Abstract
Animals that have myelin basic protein (MBP) specific lymphocytes with a Th1(+) phenotype have worse stroke outcome than those that do not. Whether these MBP specific cells contribute to worsened outcome or are merely a consequence of worse outcome is unclear. In these experiments, lymphocytes were obtained from donor animals one month after stroke and transferred to naïve recipient animals at the time of cerebral ischemia. The MBP specific phenotype of donor cells was determined prior to transfer. Animals that received either MBP specific Th1(+) or Th17(+) cells experienced worse neurological outcome, and the degree of impairment correlated with the robustness of MBP specific Th1(+) and Th17(+) responses. These data demonstrate that the immunologic phenotype of antigen specific lymphocytes influences stroke outcome.
Keywords: Stroke, Immune response, Th1, Th17, Lymphocyte
1. Introduction
Following stroke there is a transient breakdown in the blood–brain barrier (BBB) that allows cells of the immune system to gain access to the brain (Jander et al., 1995). In addition, dying neurons and glia release proteins into the systemic circulation allowing cells of the immune system to come into contact with CNS specific proteins in the periphery (Jauch et al., 2006). The possibility for developing an immune response to brain antigens thus exists, and the nature of that response depends upon the microenvironment at the site of antigen presentation. In animal models, we showed that Th1 type immune responses to antigens such as myelin basic protein (MBP) were uncommon after stroke, but that Th1 responses could be enhanced by an inflammatory insult (systemic injection of lipopolysaccharide [LPS]) during the period of BBB compromise (Becker et al., 2005). In these experiments, a Th1(+) response to MBP was associated with worse stroke outcome (Becker et al., 2005; Gee et al., 2008, 2009). Whether the Th1 response to brain antigens contributed to the poor outcome or was merely a marker of poor outcome has not yet been adequately addressed. In the current study, the influence of lymphocytes primed to MBP by stroke (and injection of LPS) on stroke outcome was assessed by adoptive transfer of these cells to naïve animals. Lymphocytes were harvested from donor animals one month after stroke and injected into recipient animals at the time of middle cerebral artery occlusion (MCAO). The immunologic phenotype of the transferred cells (MBP specific Th1[+] or Th17[+]) was determined and their effect on the outcome of recipients assessed.
2. Methods
2.1. Animals
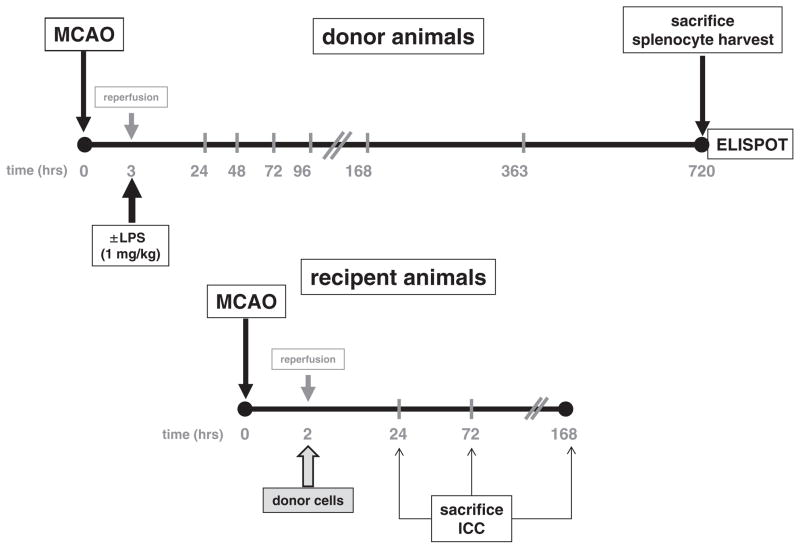
All protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Donor animals (male Lewis rats, 325–375 g, Charles River) underwent 3 h middle MCAO and received either an intraperitoneal (IP) injection of lipopolysaccharide (LPS; 1 mg/kg) or normal saline (1 mL/kg) at the time of reperfusion. The prolonged period of ischemia (3 h) and LPS was used to skew the immune response towards MBP to that of a Th1(+) response (Becker et al., 2005). Sham-operated animals underwent ligation of the common carotid artery without MCAO and were similarly treated with either LPS or saline. Donor animals were sacrificed at 1 month after MCAO — the optimal time point to detect the Th1 immune response to MBP based on our prior studies (Becker et al., 2005). Recipient animals (male Lewis rats, 325–375 g, Charles River) underwent MCAO (2 h) and at the time of reperfusion received an IP injection of splenocytes (1 × 108 cells in 1 mL normal saline) from donor animals. Recipient animals received cells from only 1 donor animal and were sacrificed at 1 day, 3 days or 1 week after MCAO (Fig. 1). Temperature was maintained at normothermia during MCAO, but animals were allowed to spontaneously thermoregulate thereafter. A shorter period of ischemia (2 h) was used in recipient animals so that a detrimental effect of the adoptively transferred cells could be detected.
