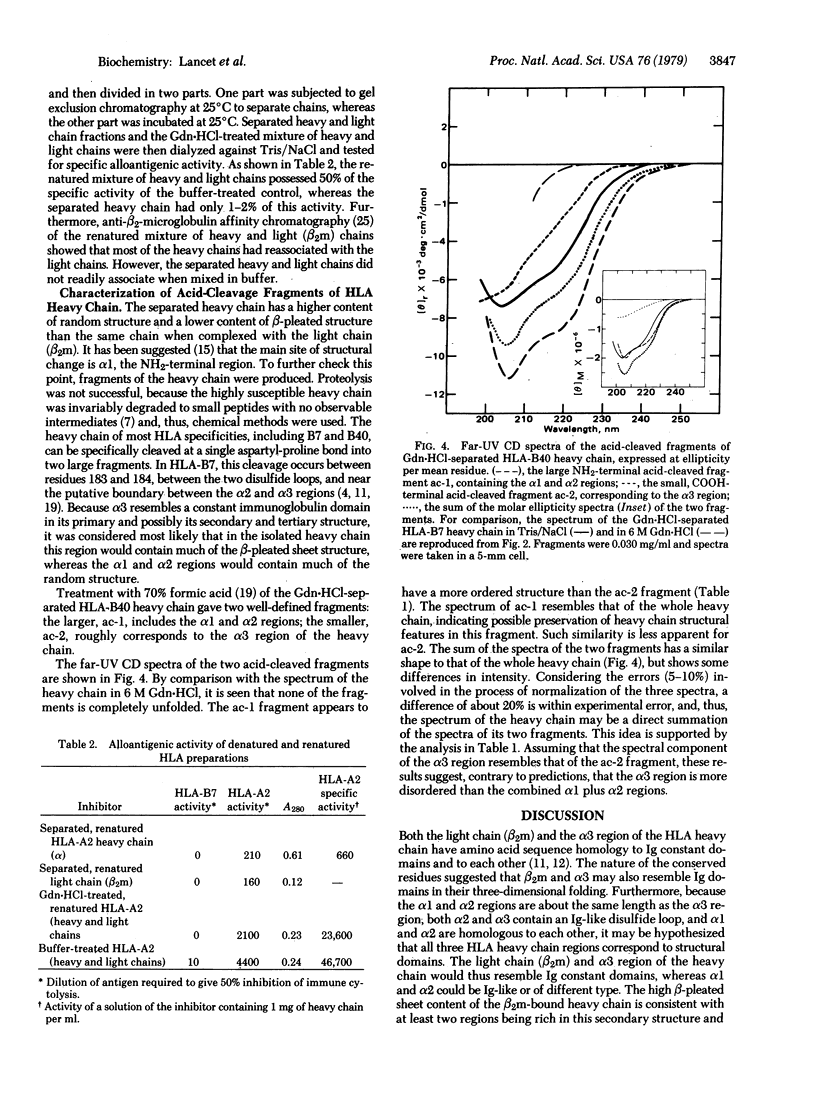
The three-dimensional organization of HLA antigens has been investigated by spectroscopic and immunochemical techniques. Measurement of the circular dichroism shows that in papain-solubilized HLA the heavy chain as well as the previously studied light chain (beta 2-microglobulin) consists predominantly of beta-pleated sheet structures. When heavy chain is separated from the light chain under denaturing conditions and is allowed to renature, about 50% of the beta structure is lost, concomitantly with most of the alloantigenic activity. Analysis of the two acid-cleaved fragments of HLA-B7 heavy chain shows that beta structure is preferentially lost from the COOH-terminal region of the heavy chain. Exposure to denaturants per se does not inevitably result in irreversible loss of antigenic activity. However, recovery of antigenic properties does seem to depend on reassociation of the two chains. The results reported here provide further evidence for (i) the similarity of HLA antigens and immunoglobulins at the three-dimensional level and (ii) two distinct and physiologically important conformations of the HLA heavy chain, depending upon whether it is associated with the light chain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Bodmer W. F., Bodmer J. G. Evolution and function of the HLA system. Br Med Bull. 1978 Sep;34(3):309–316. doi: 10.1093/oxfordjournals.bmb.a071518. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Ewenstein B. M., Uehara H., Martinko J. M., Nathenson S. G. Further structural analysis of the murine H-2Kb glycoprotein using radiochemical methodology. Mol Immunol. 1979 Jan;16(1):3–8. doi: 10.1016/0161-5890(79)90021-x. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Snary D. Isolation and structure of human histocompatibility (HLA) antigens. Contemp Top Mol Immunol. 1977;6:53–82. doi: 10.1007/978-1-4684-2841-4_2. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. The genetic control of immunoglobulin synthesis. Annu Rev Genet. 1972;6:1–46. doi: 10.1146/annurev.ge.06.120172.000245. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Lancet D., Pecht I. Folding pathways of immunoglobulin domains. The folding kinetics of the Cgamma3 domain of human IgG1. Biochemistry. 1979 Jul 24;18(15):3327–3336. doi: 10.1021/bi00582a020. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Painter R. H., Dorrington K. J. The structure and function of immunoglobulin domains: studies with beta-2-microglobulin on the role of the intrachain disulfide bond. Proc Natl Acad Sci U S A. 1975 Feb;72(2):548–552. doi: 10.1073/pnas.72.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F. A. Physical-chemical properties of beta 2-microglobulin. Immunochemistry. 1974 Mar;11(3):111–114. doi: 10.1016/0019-2791(74)90207-9. [DOI] [PubMed] [Google Scholar]
