Abstract
Lead remains a significant environmental toxin, and we believe we may have identified a novel target of lead toxicity in articular chondrocytes. These cells are responsible for the maintenance of joint matrix, and do so under the regulation of TGF-β signaling. As lead is concentrated in articular cartilage, we hypothesize that it can disrupt normal chondrocyte phenotype through suppression of TGF-β signaling. These experiments examine the effects of lead exposure in vivo and in vitro at biologically-relevant levels, from 1nM–10µM on viability, collagen levels, matrix degrading enzyme activity, TGF-β signaling, and articular surface morphology. Our results indicate that viability was unchanged at levels ≤100µM Pb, but low and high level lead in vivo exposure resulted in fibrillation and degeneration of the articular surface. Lead treatment also decreased levels of type II collagen and increased type X collagen, in vivo and in vitro. Additionally, MMP13 activity increased in a dose-dependent manner. Active caspase3 and 8 were dose-dependently elevated, and treatment with 10µM Pb resulted in increases of 30% and 500%, respectively. Increasing lead treatment resulted in a corresponding reduction in TGF-β reporter activity, with a 95% reduction at 10µM. Levels of phosphoSmad2 and 3 were suppressed in vitro and in vivo and lead dose-dependently increased Smurf2. These changes closely parallel those seen in osteoarthritis. Over time this phenotypic shift could compromise maintenance of the joint matrix.
Keywords: Lead, Osteoarthritis, TGF-β, Articular chondrocytes
INTRODUCTION
Elemental lead (Pb2+) is a chemically stable and ubiquitous environmental toxin. Once deposited, it persists in the environment until it is actively removed. In addition, in humans exposed to lead, most of the heavy metal is deposited in the skeleton (95% of body burden) where its half-life in the bone compartment is 25–30 years [1]. This creates a window for secondary exposure that will increase during times of rapid bone resorption such as menopause, pregnancy, or immobilization [2]. No beneficial function or safe limit of exposure has been identified for lead in the body, and exposure to lead results in anemia, cognitive deficit, kidney damage, as well as cancer and other pathologies [3]. Through investigation of clinical and epidemiological findings, we believe that we have uncovered a previously unknown effect of lead in the joint at levels that are not acutely toxic: the disruption of normal articular chondrocyte function. Over the last few decades the association of lead with joint degeneration has surfaced with increasing frequency. Duration of lead exposure in lead smelter workers was found to correlate significantly with incidence of joint pain [4]. Additionally, case study analyses have found an association between retention of lead pellets and joint degeneration [5, 6]. Furthermore, controlled experiments implanting periarticular lead pellets found a significant increase in joint damage beyond that seen with steel implants and sham surgeries [7, 8].
Lead has been shown to disrupt TGF-β signaling in other tissues [9, 10]. At the molecular level, signaling by the cytokine TGF-β constitutes the preeminent constraint in the continued maintenance of articular cartilage by articular chondrocytes [11]. TGF-β directs articular chondrocytes to maintain a stable phenotype and continue cellular functions associated with joint maintenance, such as production and organization of type II collagen (Col2) and glycosaminoglycans into the articular surface [12] and suppression of type X collagen (ColX) [13] and matrix degrading enzymes (MMPs) [14]. Canonical TGF-β signaling functions through a complex, but well defined, pathway. Exogenous TGF-β binds the cell surface receptor, TGF-βRII, and upon binding, this receptor dimerizes with TGF-βRI and phosphorylates it. Activated TGF-βRI can subsequently phosphorylate transcription factors Smad2 or Smad3. Once activated these Smads are able to form heteromeric complexes with the co-factor Smad4, allowing for translocation to the nucleus where they directly influence gene transcription via Smad binding elements (SBEs). TGF-β signaling can be endogenously inhibited via alterations to pathway intermediates in part by the Smad ubiquitination regulatory factor, Smurf2, which marks Smad2 and Smad3 for degradation [15–18]. The importance of TGF-β signaling to joint maintenance has been further illustrated by transgenic animals. Three examples are: 1) expression of a dominant-negative TGF-β receptor induces osteoarthritis-like symptoms [19], 2) Smad3 knockout mice show progressive degeneration of the articular surface [20], and 3) heterozygous constitutive expression of Smurf2 results in strong osteophyte production in and around the joint space [17].
Due to their proximity to lead sequestered in the subchondral bone and synovial fluid [21], we hypothesized that articular chondrocytes are vulnerable to lead toxicity. This vulnerability should manifest itself via changes in phenotype and joint morphology. Several findings point to the TGF-β receptor, specifically, as a target of lead toxicity. Projected susceptibility stems from studies showing lead disruption of serine/threonine receptor kinases in other cell types [22]. The likelihood of interaction between lead and TGF-βRI or TGF-βRII is further increased due to the presence of Cys-box domains in these receptors. These domains have proven especially vulnerable to lead binding and inhibition [23–25]. We further hypothesized that lead may be acting upon downstream elements in the TGF-β signaling pathway. We, therefore, investigate the ability of lead to shift articular chondrocytes away from a normal phenotype and towards an osteoarthritis-like phenotype. Furthermore, we explore the disruptive effects of lead on TGF-β signaling.