Fig. 1. Experimental protocol.
Donor animals underwent 3 h of MCAO (or sham surgery) and received either LPS or saline at the time of reperfusion. Animals were sacrificed at 720 h (1 month), splenocytes harvested and cells cultured with MBP for 48 h. A subset of cells was subjected to ELISPOT assay to determine the Th1 and Th17 responses to MBP. Recipient animals underwent 2 h MCAO and donor cells were injected into recipient animals at the time of reperfusion. Neurologic function was assessed at 24 h (1 day), 72 h (3 days) and 168 h (1 week) after MCAO. Subsets of animals were sacrificed at each time point for histological analysis.
2.2. Donor cell preparation
Spleens were removed from donor animals at the time of sacrifice and processed into single cell suspensions. Cells (5 × 106/mL) were cultured in media supplemented with bovine MBP (2 μg/mL, Sigma Aldrich) for 48 h to expand the population of MBP reactive cells. Cells were washed extensively prior to injection. Splenocytes from a subset of animals were labeled with carboxy-fluorescein diacetate succinimidyl ester (CFDA SE) according to manufacturer’s recommendations (Invitrogen) to track their migration after transfer. One donor animal generally provided enough cells for transfer to up to two different recipient animals.
2.3. ELISPOT assays
At the time of sacrifice, ELISPOT assays were done on isolated donor lymphocytes to detect the MBP specific secretion of IFN-γ, IL-17 and TGF-β1 (R&D Systems). Briefly, cells were cultured in media alone or in media supplemented with human MBP (50 μg/mL, Sigma Aldrich) for 48 h in 96 well plates (Multiscreen®-IP, Millipore). Plates were developed using standard protocols (R&D Systems). After plate development, spots were counted with the aid of a semi-automated system (Metamorph®) and expressed as the ratio of the relative increase in the number of MBP specific IFN-γ to the relative increase in the number of MBP specific TGF-β1 secreting cells (Th1 response) or as the ratio of the relative increase in the number of MBP specific IL-17 to the relative increase in the number of MBP specific TGF-β1 secreting cells (Th17 response).
The ratios of the number of MBP specific IFN-γ and IL-17 secreting cells to that of MBP specific TGF-β1 secreting cells (Th1 and Th17 responses, respectively) were used to better reflect the overall immunologic phenotype of the adoptively transferred cells. The most robust Th1 response seen in any naïve/sham-operated animal was 1.16 and the most robust Th17 response seen in any naïve/sham-operated animal was 1.15. Animals were thus considered to be Th1(+) or Th17(+) if they had responses that exceeded these values (1.16 and 1.15, respectively).
2.4. Behavioral outcomes
The neurological score of recipient animals was determined at 3 h, 1 day, 3 days and 1 week after MCAO (Bederson et al., 1986). Recipient animals were trained on the rotarod for 5 sessions over 5 days prior to MCAO. After MCAO, rotarod performance was assessed at 1 day, 3 days and 1 week and expressed as a percent of pre-stroke baseline. Performance of the foot fault test was also assessed at these time points and the results expressed as a percentage of foot faults per total number of steps taken (Lubics et al., 2005).