- Kvist S., Sandberg-Trägårdh L., Ostberg L., Peterson P. A. Isolation and partial characterization of papain-solubilized murine H-2 antigens. Biochemistry. 1977 Oct 4;16(20):4415–4420. doi: 10.1021/bi00639a014. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Sege K., Klareskog L., Anundi H., Ostberg L. Evolutionary relationship between immunoglobulins and transplantation antigens. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1612–1616. doi: 10.1073/pnas.72.4.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Cannon L. E., Strominger J. L. Cell-free translation of the mRNAs for the heavy and light chains of HLA-A and HLA-B antigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2273–2277. doi: 10.1073/pnas.76.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Strominger J. L. Rapid purification of detergent-solubilized HLA antigen by affinity chromatography employing anti-beta2-microglobulin serum. J Biol Chem. 1976 Sep 10;251(17):5427–5428. [PubMed] [Google Scholar]
- Sanderson A. R., Batchelor J. R. Transplantation antigens from human spleens. Nature. 1968 Jul 13;219(5150):184–186. doi: 10.1038/219184a0. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Cresswell P., Grey H., Humphreys R. E., Mann D., McCuneJ, Parham P., Robb R., Sanderson A. R., Springer T. A. The immunoglobulin-like structure of human histocompatibility antigens. Transplant Rev. 1974;21(0):126–143. doi: 10.1111/j.1600-065x.1974.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Mann D. L., Parham P., Robb R., Springer T., Terhorst C. Structure of HL-A A and B antigens isolated from cultured human lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):323–329. doi: 10.1101/sqb.1977.041.01.038. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Robb R., Jones C., Strominger J. L. Further structural studies of the heavy chain of HLA antigens and its similarity to immunoglobulins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4002–4006. doi: 10.1073/pnas.74.9.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trägårdh L., Curman B., Wiman K., Rask L., Peterson P. A. Chemical, physical-chemical, and immunological properties of papain-solubilized human transplatation antigens. Biochemistry. 1979 May 29;18(11):2218–2226. doi: 10.1021/bi00578a013. [DOI] [PubMed] [Google Scholar]
- Trägårdh L., Wiman K., Rask L., Peterson P. A. Fragmentation of the human transplantation antigen heavy chain by limited proteolysis, acid cleavage, and cyanogen bromide treatment. Biochemistry. 1979 Apr 3;18(7):1322–1328. doi: 10.1021/bi00574a031. [DOI] [PubMed] [Google Scholar]
- Turner M. J., Cresswell P., Parham P., Strominger J. L., Mann D. L., Sanderson A. R. Purification of papain-solubilized histocompatibility antigens from a cultured human lymphoblastoid line, RPMI 4265. J Biol Chem. 1975 Jun 25;250(12):4512–4519. [PubMed] [Google Scholar]