MATERIALS AND METHODS
Chondrocyte Isolation, Cell Lines and Culture, and Viability Assay
Chick articular chondrocytes were isolated from the femora and tibiae of 4–6 week old Gallus domesticus chickens as previously described [26, 27]. C5.18 cells were used as an articular chondrocyte-like cell culture model. These rat-derived cells normally express high levels of Col2 and aggrecan and low ColX [28]. Additionally, they form cartilage nodules and do not mineralize [29, 30]. Cells were cultured at 37° C in low glucose DMEM medium supplemented with 10% FBS, 1% pen/strep, 4.7g/L sodium bicarbonate, and 50ug/ml ascorbic acid. Viability for all cell experiments was assessed using CellTiter-Blue cell viability assay (Promega). The manufacturer’s protocol was followed and fluorescence was measured (560abs/590em) on a plate reader.
Western blot
Western blots were performed as previously described by our lab [17, 31]. The blots were probed overnight at 4°C with the following antibodies: rabbit-anti-mouse Col2 monoclonal (Rockland), rabbit-anti-mouse phospho-Smad2 and phosphoSmad3 polyclonal (Cell Signaling), rabbit-anti-mouse Smurf2 monoclonal (Upstate Biotechnology, Lake Placid, NY), mouse monoclonal ColX (Quartett), and rabbit-anti-mouse actin monoclonal (Sigma), all at a 1:1,000 dilution. HRP-conjugated secondary antibodies against rabbit (Sigma), mouse (BioRad, Hercules, CA) or sheep (Santa Cruz) were used at a dilution of 1:5,000. The immune complexes were detected using Supersignal West Pico (Thermo Scientific Rockford, IL) and visualized following exposure to XOMAT AR film (Kodak).
P3TP TGF-β Signaling Assay
P3TP-lux is a synthetic reporter construct that consists of firefly luciferase under the control of 3 consecutive TREs (TPA response elements) and has been shown to be TGF-β responsive [32, 33]. Cells were transiently transfected with the P3TP-lux plasmid using Superfect (Qiagen), according to the manufacturer’s instructions. The SV40 renilla-luc plasmid was co-transfected to determination transfection efficiency as previously described [34]. The DNA to transfection reagent ratio used for all experiments was 1:3 (w/v) with the following amounts of DNA in 0.6 ml medium: P3TP-lux = 2 µg, SV40 renilla-luc = 10ng. Three hours after transfection, cultures were exposed to experimental conditions for 24 hours. Cells were then lysed and extracts were prepared using the Dual Luciferase Assay System (Promega) as directed by the manufacturer. Luminescence was recorded with an Optocomp luminometer (MGM Instruments).
Promega Caspase Assay
Fluorescent indicator dyes were used as a biomarker for caspase activation. The dyes contain an activation site specific for either caspase3 or 8 and must be cleaved at this site to fluoresce. After adherence cells were treated with either control media, DMEM containing 10% FBS, or control media with lead added at given concentrations for 24 hours. After 24hrs caspase3 or 8 detection reagents (Promega Madison, WI) were added and detected as per the company’s suggested protocol.
MMP13 Assay
Media was collected from confluent C5.18 cells treated in a standard 12-well plate and MMP13 activity was assessed using the SensoLyte plus 520 MMP-13 Assay Kit (AnaSpec, CA). The assay was conducted per the manufacturer’s recommended protocol. This assay can detect the activity of sub-nanogram concentrations of MMP13 without cross reactivity of MMPs 1,2,3,7,8,9,10,12, or 14 [35]. Fluorescence was graphed as a function of time and the rate of increase was calculated via linear regression analysis and then normalized to total protein concentration by Coomassie Plus Protein Assay kit (Pierce Chemical, Rockford, IL).
Histology and staining
For collagen type 2 and type 10 immunohistochemistry, sections were first deparaffinized in xylene and digested for 10 minutes, in 10% pepsin. Slides were then quenched with 3% hydrogen peroxide then incubated overnight at 4°C with anti-col II (USA) or anti-col X (USA) at 1:50 dilution. Collagen proteins were detected using rabbit anti-goat secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA), followed by horseradish peroxide streptavidin (1:750; Zymed, San Francisco, CA, USA) for 30 min treatment at room temperature and developed with AEC chromogen for 10 minutes.
Lead exposure
The animals were exposed via lead acetate in the drinking water administered ad libitum. Short-term exposure consisted of 500ppm for 3 months, long-term exposure of 50ppm for 18 months. Blood lead levels of 45 and 9µg/dL (respectively) were achieved within one week and maintained throughout treatment.
