Abstract
Background
Chronic kidney disease (CKD) is highly prevalent in patients with diabetes or hypertension in primary care. A shared care model could improve quality of care in these patients
Aim
To assess the effect of a shared care model in managing patients with CKD who also have diabetes or hypertension.
Design and setting
A cluster randomised controlled trial in nine general practices in The Netherlands.
Method
Five practices were allocated to the shared care model and four practices to usual care for 1 year. Primary outcome was the achievement of blood pressure targets (130/80 mmHg) and lowering of blood pressure in patients with diabetes mellitus or hypertension and an estimated glomerular filtration rate (eGFR)<60ml/min/1.73m2.
Results
Data of 90 intervention and 74 control patients could be analysed. Blood pressure in the intervention group decreased with 8.1 (95% CI = 4.8 to 11.3)/1.1 (95% CI = −1.0 to 3.2) compared to −0.2 (95% CI = −3.8 to 3.3)/−0.5 (95% CI = −2.9 to 1.8) in the control group. Use of lipid-lowering drugs, angiotensin-system inhibitors and vitamin D was higher in the intervention group than in the control group (73% versus 51%, 81% versus 64%, and 15% versus 1%, respectively, [P = 0.004, P = 0.01, and P = 0.002]).
Conclusion
A shared care model between GP, nurse practitioner and nephrologist is beneficial in reducing systolic blood pressure in patients with CKD in primary care.
Keywords: chronic renal insufficiency, diabetes, glomerular filtration rate, hypertension, primary health care
INTRODUCTION
The high and rising prevalence of chronic kidney disease (CKD) — which amounts to 13% in the general population in the US and 7% in primary care in the UK — places a burden on healthcare facilities.1,2 Of the risk factors contributing to CKD, diabetes and hypertension are the most common.3 CKD can progress to end-stage renal disease. The awareness of CKD is predominantly fostered by the recognition that it is an important risk predictor for coronary events and cardiovascular mortality.4,5 Timely intervention that is directed at cardiovascular risk factors can decrease the loss of renal function and the incidence of cardiovascular disease.6–8 Guidelines provide recommendations for treatment of CKD;9,10 however, treatment targets are often not met.11–13
There is a significant evidence gap in how to best organise the care for patients with CKD.14 A multidisciplinary approach has been proposed;15 shared care between primary and secondary care has been successful in the treatment of other chronic conditions.16 Observational studies on shared care for patients with CKD show promising results,17 but the effectiveness of shared care for patients with CKD has not yet been proved in randomised trials.18
This study developed a shared care model for patients with CKD in primary care in which the nurse practitioner played a central role and a nephrologist and a nephrology nurse could be consulted. In a cluster randomised controlled trial the study tested whether the model led to improved quality of care in patients with CKD and diabetes or hypertension. Lowering of blood pressure was the primary outcome.
METHOD
Setting
The study involved nine general practices (54 231 patients) that are part of the Academic Practice-based Research Network of Radboud University Nijmegen Medical Centre in The Netherlands.19 Usual care for patients with diabetes and hypertension in these practices is given in a structured setting with the help of nurse practitioners. Patients with diabetes or hypertension are seen every 3 months. Once a year an extensive control including renal function monitoring is performed according to the national evidence-based practice guidelines.20,21 Blood pressure measurements are performed according to a protocol, which requires a rest period and noting the mean of two measurements.
Adult patients (aged >18 years) who were treated for hypertension or type 2 diabetes mellitus by their GP and had an estimated glomerular filtration rate (eGFR) measurement of <60ml/min/1.73m2 were included. GPs were informed which patients lacked the annual information on renal function, so they could have them tested and include them if an eGFR of <60ml/min/1.73m2 was newly found. Exclusion criteria were:
serious medical or psychiatric conditions;
drug or alcohol abuse;
specialist CKD care in the last year;
inability to understand Dutch (including cognitive disorders); and
participation in another intervention trial.
How this fits in
Chronic kidney disease (CKD) is highly prevalent in patients with diabetes or hypertension in the primary care setting and leads to a large rise in cardiovascular risk. Although CKD guidelines are clear, implementation in primary care is poor, partly because of lack of confidence from GPs and partly because of lack of time. A shared care model between the GP, nurse practitioner, and a nephrology team is an effective way to reduce blood pressure in patients presenting to primary care who have CKD and diabetes or hypertension. Given the societal burden of CKD, this model may prove to be cost effective in lowering cardiovascular morbidity and mortality.
Eligible patients were invited to take part in the study when they visited the practice for a regular consultation until a minimum of 20 and a maximum of 28 patients per practice were recruited. Patients were included if they had given written informed consent and if a second eGFR-measurement was still <60ml/min/1.73m2.
Randomisation was carried out at the general practice level because the intervention involved changes to the practice organisation. Practices were stratified by the mean blood pressure of all eligible patients and then randomly allocated to intervention or control group. In the control practices, patients were identified and included at the start of the study. To avoid bias by study inclusion, patients were asked to give written informed consent only at the time of the final measurement at the end of the trial; their GPs and nurse practitioners were informed of the patient’s study inclusion or exclusion status at that time.
To show a clinically relevant difference in the decline of blood pressure of 5 mmHg (standard deviation of blood pressure difference 10 mmHg, α = 0.05, β = 0.20, and intracluster correlation coefficient (ICC) 0.03) the study was powered to contain nine practices with 25 patients per practice.