2.5. Histology
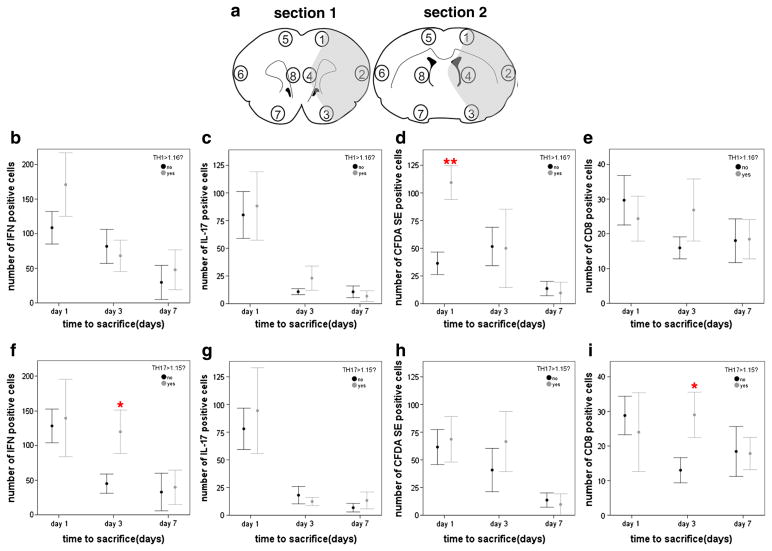
At the time of sacrifice, recipient animals were perfused with saline followed by 4% paraformaldehyde. Brains were removed, post-fixed in 4% paraformaldehyde, cryoprotected in 25% sucrose and frozen at −80 °C until sectioning. Sections (20 μm) were stained for IFN-γ and IL-17 (Abcam). Additional sections were stained for lymphocytes using a CD3 antibody (Abcam) detected with a rhodamine tagged secondary antibody (Jackson labs); CFDA SE labeled lymphocytes were identified with an anti-fluorescein antibody (Invitrogen) and detected with a fluorescein tagged secondary antibody (Abcam). CD8+ cells were detected using a mouse anti-rat CD8 antibody (Abcam). Cells were counted in coronal brain sections correlating to bregma 2.70 mm and −0.26 mm. The number of cells within 6 high power fields (100×) in each of the 8 different brain regions outlined in Fig. 3a was determined.
Fig. 3.
The numbers of cells staining for IFN-γ, IL-17, fluorescein and CD8 were determined in 4 regions within the infarcted hemisphere and in 4 regions within the non-infarcted hemisphere in two different coronal brain sections (a). Cells were counted in 6 adjacent high power fields (100×) within each of the 4 brain regions in the infarcted and non-infarcted hemispheres. The graphs show the total cell counts for regions 1 through 4 in both coronal sections (as there were virtually no identifiable cells in non-infarcted hemisphere). Animals receiving cells from a Th1(+) donor had more CFDA SE positive (or fluorescein+) cells in the infarcted hemisphere at day 1 after MCAO (d), but there were no differences in the number of IFN-γ+ (b), IL-17+ (c) or CD8+ (d) cells. Animals receiving cells from a Th17(+) donor had more IFN-γ+ and CD8+ cells at day 3 after MCAO (f and i). Data are presented as the mean and SEM. *P < 0.05 and **P < 0.01 by t-test.
2.6. Statistics
Non-parametric data are displayed as the mean and (interquartile range [IQR]) and compared using the Mann–Whitney U test. Parametric data are displayed as the mean and standard deviation (unless otherwise indicated) and compared by t-test. Categorical data are compared using the χ2-test statistic. Correlations are performed using Pearson’s r for parametric data and Spearman’s rho (ρ) for non-parametric data. Significance was set at P < 0.05.
3. Results
The absolute change in the numbers of cells secreting IFN-γ, TGF-β1 and IL-17 upon culture with MBP (in comparison to culture in media alone) after stroke is depicted in Fig. 2. The fact that there was a decrease in the number of cells secreting cytokines after culture with MBP in some animals suggests that MBP downregulated secretion of those cytokines. The horizontal line in each panel represents the highest value seen in sham-operated animals. Stroke was associated with an increase in the proportion of animals that had an MBP dependent upregulation in the secretion of IFN-γ and IL-17. The effect of LPS administration at stroke onset was notably associated with an increase in the MBP specific IFN-γ response (Fig. 2a).
Fig. 2.
Change in numbers of cells secreting IFN-γ (a), TGF-β1 (b) and IL-17 (c) after culture with MBP (in comparison to culture in media alone). The Y-axis shows the increase or decrease in cell number (per 100,000 cells) following culture with MBP and the X-axis shows the treatment of the donor animals. The horizontal line indicates the highest number of MBP specific cells seen in sham-operated animals. The proportion of animals with stroke induced increases in MBP specific IFN-γ and IL-17 responses (in comparison to highest number seen in sham-operated animals) is displayed in panels a and c. LPS administration to donor animals was also associated with an increase in the absolute numbers of MBP specific IFN-γ secreting cells (a; P = 0.03 by ANOVA). Stroke (with or without LPS administration) did not result in a significant change in the number of MBP specific TGF-β1 secreting cells (b). * indicates that the treatment groups differ by P < 0.05 (χ2).