Statistical Analysis and ImageJ Quantification of Western Blots
Statistical analyses were performed using ANOVA. p values less than 0.05 were viewed as significant and denoted by “*” unless otherwise indicated.
Images were analyzed using ImageJ software and according to the protocol found at http://lukemiller.org/index.php/2010/11/analyzing-gels-and-western-blots-with-image-j/.
RESULTS
Long-term low-level lead exposure results in disruption of articular surface
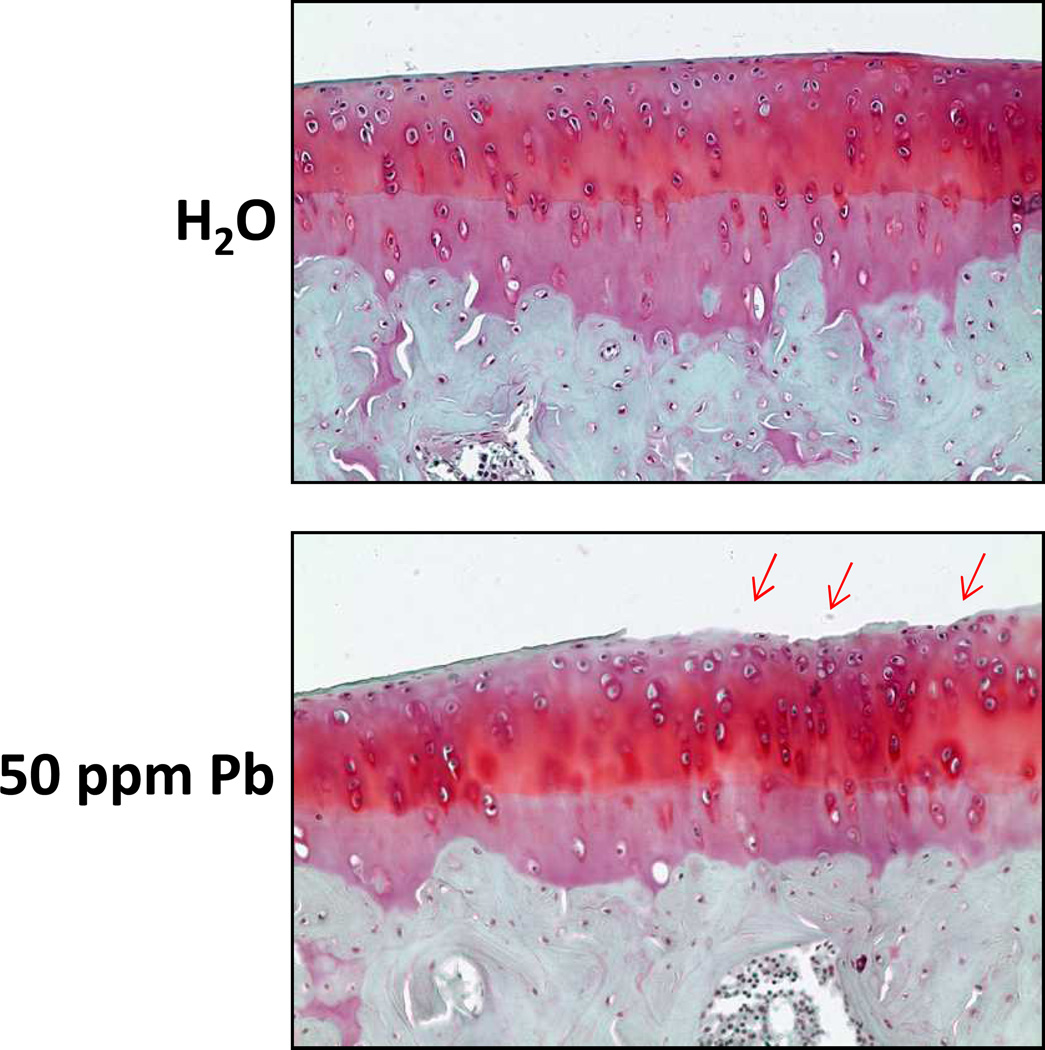
Safranin O staining revealed a loss of proteoglycans in the most superficial zone of the articular surface in tibiofemoral joints. Consistent with this, we found an increase in the presence of cartilage abrasions, specifically in the lamina splendens, 22.2% in Pb treatment to 0% in controls (Figure 1).
Figure 1.
Rats subjected to long-term low-level lead exposure exhibit cartilage degeneration in the tibiofemoral articulation. Ad libitum treatment with 50 ppm Pb via lead acetate in the drinking water resulted in blood lead levels of 9µg/dL. These levels were achieved within 1 week of treatment and maintained for 18 months. Histological examination after Safranin O staining revealed degradation of the lamina splendens and changes in cell morphology in the lead treated group but not in unexposed controls.