Intervention
The multifaceted intervention consisted of the training of professionals, structured care by nurse practitioners, and the opportunity to ask advice from a nephrology team. In spring 2008, nurse practitioners and GPs of intervention practices were trained by a nephrology team. Blood pressure measurement and treatment, proteinuria, cholesterol lowering, blood–glucose management, and lifestyle advice were the main issues. A protocol, based on the Kidney Disease Outcomes Quality Initiative (KDOQI) guideline, was provided with treatment goals and treatment advice.10
During the following intervention year, nurse practitioners received two extra training sessions on treatment of hyperparathyroidism and anaemia. The nurse practitioner saw patients every 3 months for a 20-minute consultation, in which blood pressure treatment was the main aim. Patients and nurse practitioners decided together which other treatment goals were to be prioritised. GPs supervised the consultation afterwards. GPs and nurse practitioners could, if necessary, consult a nephrology team in a protected digital environment.22
Outcome
Lowering of blood pressure was the primary outcome and was ascertained according to the difference between the usual blood pressure measurement at baseline and the study blood pressure measurement after 1 year. At the end of the trial, blood pressure and the number of patients meeting the blood pressure target (130/80 mmHg) were compared between the control and intervention groups. Other quality-of-care variables were kidney-disease measures and the number of patients that reached the treatment goals. Additionally, functional status and the use of angiotensin system inhibitors and lipid-modifying agents were measured. The number of consultations with the nephrologist and the number of referrals were described.
At baseline, the nurse practitioner collected data in the intervention group. After 1 year the same measurements were performed in patients in both intervention and control practices. Study blood pressure was measured with an oscillometric device (Stabil-O-Graph). After a 5-minute rest, three measurements were taken with the patient in a sitting position; the mean of the last two measurements was used for analysis. In patients with atrial fibrillation, blood pressure was measured manually with a sphygmomanometer. The latest noted usual blood pressure measurement before inclusion was used as the baseline value for blood pressure.
Clinical chemical analyses were performed by the laboratory of the Canisius Wilhelmina Hospital in Nijmegen, The Netherlands. Creatinine, calcium, phosphate, and parathyroid hormone (PTH) were measured by a Roche modular analyser. Blood samples for PTH analysis were put on ice immediately after blood sampling and, where possible, analysed within 2 hours. If this was not possible, samples were centrifuged and saved in a refrigerator until analysis. Serum creatinine was measured enzymatically with calibration traceable to the international standard (isotope dilution mass spectrometry [IDMS]) reference material. The eGFR was calculated from the Modification of Diet in Renal Disease (MDRD) equation.23 Calcium levels were corrected for albumin levels. Haemoglobin was measured on a Sysmex XE-2100 instrument. Albuminuria was defined as an albumin:creatinine ratio of ≥2.5mg/mmol or ≥3.5mg/mmol in male or female patients respectively.
COOP-WONCA charts were used to obtain additional information about the patient’s functional capacity.24
Statistical analysis
Descriptive analyses were used to describe the characteristics of the patients in both groups. As a result of the hierarchical structure of the study (patient nested within practices), multilevel analyses were performed that took account of the variability associated with each level of nesting.
A random intercept model with other variables that were fixed was used. For dichotomous variables, a multilevel logistic model was performed. Blood pressure change between intervention and control group was analysed by analysis of covariance with the follow-up blood pressure measurement as an outcome and the baseline blood pressure measurement (last noted blood pressure in the patient file) as a covariate. As the number of practices was relatively small, a cluster-level analysis was performed by analysing summary measures from each cluster as a sensitivity analysis.25
The ICC was calculated from pre-intervention blood pressure data from both intervention and control group.
SAS Proprietary Software 9.2 was used for all analyses and multilevel analyses were performed with PROC MIXED for continuous outcomes and PROC GLIMMIX for dichotomous variables.
RESULTS
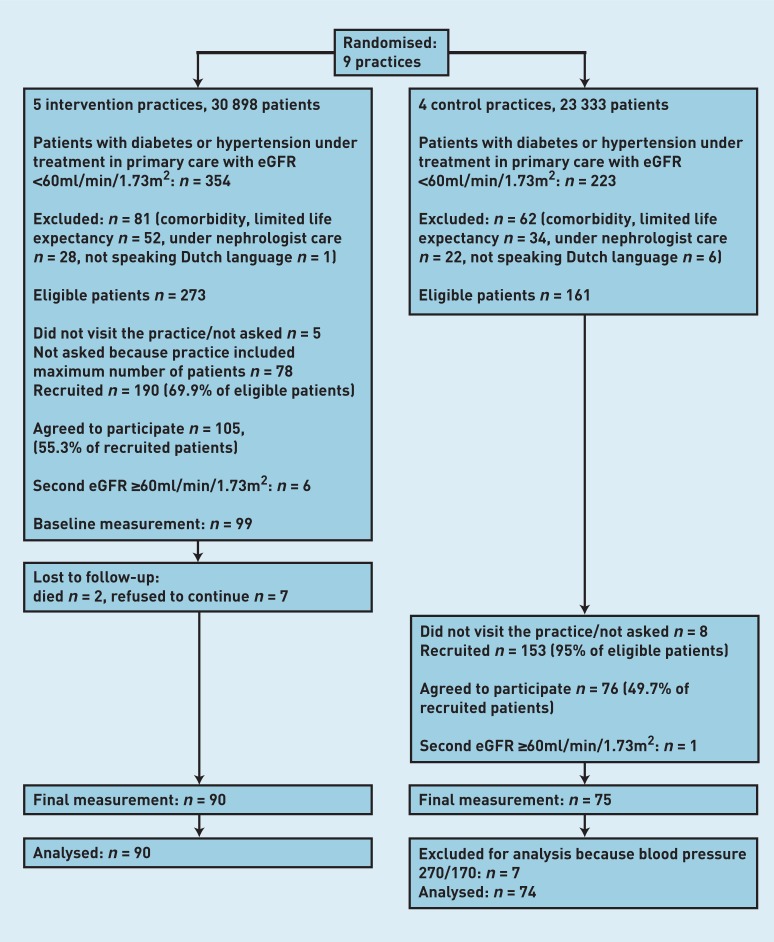
Figure 1 shows a flowchart of participating practices and patients. Five intervention practices included 16, 12, 20, 23, and 28 patients respectively; four control practices included 6, 23, 19, and 27 patients. Nine patients from four practices in the intervention group did not finish the trial: two died (of lung carcinoma and heart failure), three stopped because their general condition worsened, and four no longer wished to come for extra control visits. One patient in the control group was excluded from the analysis because of extreme blood pressure values that were considered to be invalid measurements (270/170 mmHg). Usual systolic blood pressure at baseline did not differ between the intervention and control groups, whereas diastolic blood pressure was lower in the intervention group (Table 1).