To better reflect the immunologic status of this pool of splenocytes, the ratio of the relative increase in the number of MBP specific cells secreting IFN-γ to that of the relative increase in the number of MBP specific cells secreting TGF-β1 was calculated (as a marker of the Th1 response) and the ratio of the relative increase in the number of MPB specific cells secreting IL-17 to that of the relative increase in the number of MBP specific cells secreting TGF-β1 was calculated (as a marker of the Th17 response). The immunologic phenotype of donor cells, Th1(+) versus Th17(+), is displayed in Table 1. The proportion of animals with a Th1(+) response was increased by the administration of LPS at the time of MCAO, while Th17(+) responses were associated with MCAO alone.
Table 1.
Th1 and Th17 responses of donor animals based on treatment status.
| Number of animals with Th1(+) and Th17(+) responses
|
|||
|---|---|---|---|
| Naïve/sham | MCAO; LPS(−) | P | |
| Th1(+) | 0/9 | 2/8 (25%) | 0.11 |
| Th17(+) | 0/9 | 5/8 (62%) | <0.01 |
| Naïve/sham | MCAO; LPS(+) | P | |
|
| |||
| Th1(+) | 0/9 | 10/18 (56%) | <0.01 |
| Th17(+) | 0/9 | 9/18 (50%) | <0.01 |
| MCAO; LPS(−) | MCAO; LPS(+) | P | |
|
| |||
| Th1(+) | 2/8 (25%) | 10/18 (56%) | 0.15 |
| Th17(+) | 5/8 (62%) | 9/18 (50%) | >0.20 |
Th1(+) indicates a Th1 response > 1.16, Th17(+) response indicates a Th17(+) response >1.15 as defined in the methods section. MCAO = middle cerebral artery occlusion, LPS = lipopolysaccharide, LPS(+) = treated with LPS at the time of MCAO, LPS(−) = treated with saline at the time of MCAO. Statistics are by χ2.
Bold indicates significant results (P < 0.05).
Neither the stroke nor the LPS treatment status of the donor animals affected the outcome of recipient animals (data not shown). Animals that received Th17(+) cells at the time of MCAO, however, had higher temperatures in the post-stroke period and higher (worse) neurological scores 1 week after MCAO (Table 2). Animals that received cells with either a Th1(+) or Th17(+) phenotype also performed less well on the rotarod after stroke (Table 2). To further test the relationship between the immunologic phenotype of the donor cells and their effect of the outcome of recipient animals, the robustness of the Th1 and Th17 response was correlated to each outcome measure. Table 3 shows that the more robust the Th1 and Th17 response in donor cells the worse the performance on the rotarod in recipient animals. Additionally, more robust Th17 responses to MBP were associated with worse (higher) neurological scores in recipient animals.
Table 2.
Differences in neurological outcomes of recipient animals based on the phenotype of donor cells.
| Donor status: | Th1(+) | Th1(−) | P | Th17(+) | Th17(−) | P |
|---|---|---|---|---|---|---|
| Temperature | ||||||
| Baseline | 36.3 (36.1, 36.6) N = 18 |
36.4 (36.1, 36.6) N = 25 |
>0.20 | 36.4 (36.0, 36.6) N = 21 |