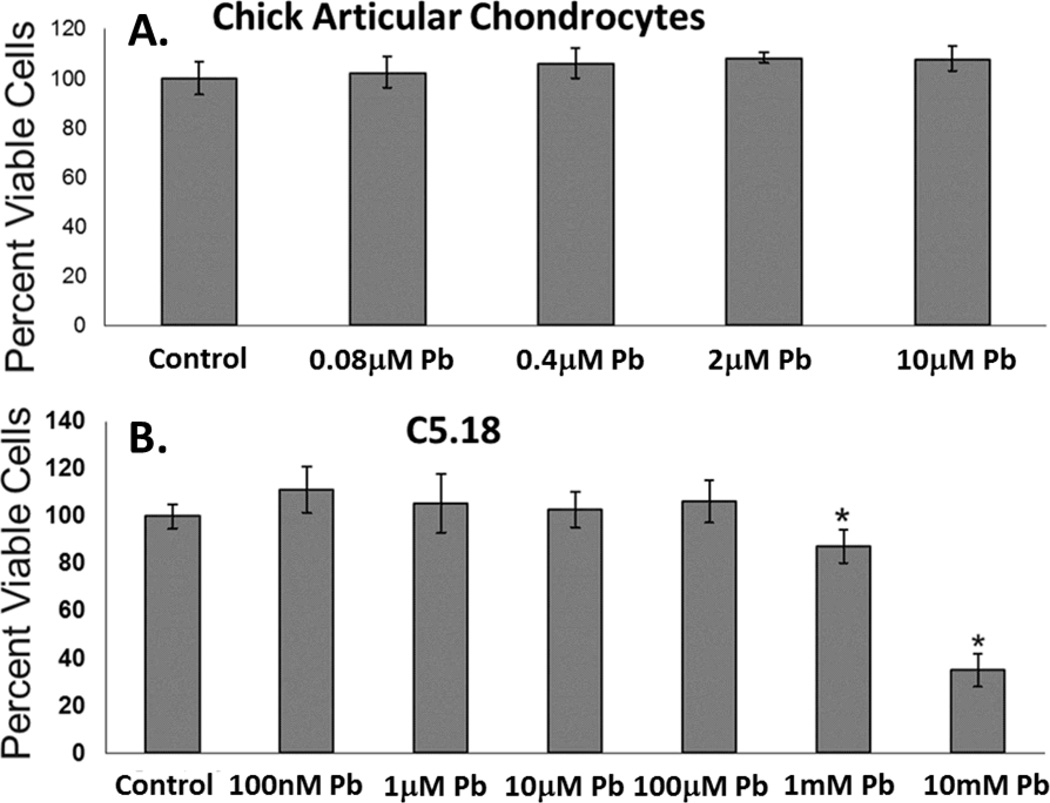
Lead is not acutely cytotoxic in vitro at physiologically relevant levels
Cultured primary articular chondrocytes demonstrated no decrease in viability when treated with increasing levels of lead up to 10µM for 24hrs, as measured by cell titer blue assay (Figure 2A). Likewise, C5.18 cells, an articular chondrocyte-like cell line, survived at biologically relevant lead concentrations and only demonstrated a decrease in viability at lead levels greatly exceeding this range, i.e. >100µM (Figure 2B).
Figure 2.
Lead does not exert acute toxicity on articular chondrocytes at relevant experimental doses (≤10µM). In an articular chondrocyte cell model, C5.18 cells exhibited no reduction in viability compared to control (CellTiter-Blue assay). When treated with lead concentrations from 100nM–100µM a reduction in viability was observed only at lead concentrations of 1mM and 10mM (i.e. biologically irrelevant concentrations and 100 times greater than our highest experimental dose) (A.). Cell viability was unchanged in articular chondrocytes treated with lead concentrations from 0.08µM –10µM for 24hrs (B.) (*=p<0.05 from control, n=8).