Figure 1. Flow chart of participating practices and patients.
Table 1.
Patient characteristics at baseline, derived from the electronic patient record
| Characteristic | Control group, na (%) | Intervention group na (%) |
|---|---|---|
| Sex, male | 39 (52.7) | 34 (37.8) |
| Age, years | 72.4 (8.2) | 73.9 (8.0) |
| Creatinine, μmol/l | 117.6 (21.2) | 109.0 (24.9) |
| eGFR, ml/min/1.73m2 | 50.0 (6.7) | 49.1 (7.9) |
| Systolic usual office blood pressure, mmHgb | 142.5(15.1) | 142.7 (17.6) |
| Diastolic usual office blood pressure, mmHgb | 80.4 (8.2) | 74.9 (9.2) |
| Diabetes, % | 26 | 34 |
| Hypertension, % | 69 | 81 |
| Myocardial infarction, % | 4 | 12 |
| Heart failure, % | 3 | 5 |
| Transient ischaemic attack, % | 6 | 6 |
| Cerebrovascular accident, % | 6 | 10 |
| Peripheral artery disease, % | 5 | 13 |
Comorbidity is based on ICPC (International Classification of Primary Care) coding in the problem list in the electronic patient record.
Values are given as mean and standard deviation, or number (percentage).
In one patient in the control group, a usual blood pressure at baseline could not be found in the electronic patient file. eGFR = estimated glomerular filtration rate. Usual office blood pressure is the blood pressure as noted in the electronic patient record.
The decrease in systolic blood pressure in the intervention group was 8.1 mmHg (95% confidence interval [CI] = 4.8 to 11.3) compared with 0.2 mmHg (95% CI = −3.8 to 3.3) in the control group (Table 2) . The decrease in diastolic blood pressure did not differ between intervention and control group. The ICC was 0.11 for systolic blood pressure and 0.15 for diastolic blood pressure. Blood pressure after 1 year was lower in the intervention group than in the control group: systolic blood pressure was 8.2 mmHg (95% CI = 3.6 to 12.9) lower, diastolic blood pressure was 4.7 mmHg (95% CI = 1.1 to 8.4) lower (Appendix 1) The number of patients that reached the treatment goal for systolic blood pressure in the intervention group (44.4%) was higher than in the control group (21.6%) (odds ratio [OR] 2.9 [95% CI = 1.4 to 5.8]; P = 0.003). For diastolic blood pressure these percentages were 71.1% and 50.0% respectively (OR 2.5; 95% CI = 1.3 to 4.7; P = 0.007) (Table 2). More patients in the intervention group received renin-angiotensin system inhibitors, lipid-lowering drugs, and vitamin D than patients in the control group. Laboratory values did not differ between the intervention and control groups, with the exception of PTH, which was lower in the intervention group (Table 2).
Table 2.