36.4 (36.1, 36.6) N = 22 |
>0.20 |
| 3 h | 38.4 (38.2, 39.0) N = 18 |
38.8 (38.4, 39.2) N = 25 |
>0.20 | 39.0 (38.4, 39.3) N = 21 |
38.5 (38.1, 39.0) N = 22 |
0.09 |
| 1 day | 38.0 (37.8, 38.3) N = 18 |
38.0 (37.6, 38.5) N = 25 |
>0.20 | 38.2 (38.0, 38.5) N = 21 |
37.7 (37.2, 38.1) N = 22 |
<0.01 |
| 3 days | 37.7 (37.4, 37.8) N = 12 |
37.6 (37.3, 37.7) N = 17 |
>0.20 | 37.7 (37.5, 37.9) N = 14 |
37.4 (37.2, 37.7) N = 15 |
0.03 |
| 1 week | 37.3 (37.0, 37.7) N = 5 |
37.6 (37.1, 37.7) N = 8 |
>0.20 | 37.2 (36.7, 37.6) N = 6 |
37.6 (37.1, 37.7) N = 7 |
>0.20 |
| Neuroscore | ||||||
| Baseline | – | – | – | – | – | – |
| 3 h | 3 (2, 3) N = 18 |
3 (2, 3) N = 25 |
>0.20 | 3 (2, 3) N = 21 |
3 (2, 3) N = 22 |
0.16 |
| 1 day | 3 (3, 4) N = 18 |
3 (3, 4) N = 25 |
>0.20 | 3 (3, 4) N = 21 |
3 (2, 4) N = 22 |
>0.20 |
| 3 days | 3 (2, 3) N = 12 |
3 (2, 3) N = 17 |
>0.20 | 3 (2, 3) N = 14 |
3 (2, 3) N = 15 |
>0.20 |
| 1 week | 2 (2, 2) N = 5 |
2 (0, 3) N = 8 |
>0.20 | 2 (2, 3) N = 6 |
2 (0, 2) N = 7 |
<0.05 |
| Foot fault (% of total steps) | ||||||
| Baseline | 0 (0, 2) N = 18 |
0 (0, 1) N = 25 |
>0.20 | 0 (0, 2) N = 21 |
0 (0, 0) N = 22 |
0.17 |
| 3 h | – | – | – | – | – | – |
| 1 day | 87 (73, 96) N = 18 |
80 (58, 90) N = 25 |
0.14 | 88 (72, 94) N = 21 |
80 (59–89) N = 22 |
0.15 |
| 3 days | 86 (61, 96) N = 12 |
71 (46, 85) N = 17 |
0.08 | 82 (62, 94) N = 14 |
71 (44, 84) N = 15 |
0.05 |
| 1 week | 25 (18, 48) N = 5 |
32 (11, 51) N = 8 |
>0.20 | 31 (19, 45) N = 6 |
25 (10, 53) N = 7 |
>0.20 |
| Rotarod performance (% of baseline) | ||||||
| Baseline | – | – | – | – | – | – |
| 3 h | – | – | – | – | – | – |
| 1 day | 9.7 (5.9, 19.1) N = 18 |
10.8 (7.2, 60.7) N = 25 |
0.18 | 10.3 (5.9, 21.4) N = 21 |
9.8 (6.9, 78.7) N = 22 |
>0.20 |
| 3 days | 5.8 (5.0, 7.6) N = 12 |
6.6 (5.9, 80.0) N = 17 |
0.18 | 5.6 (4.8, 7.0) N = 14 |
10.0 (6.0, 100) N = 15 |
0.01 |
| 1 week | 6.5 (6.0, 48.8) N = 5 |
23.6 (11.0, 100) N = 8 |
0.04 | 7.5 (6.0, 35.9) N = 6 |
27.9 (8.6, 100) N = 7 |
0.04 |
Th1(+) represents a value greater than sham-operated (>1.16), Th17(+) represents a value greater than all sham-operated animals (>1.15). Statistics are by Mann–Whitney U test.
Table 3.
Correlation between the robustness of the Th1 and Th17 responses of donor cells and the neurological outcome of recipients.
| Correlations | 1 day
|
3 days
|
1 week
|
|||
|---|---|---|---|---|---|---|
| Th1 | Th17 | Th1 | Th17 | Th1 | Th17 | |
| Temperaturea | −0.22 | 0.15 | 0.07 | 0.13 | −0.18 | −0.32 |
| 0.12 | >0.20 | >0.20 | >0.20 | >0.20 | >0.20 | |
| Neurological scoreb | −0.02 | −0.01 | 0.17 | 0.42 | 0.23 | 0.51 |
| >0.20 | >0.20 | >0.20 | 0.01 | >0.20 | P = 0.05 | |
| Foot fault testb | 0.12 | 0.15 | 0.30 | 0.33 | 0.04 | 0.28 |
| >0.20 | >0.20 | 0.09 | 0.06 | >0.20 | >0.20 | |
| Rotarodb | −0.10 | −0.07 | −0.36 | −0.32 | −0.65 | −0.52 |
| >0.20 | >0.20 | 0.04 | 0.07 | <0.01 | <0.05 | |
Pearson’s r.
Spearman’s rho.