Lead alters articular chondrocyte phenotype
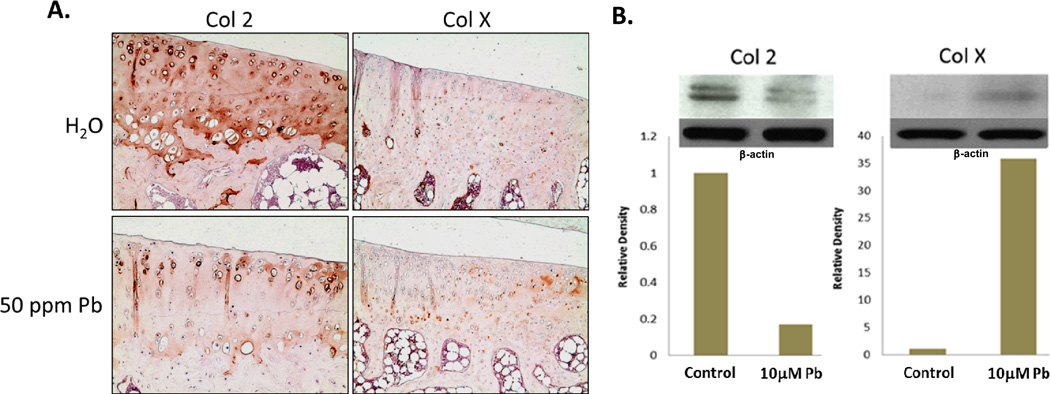
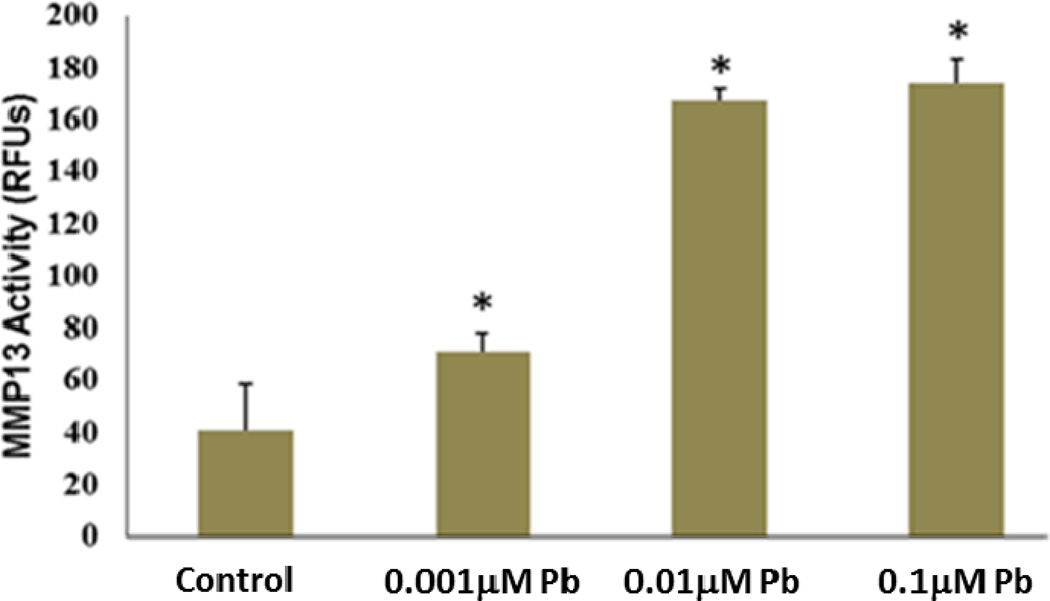
Long-term low-level lead treated rats displayed decreased staining for Col2 and increased ColX in articular cartilage (Figure 3A). At the protein level, articular chondrocytes exposed to 10µM lead demonstrated a 5 fold decrease in Col2 production (Figure 3B). In contrast, expression of ColX was elevated 35 fold above control (Figure 3B). Message level of ColX was similarly found to be elevated (data not shown). Additionally, MMP13 activity in C5.18 cells, assayed via fluorescent indicator dye, was found to be elevated over a lead treatment range from 100nM–10µM, with the highest levels of induction being approximately 4 fold at lead concentrations of 0.01µM and 0.1µM (Figure 4).
Figure 3.
Protein expression of the normal cartilage phenotype was disrupted by lead treatment. The articular cartilage of long-term low-level lead exposed rats displayed reduced staining for type II collagen (Col2) and increased type X collagen (ColX) (A.). After 24hrs of treatment with 10µM Pb, Col2 levels were suppressed to 20% of normal while ColX increased by 35 fold in articular chondrocytes, as quantified by ImageJ analysis. Band intensity was normalized to β-actin.
Figure 4.
Lead treatment significantly and dose-dependently increased MMP13 activity across the range of 1nM–0.1µM from 2 fold to 4 fold, respectively, and MMP13 activity was also significantly elevated by treatment with 10µM Pb (*=p<0.05 from control, n=4).