Outcome measures in the intervention and control group at t = 1 year
| Variable | Treatment goal | Control group | Intervention group | Difference for continuous variablesa between intervention and control group (95% CI) | P-value |
|---|---|---|---|---|---|
| Sample, n | 74 | 90 | |||
|
| |||||
| Systolic study blood pressure | <130mmHg | 142.9 (16.8) | 134.7 (15.7) | −8.2 (−12.9 to −3.6) | <0.001 |
| Treatment goal reached (n, %) | 16 (21.6) | 40 (44.4) | 2.9 (1.4 to 5.8)a | 0.003 | |
| Δ systolic blood pressure t = 1 year minus t = 0 (95% CI) | 0.2 (−3.6 to 3.8) | −8.1 (−11.3 to −4.8) | |||
|
| |||||
| Diastolic study blood pressure | <80mmHg | 80.9 (11.2) | 73.8 (9.6) | −4.7 (−8.4 to −1.1) | 0.010 |
| Treatment goal reached (n, %) | 37 (50.0) | 64 (71.1) | 2.5 (1.3 to 4.7)a | 0.007 | |
| Δ diastolic blood pressure t = 1 year minus t = 0 (95% CI) | 0.5 (−1.80 to 2.9), P = 0.64 | −1.1 (−3.2 to 1.0), P = 0.30 | |||
|
| |||||
| Weight, kg | n/a | 80.8 (15.0) | 79.8 (14.9) | −1.0 (−5.7 to 3.6) | 0.720 |
| Waist circumference, cm | <80 female, <94 male | 100.7 (14.9) | 101.2 (12.9) | 0.5 (−3.8 to 4.8) | 0.230 |
| Treatment goal reached, n (%) | 8 (10.8) | 5 (5.6) | 0.5 (0.2 to 1.6)a | 0.220 | |
| Creatinine, μmol/l | n/a | 114.2 (24.6) | 110.9 (25.4) | −3.3 (−11.1 to 4.4) | 0.640 |
| eGFR MDRD, ml/min/1.73m2 | n/a | 49.4 (8.0) | 48.6 (8.7) | −0.7 (−3.3 to 1.9) | 0.830 |
| Fasting glucose, mmol/l | <7 | 6.5 (1.4) | 6.4 (1.4) | −0.1 (−0.6 to 0.3) | 0.500 |
| Treatment goal reached, n (%) | 58 (78.4) | 68 (75.6) | 0.9 (0.4 to 1.8)a | 0.670 | |
| HbA1c, % | <7 | 6.4 (0.9) | 6.4 (0.8) | 0.01 (−0.2 to 0.3) | 0.880 |
| Treatment goal reached, n (%) | 63 (85.1) | 69 (76.7) | 0.6(0.3 to 1.3)a | 0.180 | |
| Total cholesterol, mmol/l | n/a | 4.8 (1.2) | 4.6 (1.1) | −0.2 (−0.5 to 0.2) | 0.320 |
| HDL cholesterol, mmol/l | n/a | 1.4 (0.4) | 1.4 (0.4) | −0.04 (−0.2 to 0.1) | 0.850 |
| Total cholesterol/HDL | n/a | 3.7 (1.1) | 3.6 (1.2) | −0.04 (−0.4 to 0.3) | 0.550 |
| LDL cholesterol, mmol/l | <2.5 | 2.6 (1.1) | 2.5 (0.9) | −0.1 (−0.4 to 0.2) | 0.570 |
| Treatment goal reached, n (%) | 34 (46.0) | 47 (52.2) | 1.3 (0.7 to 2.4)a | 0.430 | |
| Triglycerides, mmol/l | n/a | 1.8 (0.8) | 1.7 (0.9) | −0.1 (−0.3 to 0.2) | 0.320 |
| Haemoglobin, mmol/l | >6.8 | 8.9 (0.8) | 8.7 (0.8) | −0.2 (−0.4 to 0.1) | 0.360 |
| Treatment goal reached, n (%) | 74 (100) | 90 (100) | – | – | |
| MCV, fl | n/a | 90.7 (4.0) | 91.7 (4.0)b | 0.9 (−0.3 to 2.2) | 0.060 |
| Serum albumin, g/L | 35–50 | 43.7 (2.5) | 43.3 (2.3) | −0.5 (−1.2 to 0.3) | 0.820 |
| Treatment goal reached, n (%) | 73 (98.7) | 90 (100) | – | 0.270 | |
| Sodium, mmol/l | n/a | 140.8 (2.7) | 140.4 (2.1) | −0.4 (−1.2 to 0.3) | 0.420 |
| Potassium, mmol/l | n/a | 4.38 (0.42) | 4.89 (0.54)c | 0.51 (0.36 to 0.66) | 0.030 |
| Calcium, mmol/l | <2.54 l | 2.32 (0.12) | 2.28 (0.09) | −0.04 (−0.08 to −0.01) | 0.050 |
| Treatment goal reached, n (%) | 71 (96.0) | 90 (100) | – | 0.050 | |
| Phosphate, mmol/l | <1.5 l | 1.01 (0.17) | 1.04 (0.14) | 0.03 (−0.02 to 0.08) | 0.660 |
| Treatment goal reached, n (%) | 74 (100) | 90 (100) | – | – | |
| Parathyroid hormone, pmol/l | <7.7 ; if eGFR 15–30:<12 | 8.2 (3.7)b | 6.1(2.6)d | −2.1 (−3.2 to −1.1) | 0.020 |
| Treatment goal reached, n (%) | 43 (59.7) | 62(87.3) | 3.2 (1.5 to 6.8)a | 0.002 | |
| Urine albumin/creatinine, mg/mmol | <25 male, <35 female | 6.8 (33.4)c | 3.9 (6.6)c | –2.9(–10.8 to 5.0) | 0.560 |
| Treatment goal reached, n (%) | 72 (98.6) | 82 (97.6) | 0.6 (0.01 to 7.0)a | 0.680 | |
| Body mass index, kg/m2 | 25 | 28.4 (4.6) | 28.9 (4.7) | 0.4 (−1.0 to 1.9) | 0.680 |
| Treatment goal reached, n (%) | 13 (17.6) | 15 (16.7) | 0.9 (0.4 to 2.1)a | 0.880 | |
| Positive smoking status, n (%) | Not smoking | 10 (13.5) | 11 (12.2) | – | 0.810 |
|
| |||||
| WONCA functional health status | |||||
| Overall health | n/a | 3.0 (0.8)c | 3.0 (0.8)c | −0.04 (−0.28 to 0.22) | 0.420 |
| Daily activities | n/a | 1.7 (1.0)c | 2.1 (1.2)c | 0.34 (0.03 to 0.73) | 0.270 |
| Feelings | n/a | 1.7 (0.9)b | 1.8 (1.0)c | 0.09 (0.98 to 0.16) | 0.970 |
| Physical fitness | n/a | 3.1 (0.8) | 3.4 (1.0)c | 0.22 (−0.07 to 0.52) | 0.150 |
| Social activities | n/a | 1.2 (0.7) | 1.6 (1.0)c | 0.33 (0.06 to 0.60) | 0.120 |
| Change in health | n/a | 3.0 (0.5) | 2.8 (0.6)c | −0.17 (−0.34 to −0.003) | 0.060 |
|
| |||||
| Medication | n/a | 47 (63.5) | 73 (81.1) | n/a | 0.010 |
| C09 agents acting on the RAAS system | n/a | n/a | n/a | n/a | n/a |
| Lipid modifying agents | n/a | 38 (51.4) | 66 (73.3) | n/a | 0.004 |
| Vitamin D | n/a | 1 (0.6) | 14 (15.5) | n/a | 0.002 |
Values are given as mean and standard deviation (or standard deviation of difference), or number (percentage).