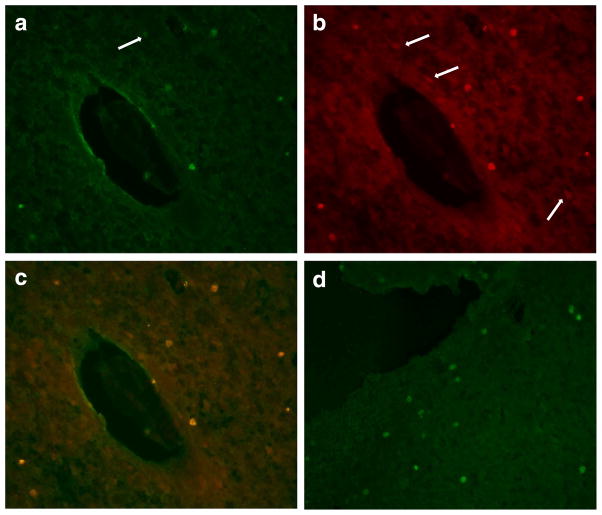
Immunocytochemistry was done for IFN-γ, IL-17, fluorescein (to identify CFDA SE labeled cells) and CD8 at the time of sacrifice (1, 3, or 7 days after MCAO). Fig. 3 shows compares the number of these cells in the brain based on the phenotype (Th1[+] or Th17[+]) of the cells. There were more fluorescein+ cells in the infarcted hemisphere 1 day after MCAO in animals receiving Th1(+) donor cells (Fig. 3d). Among animals receiving Th17(+) donor cells, there were more IFN-γ+ and more CD8+ cells in the infarcted hemisphere 3 days after MCAO (Fig. 3f and i). No fluorescein labeled cells were found in the brains of sham-operated animals or in the non-infarcted hemisphere in animals undergoing MCAO. At 3 days after MCAO/adoptive transfer, there was a strong correlation between the number of fluorescein labeled cells and the number of IFN-γ+ (r =0.76, P = 0.001), IL-17+ (r = 0.54, P = 0.04), and CD8+ (r = 0.70, P = 0.007) cells. The correlation between the number of fluorescein labeled cells and the number of CD8+ cells persisted to 7 days (r = 0.64, P = 0.04). Fig. 4 shows the infiltration of donor (fluorescein+) cells 1 day after MCAO in recipients of MBP specific Th1(+) cells.
Fig. 4.
Immunocytochemistry for CFDA SE (fluorescein) (a), CD3 (b), and both (c) at 20×. The donor cells for this animal were Th1(+) but not Th17(+); the recipient animal was sacrificed 1 day after MCAO. The arrow in panel a shows a fluorescein+ cell that is not CD3+, and the arrows in panel b show CD3+ cells that are not fluorescein+. Panel d shows fluorescein+ (donor) cells in a different animal that received MBP specific Th1(+) but not Th17(+) cells and was sacrificed 1 day after MCAO.
4. Discussion
There is increasing evidence that lymphocytes contribute to ischemic brain injury (Iadecola and Anrather, 2011). There is also ample evidence demonstrating the occurrence of autoimmune responses to brain antigens after stroke, but the pathologic consequences of these responses are unknown (Rocklin et al., 1971; Youngchaiyud et al., 1974; Kallen et al., 1977; Wang et al., 1992). We previously showed that a Th1 type immune response to MBP is associated with worse outcome in experimental models of stroke (Becker et al., 2005; Gee et al., 2008; Gee et al., 2009) and that Th1 responses to MBP are associated with worse clinical outcome in a cohort of stroke patients 3 months after stroke onset (Becker et al., 2011). The association of the Th1 response to MBP and worse outcome, however, does not prove that the lymphocytes with the Th1 phenotype mediate the worse outcome. The purpose of this study was to more definitively address the role of MBP specific lymphocytes in modulating outcome from stroke. The contribution of MBP specific lymphocytes was evaluated by adoptive transfer into recipient animals. For clinical relevance, the donor lymphocytes were generated in animals undergoing severe stroke (3 h MCAO) and skewed towards a Th1(+) response by the administration of LPS. Animals that received donor cells with either an MBP specific Th1(+) or Th17(+) phenotype at the time of stroke experienced worse clinical outcome than animals that received donor cells without a Th1(+) or Th17(+) phenotype. Further, there was a direct correlation between the robustness of both the Th1 and Th17 responses to MBP and functional outcome at multiple time points after stroke. These observations suggest that adoptively transferred cells mediate the worsened outcome.