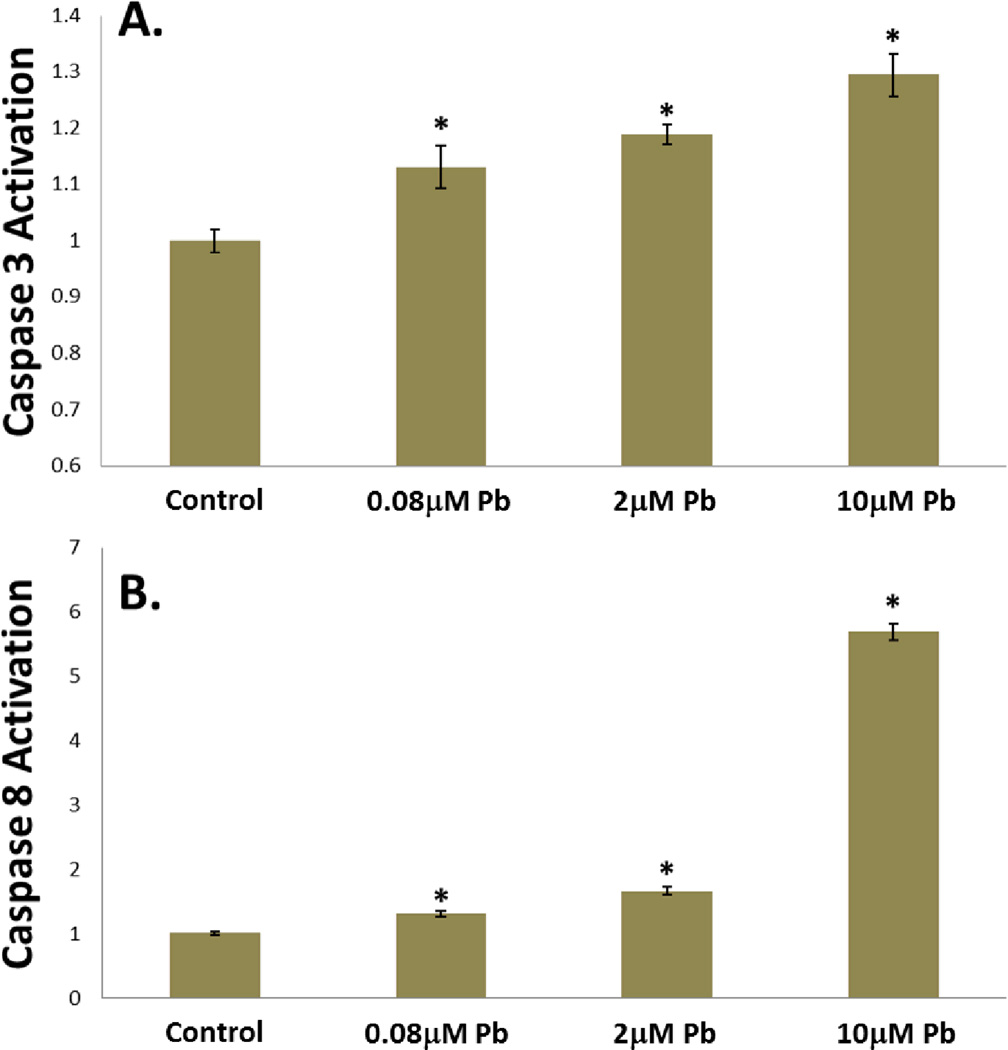
Loss of chondrogenic potential has been associated with increased caspase activation [36], and in response to lead treatment a significant and dose-dependent increase in the activities of caspase3 (Figure 5A) and caspase 8 (Figure 5B) was seen, with caspase 3 increasing by 30% and caspase8 increasing by 500% in the 10µM Pb treatment group. TGF-β is a known survival factor in articular chondrocytes, therefore caspase activation is consistent with its disruption [37].
Figure 5.
Lead treatment resulted in dose-dependent caspase activation. As evidenced by high-specificity fluorescent indicator dyes, after 24hrs of lead treatment caspase3 activation was elevated approximately 15% at 0.08µM and significantly increased by 20% at 2µM and 30% at 10µM in chick articular chondrocytes (A.). Similarly, when treated with lead for 24hrs, caspase8 activation was increased in a dose-dependent manner, with a 6 fold increase seen in the 10µM treatment group (B.). (*=p<0.05 from control, n=8).
Lead disrupts TGF-β signaling
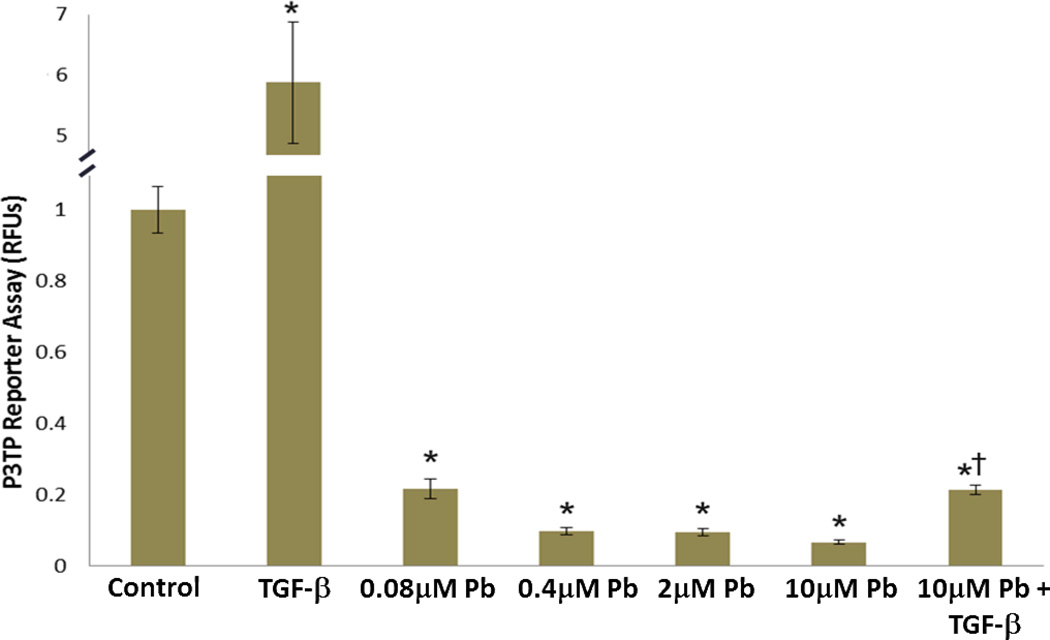
For this experiment articular chondrocytes were transfected with the TGF-β/Smad3 responsive reporter, P3TP-Luc, and treated with Pb in the presence and absence of 5ng/ml TGF-β. Upon lead treatment we observed a dose-dependent decrease in the ability of articular chondrocytes to activate this reporter. A 95% reduction was seen in the 10µM Pb treatment group and interestingly, exogenous addition of TGF-β was unable to overcome this inhibition (Figure 6).