Odds ratio for discrete variables
Two missing values.
One missing value.
Three missing values. eGFR = estimated glomerular filtration rate. HbA1c = glycated haemoglobin. HDL = high-density lipoprotein. LDL = low-density lipoprotein. MCV = Mean Corpuscular Volume. MDRD = Modification of Diet in Renal Disease. RAAS = renin-angiotensin-aldosterone system. SD = standard deviation. WONCA = World Organisation of National Colleges, Academies and Academic Associations of General Practitioners/Family Physicians.
During the intervention, cholesterol and low-density lipoprotein (LDL) levels decreased in the intervention group, parallel with an increase in the use of lipid-lowering drugs (Appendix 2).
In 31 patients in the intervention group, 50 consultations were performed between the GP and nephrologist; none of these resulted in a referral. In the intervention group, two patients were referred to a nephrologist; in the control group, one patient was referred.
DISCUSSION
Summary
The shared care model for patients with CKD and diabetes or hypertension leads to a significant and clinically relevant systolic lowering of blood pressure in the intervention group compared with the control group; in addition, there was also a better achievement of blood pressure targets along with increased use of renin-angiotensin system inhibitors. The intervention also led to lower PTH levels, along with an increased use of vitamin D, as well as leading to an increased use of lipid-lowering drugs. Although LDL cholesterol decreased in the intervention group, LDL levels at the end of the study did not differ between the intervention and control groups.
It is promising that blood pressure targets were better met in the shared care practices, because a lower blood pressure is associated with better patient outcome.6 Hypertension management is generally recognised as a primary care task, but blood pressure management in patients with CKD in primary care is not as effective as it is in nephrology.26 Underlying factors are that the GP’s confidence in treating CKD is lower than in treating diabetes and hypertension, and that blood pressure targets in CKD are often regarded with scepticism.27,28 A discussion is ongoing with regard to optimal blood pressure targets.29 Systolic blood pressure of <120 mmHg is associated with stroke and diastolic blood pressure of <60 mmHg is associated with increased mortality in older people who are frail.30,31 Nephrologists could be of help in the titration of antihypertensive agents.
Albuminuria did not change during the study. This was due to the fact that albuminuria treatment goals had been met by a large number of patients at baseline. In a sub-analysis no albuminuria differences between patients with and without diabetes were found.
Strengths and limitations
A strength of the study is that it used a cluster randomised trial design. Usual care for patients with diabetes and hypertension in the practices was already well organised, which makes the additive value of the shared care model robust. Baseline blood pressure values were at a relatively low level; in practices with less-favourable baseline blood pressure levels, it may be possible to see even more improvement.
Potential bias in the usual-care group was reduced by informing these patients, GPs, and nurse practitioners about the study at the end of the trial.
The setting in research practices enabled retrospective collection of usual blood pressure measurements to serve as baseline measurements for the control group. A further strength is that, before entry, participants had had two consecutive measurements of eGFR <60ml/min/1.73m2 to confirm a diagnosis of CKD.
Several limitations need to be mentioned. The first is a potential selection bias: although identified at the beginning of the trial, patients in the control group were asked for their informed consent 1 year after randomisation of their practice took place.
As a second limitation, it should be mentioned that a pragmatic recruitment procedure was followed: when the maximum number of patients in one cluster was reached, the inclusion in that practice was stopped. This may have caused a selection of patients who were relatively healthy to adhere to the control visits. On the other hand, not all practices reached the required minimum of 20 patients.
A third limitation is that it was necessary to rely on usual blood pressure measurements to serve as baseline values. It is well known that usual blood pressure measurements lead to higher results than blood pressure measurements in a study setting.32 The fact that the usual blood pressure measurement in the control group did not differ from the study blood pressure levels at the end of the study reduces concerns about comparability of usual and study blood pressure measurements in this trial.
A further point to be noted concerns generalisability. The population in this study was mainly white, so the results are not representative of a population with a greater proportion of patients of other ethnicities or racial groups, who may have different blood pressure outcomes.
