Abstract
The conformation of the linear peptide antibiotic alamethicin in dipalmitoyl phosphatidylcholine multilayers was investigated in the absence of an electric field by means of infrared attenuated total reflection spectroscopy. Alamethicin was found to be incorporated into the lipid membrane not only in the dry state but also in an aqueous environment. Its molecular conformation, however, changed from a helix when dry to an extended chain when aqueous. The extended chain aggregated to di- and multimers spanning the lipid bilayer. The equilibrium concentration of alamethicin in the surrounding water was 90 nM, which is in the range of concentrations used in black film experiments. The corresponding molar ratio of lipid to peptide was 80:1. Concerning the molecular mechanism of electric field-induced pore formation, one has to conclude that the dipole model proposed by several authors is very unlikely because it is based on the assumption that the major part of alamethicin is adsorbed on the membrane surface, from which small amounts flip into the membrane under the influence of an electric field. An alternative mechanism is proposed, based on a field-induced conformational change of the peptide from the extended state to a helix. This transition is favored by the resulting dipole moment of the alamethicin helix.
Full text
PDF
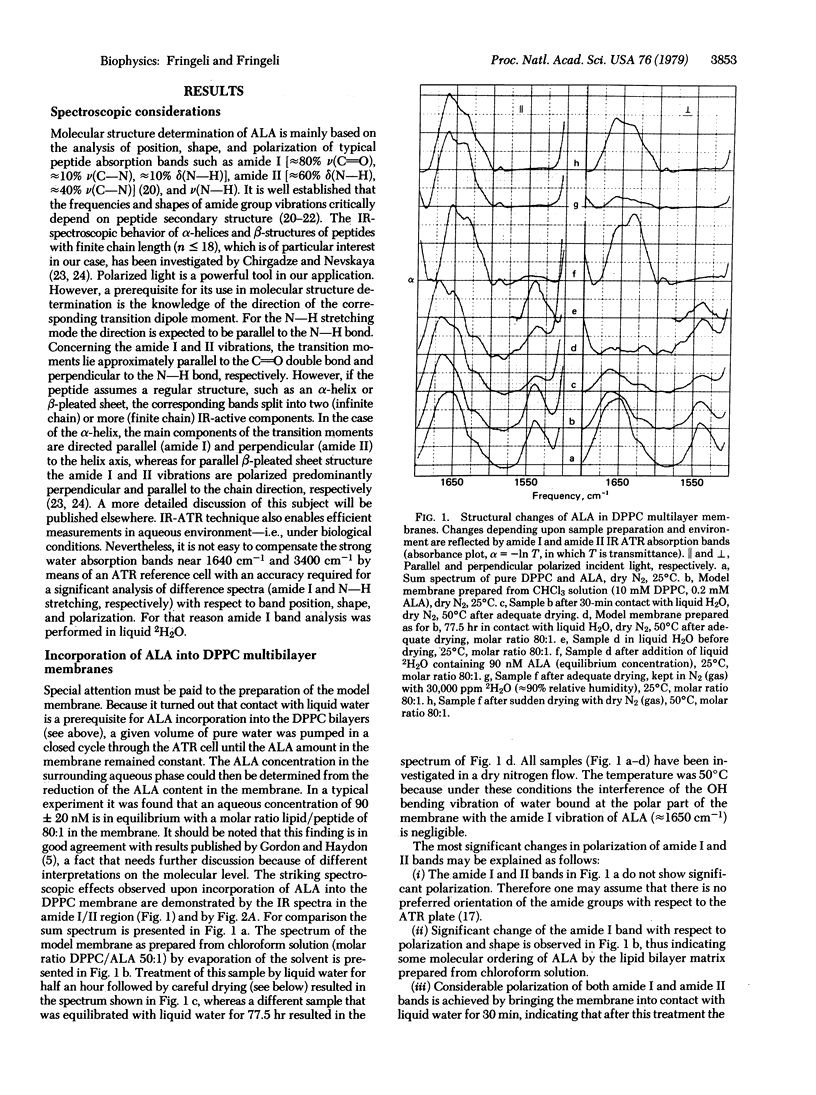
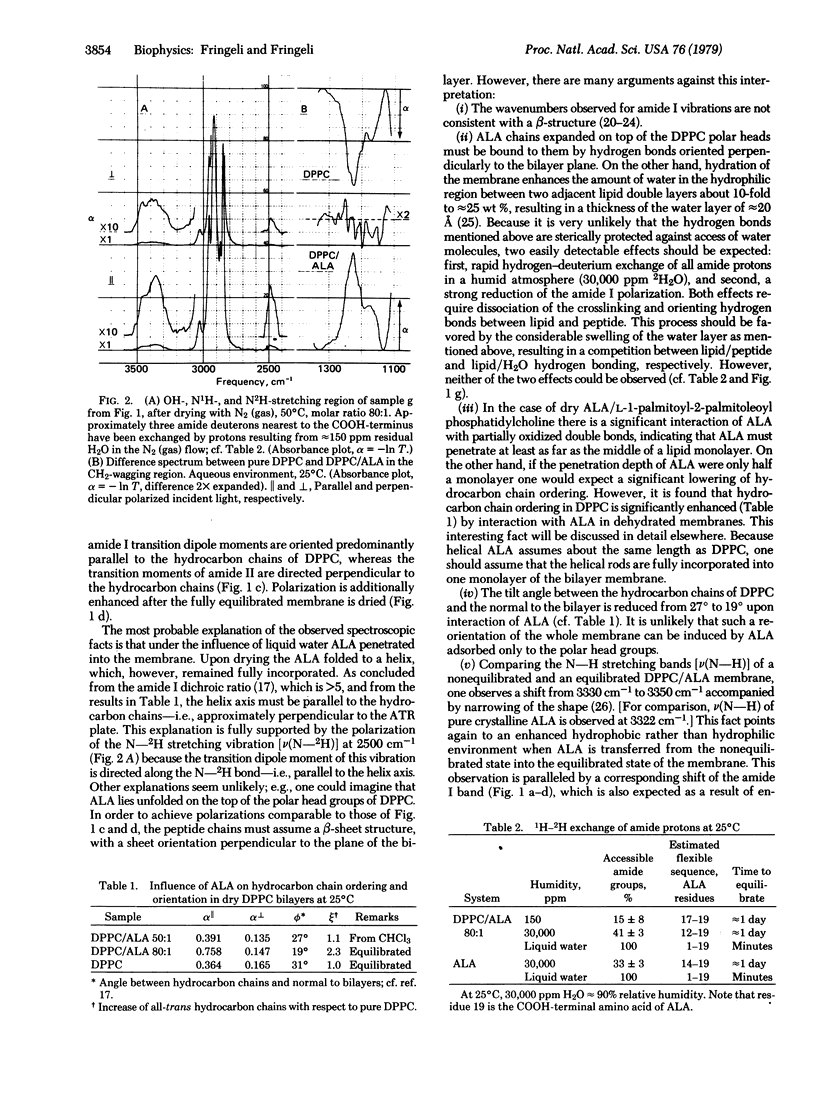


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann G., Mueller P. A molecular model of membrane excitability. J Supramol Struct. 1974;2(5-6):538–557. doi: 10.1002/jss.400020504. [DOI] [PubMed] [Google Scholar]
- Boheim G., Benz R. Charge-pulse relaxation studies with lipid bilayer membranes modified by alamethicin. Biochim Biophys Acta. 1978 Feb 21;507(2):262–270. doi: 10.1016/0005-2736(78)90421-2. [DOI] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Chirgadze Y. N., Nevskaya N. A. Infrared spectra and resonance interaction of amide-I vibration of the paraellel-chain pleated sheets. Biopolymers. 1976 Apr;15(4):627–636. doi: 10.1002/bip.1976.360150403. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Hall J. E., Mead C. A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol. 1973 Dec 31;14(2):143–176. doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Fringeli U. P. The structure of lipids and proteins studied by attenuated total reflection (ATR) infrared spectroscopy. II. Oriented layers of a homologous series: phosphatidylethanolamine to phosphatidylcholine. Z Naturforsch C. 1977 Jan-Feb;32(1-2):20–45. doi: 10.1515/znc-1977-1-205. [DOI] [PubMed] [Google Scholar]