Figure 6.
Lead suppresses basal TGF-β signaling in chick articular chondrocytes. Lead treatment was found to significantly suppress luminescence in a dose-dependent manner, from an 80% reduction at 0.08µM Pb to a 93% reduction at 10µM Pb. As expected TGF-β (5ng/ml) increased P3TP reporter activity by approximately 500%. Although cells co-treated with 10µM lead and TGF-β exhibited significantly higher reporter activity than the 10µM Pb group, their reporter activity remained significantly lower than either control or TGF-β treatment alone (*=p<0.05 from control. †=p<0.05 from TGF-β treatment and 10µM Pb, n=4).
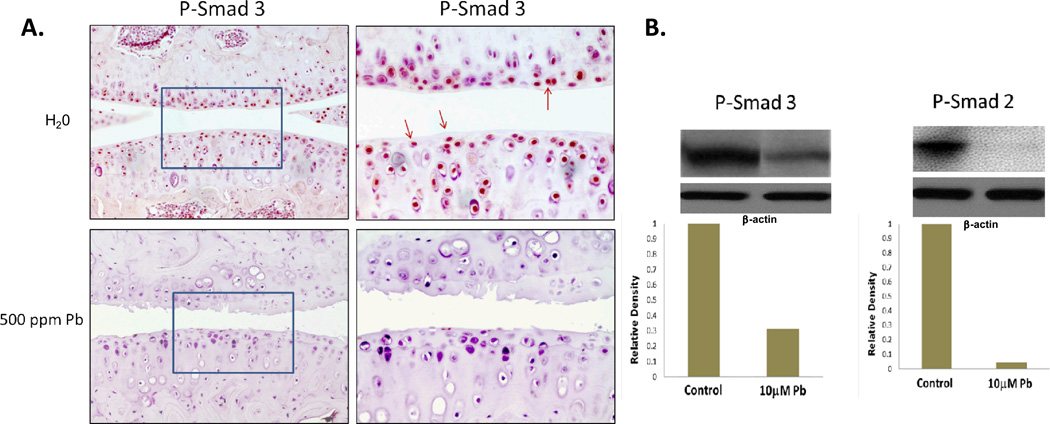
Levels of active (phosphorylated) Smad2 and 3 are relatively high in normal articular chondrocytes. However, short-term high lead exposed animals exhibited drastically decreased levels of P-Smad 3 (Figure 7A). Furthermore, these animals also exhibited fibrillation of the articular surface. In vitro, 10µM lead treatment was found by western blot to reduce levels of phosphoSmad2 by 95% and Smad3 by 67% within the cell (Figure 7B).
Figure 7.
Lead treatment reduced levels of phosphoSmad2 and 3. Mice exposed to 500ppm lead acetate in their water for 3 months had drastically reduced P-Smad 3 levels, as well as fibrillation of the articular surface (A.). These animals maintained blood lead levels of 45µg/dL. Articular chondrocytes in culture were treated with 0 or 10μM Pb acetate for 24 hrs. Cultures were then treated with 5 ng/mL TGF-β. After 45 min, as quantified by ImageJ analysis, a 67% reduction in phosphoSmad3 (P-Smad3) and a 95% reduction in phosphoSmad2 (P-Smad2) were observed in the lead treated group (B.). Blots are representative of a series of three separate experiments.