Comparison with existing literature
The effect of structured care by nurses was assessed in an observational study by Richards et al.33 Patients with CKD were enrolled in a disease management programme. Blood pressure decreased with 9/5 mmHg, but only in patients without diabetes or proteinuria. In secondary care, several studies have been performed on the nurses’ role in managing patients with CKD, with varying success. A study on older patients referred to a multidisciplinary care clinic with a nephrologist and a specialised nurse showed a 50% reduction of the risk for all-cause mortality in an observational study.34 In a comparison between additional intensive nurse-practitioner support and nephrologist care, the blood pressure decrease in the intervention group was 3/2 mmHg more (P<0.001) than in the control group.35 However, in a randomised trial in which patients were randomly assigned to a nurse-coordinated team in secondary care, or to usual care in general practice, no effect on cardiovascular risk-factor control or on clinical end points was found.36
The opportunity to ask advice from a nephrologist has been studied in a shared care system in the UK.17 Patients were treated in primary care that was sustained by continuous feedback from nephrologists on the laboratory and blood pressure results. Blood pressure decreased and the prescribing of renin-angiotensin system inhibitors increased.
In summary, the existing literature endorses this study’s findings that structuring care for patients with CKD is beneficial in reducing blood pressure. However, study designs were mainly observational with, consequentially, low levels of evidence. Cluster randomised trials like this are scarce; the Quality Improvement in CKD study (a cluster randomised trial to compare quality-improvement interventions to lower systolic blood pressure in CKD in primary care) showed that audit-based education led to blood pressure lowering of 2.4 mmHg.37
Implications for practice
It is promising that an intervention of shared care showed lowering of systolic blood pressure during a 1-year intervention, even in practices that already had a well-structured care for patients with diabetes or hypertension. Statements on more-relevant endpoints, such as cardiovascular events and hospital admissions, would need larger and longer cluster randomised trials.
Future studies should provide information on cost effectiveness. CKD has a high financial burden. This model of care aims to provide optimal care at the cheapest level possible, and may be cost-effective in lowering cardiovascular morbidity and mortality but this requires future study.
Acknowledgments
Marjan Schoneveld and Wouter Koop of the laboratory of the Canisius Wilhelmina Hospital, Nijmegen, were very helpful in organising the laboratory tests. We thank the participating patients, the GPs, and the assistants of the NMP (Nijmegen Monitoring Project) practices. Furthermore, we thank Lea Peters-van Gemert for the practical aspects of the trial and Reinier Akkermans for his statistical advice.
Appendix 1. Overview of blood pressure measurement results
| Parameter | Baseline | t= 1 year | ||
|---|---|---|---|---|
|
| ||||
| Control | Intervention | Control | Intervention | |
| n | 73 | 90 | 74 | 90 |
| Systolic usual BP, mmHg (%) | 142.5 (15.1) | 142.7 (17.6) | – | – |
| Diastolic usual BP, mmHg (%) | 80.4 (8.2) | 74.9 (9.2) | – | – |
| Systolic study BP, mmHg | NA | 137.1 (16.5) | 142.9 (16.8) | 134.7 (15.7) |
| Diastolic study BP, mmHg | NA | 75.4 (10.7) | 80.9 (11.2) | 73.8 (9.6) |
BP = blood pressure.
Appendix 2. Changes in outcome measures (other than blood pressure) in intervention group between baseline and t = 1 year (n = 90)
| Variable | Baseline | t=1 year | Difference (95% CI) between baseline andt= 1 year | P-value |
|---|---|---|---|---|
| Weight, kg | 79.5 (14.3) | 79.8 (14.9) | 0.3 (−0.4 to 1.1) | 0.340 |
| Waist circumference, cm | 101.3 (12.7) | 101.2 (12.9) | −0.1 (−1.1 to 0.9) | 0.790 |
| Creatinine, μmol/l | 109.0 (24.9) | 110.9 (25.4) | 1.8 (−2.0 to 5.6) | 0.340 |
| eGFR MDRD, ml/min/1.73m2 | 49.1 (7.9) | 48.6 (8.7) | −0.5 (−1.9 to 0.9) | 0.470 |
| Fasting glucose, mmol/l | 6.1 (1.5) | 6.4 (1.4) | 0.3 (0.07 to 0.53) | 0.010 |
| HbA1c, % | 6.3 (0.7) | 6.4 (0.8) | 0.07 (−0.01 to 0.14) | 0.080 |
| Total cholesterol, mmol/l | 4.9 (1.1) | 4.6 (1.1) | −0.3 (−0.5 to −0.1) | <0.001 |
| HDL cholesterol, mmol/l | 1.3 (0.4) | 1.4 (0.4) | 0.03 (−0.04 to 0.11) | 0.400 |
| Total cholesterol/HDL | 4.0 (1.2) | 3.6 (1.2) | −0.36 (−0.55 to −0.17) | <0.001 |