Most animals undergoing MCAO do not develop a Th1(+) response to MBP (Becker et al., 2005). Systemic administration of LPS, however, skews the immune response towards that of a Th1(+) response in animals undergoing severe stroke (3 h MCAO) (Becker et al., 2005). In accord with our prior studies, we observed an increase in the Th1 response to MBP among animals treated with LPS. LPS, however, did not appear to increase the MBP specific Th17 response after stroke. The requisites for generation of Th1 and Th17 cells differ; the former is dependent on IFN-γ in the environment of antigen presentation and the latter upon the presence of both TGF-β1 and IL-6 (Zhou et al., 2009). Few studies address stroke induced changes in plasma IFN-γ, and those that do show no appreciable changes (Urra et al., 2009) or even a decrease in IFN-γ (Vogelgesang et al., 2010) in the days after stroke. Further, IFN-γ does not appear to be reliably upregulated in the ischemic brain (Lambertsen et al., 2004). LPS, however, is known to induce the production of IFN-γ (Pulendran et al., 2001). Infections, especially those caused by Gram-negative organisms, are associated with an increase IFN-γ (Kohler et al., 1993; Lainee et al., 2005; Paats et al., 2013). These data argue that the usual scenario after stroke would not favor the development of Th1 responses, but that post-stroke infection (with generation of IFN-γ) could increase this likelihood. Unlike IFN-γ, both IL-6 and TGF-β1 are known to be markedly upregulated following ischemic stroke. Systemic IL-6 increases within hours after stroke onset (Beamer et al., 1995; Waje-Andreassen et al., 2005). There is also rapid upregulation of IL-6 and TGF-β1 expression in ischemic brain (Krupinski et al., 1996; Suzuki et al., 1999; Legos et al., 2000; Ali et al., 2001). The cytokines necessary for the development of a Th17 response are thus usually present after stroke. These observations might explain why LPS was needed to provoke the development of Th1(+) responses while stroke itself was associated with a Th17(+) response in at least 50% of animals.
In the animal model used in our experiments, LPS is used as a proxy for systemic infection. Infections, especially pneumonia and urinary tract infections, are very common following stroke (Westendorp et al., 2011). As might be expected, patients who develop infection are more likely to develop Th1 responses to MBP, and these Th1 responses are associated with worse clinical outcome (Becker et al., 2011). We have not previously evaluated the association between MBP specific Th17 responses and stroke outcome. The current study, however, suggests that both MBP specific Th1 and Th17 immune responses may not only be associated with worse stroke outcome, but may mediate this worse outcome. Importantly, the absolute number of MBP specific cells secreting IFN-γ and IL-17 in this study is similar to that seen for PLP in models of EAE (Targoni et al., 2001). Further, the numbers of MBP specific cells are similar to those seen in the circulation of patients with multiple sclerosis (Jansson et al., 2003).
CFDA SE(+) or fluorescein+ cells were found in the infarcted hemispheres of recipient animals, demonstrating that these cells traffic into ischemic brain; the infiltration of these fluorescein+ cells was most robust at 1 day after MCAO in animals receiving Th1(+) cells. Interestingly, there were more IFN-γ+ (not IL-17+) cells in the brains of animals receiving Th17(+) donor cells at day 3 after MCAO, but there appears to be a population of Th17(+) cells that also secrete IFN-γ (Suryani and Sutton, 2007; Murphy et al., 2010). We did not see a significant difference in the number of IFN-γ+ or IL-17+ cells among animals receiving Th1(+) or Th17(+) donor cells at other time points. More CD8+ cells were seen among recipients of Th17(+) cells 3 days after MCAO, suggesting these animals experienced a more robust inflammatory response, which may help to explain the worse functional outcome in recipients of Th17(+) cells. To better address the relative numbers of IFN-γ+, IL-17+, fluorescein+ and CD8+ cells, flow cytometry with intra-cellular cytokine staining of lymphocytes isolated from the ischemic brain could be done in future studies. In addition, these studies might address the contribution of inflammatory cells other than lymphocytes to the CNS inflammatory response in animals receiving MBP specific Th1(+) and Th17(+) cells.
In concert, our data argue strongly that lymphocytes are able to modulate outcome from stroke and that the phenotype of the lymphocytes is important in determining the nature of this modulation. MBP specific Th1(+) and Th17(+) cells both worsen outcome from stroke while MBP specific cells with a Treg phenotype improve outcome from stroke (Becker et al., 2003). Whether Th1(+) and Th17(+) cells differentially effect outcome was not directly evaluated in this study, but in animal models of experimental autoimmune encephalomyelitis (EAE), Th17 type cells are inducing more severe disease than Th1 type cells (Jager et al., 2009). After stroke, endogenously developing immune responses towards brain antigens may thus be either detrimental (Th1, Th17) or beneficial (Treg). This observation suggests that manipulation of the post-ischemic immune response is thus a potential therapeutic strategy for the treatment of stroke.
Footnotes
Funding was provided by NINDS 5R01NS056457.