- Gisin B. F., Kobayashi S., Hall J. E. Synthesis of a 19-residue peptide with alamethicin-like activity. Proc Natl Acad Sci U S A. 1977 Jan;74(1):115–119. doi: 10.1073/pnas.74.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. Kinetics and stability of alamethicin conducting channels in lipid bilayers. Biochim Biophys Acta. 1976 Jul 1;436(3):541–556. doi: 10.1016/0005-2736(76)90439-9. [DOI] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. Potential-dependent conductances in lipid membranes containing alamethicin. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):433–447. doi: 10.1098/rstb.1975.0021. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. H., Eanes E. D. Coexistence of rigid crystalline and liquid crystalline phases in lecithin-water mixtures. Biophys J. 1974 May;14(5):335–342. doi: 10.1016/S0006-3495(74)85920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. E. Toward a molecular understanding of excitability. Alamethicin in black lipid films. Biophys J. 1975 Sep;15(9):934–939. doi: 10.1016/S0006-3495(75)85869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Jung G., Dubischar N. Conformational changes of alamethicin induced by solvent and temperature. A 13C-NMR and circular-dichroism study. Eur J Biochem. 1975 Jun;54(2):395–409. doi: 10.1111/j.1432-1033.1975.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Lau A. L., Chan S. I. Alamethicin-mediated fusion of lecithin vesicles. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2170–2174. doi: 10.1073/pnas.72.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. R., Williams R. J. Chemical nature and sequence of alamethicin. Biochem J. 1976 Feb 1;153(2):181–190. doi: 10.1042/bj1530181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. Membrane excitation through voltage-induced aggregation of channel precursors. Ann N Y Acad Sci. 1975 Dec 30;264:247–264. doi: 10.1111/j.1749-6632.1975.tb31487.x. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Resting and action potentials in experimental bimolecular lipid membranes. J Theor Biol. 1968 Feb;18(2):222–258. doi: 10.1016/0022-5193(68)90163-x. [DOI] [PubMed] [Google Scholar]
- Nevskaya N. A., Chirgadze Y. N. Infrared spectra and resonance interactions of amide-I and II vibration of alpha-helix. Biopolymers. 1976 Apr;15(4):637–648. doi: 10.1002/bip.1976.360150404. [DOI] [PubMed] [Google Scholar]