| LDL cholesterol, mmol/l | 2.9 (1.0) | 2.5 (0.9) | −0.35 (−0.52 to −0.19) | <0.001 |
| Triglycerides, mmol/l | 1.7 (0.8) | 1.7 (0.9) | −0.01 (−0.16 to 0.14) | 0.880 |
| Haemoglobin, mmol/l | 8.8 (1.0) | 8.7 (0.8) | −0.09 (−0.13 to 0.04) | 0.170 |
| MCV, fl | 91.8 (3.7)a | 91.7 (4.0)b | −0.01 (−0.7 to 0.7) | 0.970 |
| Serum albumin (g/L) | 43.3 (2.3)c | 43.3 (2.3) | 0.02 (−0.4 to 0.4) | 0.910 |
| Sodium, mmol/l | 140.1 (2.2) | 140.4 (2.1) | 0.33 (−0.12 to 0.79) | 0.150 |
| Potassium, mmol/l | 4.7 (0.6)d | 4.89 (0.54)d | 0.14 (0.02 to 0.26) | 0.020 |
| Calcium, mmol/l | 2.36 (0.09) | 2.28 (0.09) | −0.08 (−0.10 to −0.06) | <0.001 |
| Phosphate, mmol/l | 1.15 (0.15) | 1.04 (0.14) | −0.10 (−0.14 to −0.07) | <0.001 |
| Parathyroid hormone, pmol/l | 6.2 (3.5)e | 6.1 (2.6)e | −0.36 (−0.94 to 0.22) | 0.220 |
| Urine albumin/creatinine, mg/mmol | 3.0 (5.7)a | 3.9 (6.6)a | 0.78 (−0.20 to 1.76) | 0.120 |
| Body mass index, kg/m2 | 28.9 (4.6) | 28.9 (4.7) | −0.04 (−0.30 to 0.21) | 0.740 |
| Smoking status, number of patients smoking (%) | 13(14.4) | 11(12.2) | n/a | |
|
| ||||
| WONCA functional health status: | ||||
| Overall health | 2.9(0.9) | 3.0(0.8)c | 0.10 (−0.10 to 0.31) | 0.320 |
| Daily activities | 1.8 (1.1) | 2.1(1.2)c | 0.21 (−0.02 to 0.44) | 0.070 |
| Feelings | 1.8 (1.1) | 1.8(1.0)c | −0.01 (−0.24 to 0.21) | 0.910 |
| Physical fitness | 3.5 (0.9) | 3.4(1.0)c | −0.14 (−0.33 to 0.06) | 0.170 |
| Social activities | 1.5 (0.9) | 1.6(1.0)c | 0.01 (−0.20 to 0.22) | 0.910 |
| Change in health | 2.9 (0.6) | 2.8(0.6)c | −0.08 (−0.25 to 0.09) | 0.350 |
| Agents acting on the RAAS system (%) | 66 (73.3%) | 73 (81.1%) | 0.020 | |
| Lipid modifying agents (%) | 53 (58.9%) | 66(73.3%) | <0.001 | |
| Vitamin D (%) | 1(1.1%) | 14 (15.5%) | <0.001 | |
Values are given as mean and standard deviation or number (percentage).
One missing value.
Two missing values.
Three missing values. eGFR = estimated glomerular filtration rate. HbA1c = glycated haemoglobin. HDL = high-density lipoprotein. LDL = low-density lipoprotein. MCV = Mean Corpuscular Volume. MDRD = Modification of Diet in Renal Disease. RAAS = renin-angiotensin-aldosteron system. WONCA = World Organisation of National Colleges, Academies and Academic Associations of General Practitioners/Family Physicians.
Funding
The Dutch Kidney Foundation funded this study: SHARING-study (SHARed care for patients with chronic kidney disease In Nephrology and General practice) (PV 35).
Ethical approval
This study was performed according to the Code of Conduct for Health Research which has been approved by the Data Protection Authorities for conformity with the applicable Dutch privacy legislation and was in accordance with the Helsinki Declaration of 1975, as revised in 1983. Ethical approval was not required according to the accredited Medical Research Ethics Committee Arnhem/Nijmegen (ABR NL16590.091.07). Trial registration: Sharing study: SHARed care for patients In Nephrology And General practice; Netherlands Trial Registration TC 1140 http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1140.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Jack Wetzels has received grant support from Amgen, Genzyme, and Pfizer on occasions unrelated to this study. The Department of Primary and Community Care at Radboud University Nijmegen Medical Centre received a grant from Amgen for the contact study (implementation of telenephrology).
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.de Lusignan S, Tomson C, Harris K, et al. Creatinine fluctuation has a greater effect than the formula to estimate glomerular filtration rate on the prevalence of chronic kidney disease. Nephron Clinical Practice. 2011;117(3):c213–c24. doi: 10.1159/000320341. [DOI] [PubMed] [Google Scholar]
- 3.McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. Journal of the American Society of Nephrology: JASN. 2003;14(7 Suppl 2):S65–S70. doi: 10.1097/01.asn.0000070147.10399.9e. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012 doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 7.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142(5):342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Crowe E, Halpin D, Stevens P. Early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337:a1530. doi: 10.1136/bmj.a1530.:a1530. [DOI] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. Guidelines and Commentaries 2013. http://www.kidney.org/professionals/kdoqi/guidelines_commentaries.cfm (accessed 12 Nov 2013).