References
- Ali C, Docagne F, Nicole O, Lesne S, Toutain J, Young A, et al. Increased expression of transforming growth factor-beta after cerebral ischemia in the baboon: an endogenous marker of neuronal stress? J Cereb Blood Flow Metab. 2001;21:820–827. doi: 10.1097/00004647-200107000-00007. [DOI] [PubMed] [Google Scholar]
- Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800–805. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: a role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JM, Zierath D, Hadwin J, Savos A, Kalil A, Thullbery M, et al. Long term immunologic consequences of experimental stroke and mucosal tolerance. Exp Transl Stroke Med. 2009;1:3. doi: 10.1186/2040-7378-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- Jansson A, Ernerudh J, Kvarnstrom M, Ekerfelt C, Vrethem M. Elispot assay detection of cytokine secretion in multiple sclerosis patients treated with interferon-beta1a or glatiramer acetate compared with untreated patients. Mult Scler. 2003;9:440–445. doi: 10.1191/1352458503ms951oa. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator stroke study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- Kallen B, Nilsson O, Thelin C. Effect of encephalitogenic protein on migration in agarose of leukocytes from patients with multiple sclerosis. A longitudinal study of patients with relapsing multiple sclerosis or with cerebral infarction. Acta Neurol Scand. 1977;55:47–56. [PubMed] [Google Scholar]
- Kohler J, Heumann D, Garotta G, LeRoy D, Bailat S, Barras C, et al. IFN-gamma involvement in the severity of gram-negative infections in mice. J Immunol. 1993;151:916–921. [PubMed] [Google Scholar]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Lainee P, Efron P, Tschoeke SK, Elies L, De Winter H, Lorre K, et al. Delayed neutralization of interferon-gamma prevents lethality in primate Gram-negative bacteremic shock. Crit Care Med. 2005;33:797–805. doi: 10.1097/01.ccm.0000159090.80228.57. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Gregersen R, Meldgaard M, Clausen BH, Heibol EK, Ladeby R, et al. A role for interferon-gamma in focal cerebral ischemia in mice. J Neuropathol Exp Neurol. 2004;63:942–955. doi: 10.1093/jnen/63.9.942. [DOI] [PubMed] [Google Scholar]
- Legos JJ, Whitmore RG, Erhardt JA, Parsons AA, Tuma RF, Barone FC. Quantitative changes in interleukin proteins following focal stroke in the rat. Neurosci Lett. 2000;282:189–192. doi: 10.1016/s0304-3940(00)00907-1. [DOI] [PubMed] [Google Scholar]
- Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, et al. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157:157–165. doi: 10.1016/j.bbr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Paats MS, Bergen IM, Hanselaar WE, Groeninx van Zoelen EC, Hoogsteden HC, Hendriks RW, et al. Local and systemic cytokine profiles in nonsevere and severe community-acquired pneumonia. Eur Respir J. 2013;41:1378–1385. doi: 10.1183/09031936.00060112. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin RE, Sheremata WA, Feldman RG, Kies MW, David JR. The Guillain–Barre syndrome and multiple sclerosis. In vitro cellular responses to nervous-tissue antigens. N Engl J Med. 1971;284:803–808. doi: 10.1056/NEJM197104152841501. [DOI] [PubMed] [Google Scholar]
- Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J Neuroimmunol. 2007;183:96–103. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nogawa S, Nagata E, Ito D, Dembo T, et al. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1256–1262. doi: 10.1097/00004647-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Targoni OS, Baus J, Hofstetter HH, Hesse MD, Karulin AY, Boehm BO, et al. Frequencies of neuroantigen-specific T cells in the central nervous system versus the immune periphery during the course of experimental allergic encephalomyelitis. J Immunol. 2001;166:4757–4764. doi: 10.4049/jimmunol.166.7.4757. [DOI] [PubMed] [Google Scholar]
- Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- Vogelgesang A, May VE, Grunwald U, Bakkeboe M, Langner S, Wallaschofski H, et al. Functional status of peripheral blood T-cells in ischemic stroke patients. PLoS One. 2010;5:e8718. doi: 10.1371/journal.pone.0008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waje-Andreassen U, Krakenes J, Ulvestad E, Thomassen L, Myhr KM, Aarseth J, et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88:157–162. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngchaiyud U, Coates AS, Whittingham S, Mackay IR. Cellular-immune response to myelin protein: absence in multiple sclerosis and presence in cerebrovascular accidents. Aust N Z J Med. 1974;4:535–538. doi: 10.1111/j.1445-5994.1974.tb03233.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]