- 11.Levin A. The need for optimal and coordinated management of CKD. Kidney Int Suppl. 2005(99):S7–S10. doi: 10.1111/j.1523-1755.2005.09902.x. [DOI] [PubMed] [Google Scholar]
- 12.Lenz O, Mekala DP, Patel DV, et al. Barriers to successful care for chronic kidney disease. BMC Nephrol. 2005;6:11. doi: 10.1186/1471-2369-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens PE, O’Donoghue DJ, de LS, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72(1):92–99. doi: 10.1038/sj.ki.5002273. [DOI] [PubMed] [Google Scholar]
- 14.Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14(21):1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- 15.Dean J. Organising care for people with diabetes and renal disease. Journal of Renal Care. 2012;38(Suppl 1):23–29. doi: 10.1111/j.1755-6686.2012.00272.x. [DOI] [PubMed] [Google Scholar]
- 16.van Hateren KJ, Drion I, Kleefstra N, et al. A prospective observational study of quality of diabetes care in a shared care setting: trends and age differences (ZODIAC-19) BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2012-001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones C, Roderick P, Harris S, Rogerson M. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47(1):103–114. doi: 10.1053/j.ajkd.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Ronksley PE, Hemmelgarn BR. Optimizing Care for Patients With CKD. Am J Kidney Dis. 2012;60(1):133–138. doi: 10.1053/j.ajkd.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 19.van Weel C. Longitudinal research and data collection in primary care. Ann Fam Med. 2005;3(Suppl 1):S46–S51. doi: 10.1370/afm.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouma M, Rutten GE, de Grauw WJ, et al. Samenvatting van de standaard ‘Diabetes mellitus type 2’ (tweede herziening) van het Nederlands Huisartsen Genootschap 2006. Nederlands tijdschrift voor geneeskunde 2006. 150(41):2251–2256. Epub 2006/11/02. [Summary of the practice guideline ‘Diabetes mellitus type 2’ (second revision) from the Dutch College of General Practitioners, 2006]. [PubMed] [Google Scholar]
- 21.Smulders YM, Burgers JS, Scheltens T, et al. Clinical practice guideline for cardiovascular risk management in the Netherlands. The Netherlands Journal of Medicine. 2008;66(4):169–174. [PubMed] [Google Scholar]
- 22.Scherpbier ND, de Grauw WJ, Wetzels JF, Vervoort GM. Acute nierinsufficientie bij combinatie RAAS-remmer en dehydratie. Nederlands tijdschrift voor geneeskunde. 2010;154:A1548. [Acute renal failure due to RAAS-inhibitors combined with dehydration] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 24.Van Weel C. Functional status in primary care: COOP/WONCA charts. Disabil Rehabil. 1993;15(2):96–101. doi: 10.3109/09638289309165878. [DOI] [PubMed] [Google Scholar]
- 25.Eldridge S, Kerry S. A practical guide to cluster randomised trials in health services research. Chichester: John Wiley & Sons; 2012. [Google Scholar]
- 26.Minutolo R, De Nicola L, Zamboli P, et al. Management of hypertension in patients with CKD: differences between primary and tertiary care settings. Am J Kidney Dis. 2005;46(1):18–25. doi: 10.1053/j.ajkd.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Tahir MA, Dmitrieva O, de Lusignan S, et al. Confidence and quality in managing CKD compared with other cardiovascular diseases and diabetes mellitus: a linked study of questionnaire and routine primary care data. BMC Fam Pract. 2011;12:83. doi: 10.1186/1471-2296-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crinson I, Gallagher H, Thomas N, de Lusignan S. How ready is general practice to improve quality in chronic kidney disease? A diagnostic analysis. BrJ Gen Pract. 2010;60(575):403–409. doi: 10.3399/bjgp10X502100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen DL, Townsend RR. Hypertension and kidney disease: what do the data really show? Curr hypertens Rep. 2012;14(5):462–467. doi: 10.1007/s11906-012-0285-4. [DOI] [PubMed] [Google Scholar]
- 30.Weiner DE, Tighiouart H, Levey AS, et al. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol. 2007;18(3):960–966. doi: 10.1681/ASN.2006080858. [DOI] [PubMed] [Google Scholar]
- 31.Protogerou AD, Safar ME, Iaria P, et al. Diastolic blood pressure and mortality in the elderly with cardiovascular disease. Hypertension. 2007;50(1):172–180. doi: 10.1161/HYPERTENSIONAHA.107.089797. [DOI] [PubMed] [Google Scholar]
- 32.Campbell NR, Culleton BW, McKay DW. Misclassification of blood pressure by usual measurement in ambulatory physician practices. Am J Hypertens. 2005;18(12 Pt 1):1522–1527. doi: 10.1016/j.amjhyper.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Richards N, Harris K, Whitfield M, et al. Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant. 2008;23(2):549–555. doi: 10.1093/ndt/gfm857. [DOI] [PubMed] [Google Scholar]
- 34.Hemmelgarn BR, Manns BJ, Zhang J, et al. Association between multidisciplinary care and survival for elderly patients with chronic kidney disease. J Am Soc Nephrol. 2007;18(3):993–999. doi: 10.1681/ASN.2006080860. [DOI] [PubMed] [Google Scholar]
- 35.van Zuilen AD, Bots ML, Dulger A, et al. Multifactorial intervention with nurse practitioners does not change cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2012;82(6):710–717. doi: 10.1038/ki.2012.137. [DOI] [PubMed] [Google Scholar]
- 36.Barrett BJ, Garg AX, Goeree R, et al. A nurse-coordinated model of care versus usual care for stage 3/4 chronic kidney disease in the community: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1241–1247. doi: 10.2215/CJN.07160810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Lusignan S, Gallagher H, Jones S, et al. Audit-based education lowers systolic blood pressure in chronic kidney disease: the Quality Improvement in CKD (QICKD) trial results. Kidney Int. 2013;84(3):609–620. doi: 10.1038/ki.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]