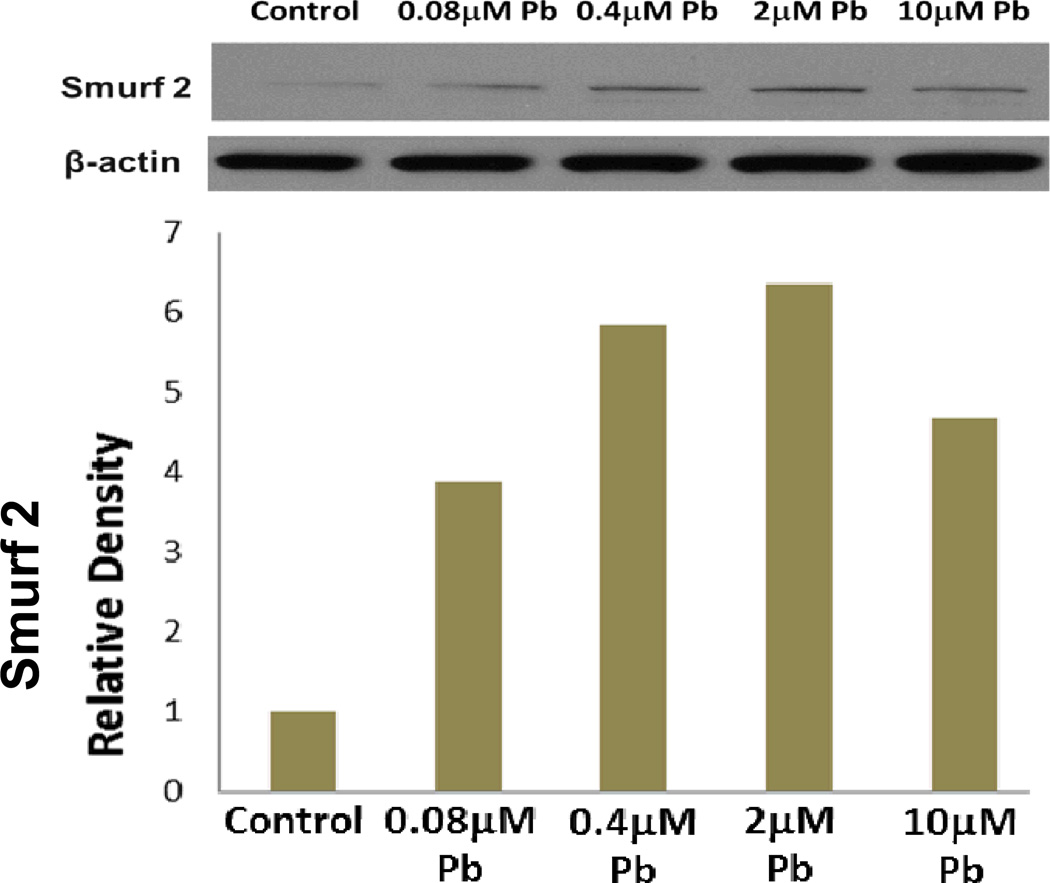
Western blot and RT-PCR analysis (data not shown) show that lead dose-dependently up-regulates Smurf2, with a maximum 6 fold induction at the 2µM concentration (Figure 8).
Figure 8.
Lead significantly elevated Smurf2 protein levels with a dose-dependent elevation above control being seen from 4 fold at 0.08µM to 6 fold at 2µM and a 4.5 fold increase at 10µM.
DISCUSSION
At acutely-nontoxic biologically-relevant doses of lead, damage to the lamina splendens is evident and articular chondrocytes alter their protein expression profile: expressing reduced levels of Col2, and elevated levels of ColX and caspases3 and 8. Additionally, lead treatment elevated the activity of secreted MMP13. TGF-β signaling, as assayed through the P3TP-lux reporter, was inhibited by lead and this inhibition could not be overcome by exogenous addition of TGF-β. Examination of the TGF-β signaling pathway revealed that lead lowered levels of phosphoSmad2 and 3 while elevating levels of Smurf2.
The function of normal articular chondrocytes is to maintain the extracellular articular matrix. To do this they produce and secrete molecules such as Col2, aggrecan, and other proetoglycans and glycoproteins that maintain the low friction coefficient of the lamina splendens and impart load-bearing and impact-dampening properties. Lead treatment degraded the lamina splendens in vivo and lowered levels of Col2 and increased levels of ColX, a collagen expressed in growth plate chondrocytes and osteoarthritic articular cartilage. Interestingly, as observed in the past, mRNA levels of colX increased by only 2-fold and protein by 35-fold. This suggests that there is either accelerated initiation of colX translation, decreased stability of colX mRNA, increased protein stability, or a combination thereof. Normal articular chondrocytes also express very low levels of matrix degrading enzymes, but lead treatment increased MMP13 activity. Lead treatment resulted in changes to the articular surface and in the protein expression profile that mimics endochondral ossification seen in osteoarthritis.
The absence of cytotoxicity in these lead treated cells supports the idea that observed lead effects must arise through a specific disruption of cellular pathways or processes, as they are not the result of general cell death. It seems that lead treatment, in addition to altering protein expression, results in cellular activity that parallels what is seen in osteoarthritis (OA). Caspase activation in articular chondrocytes has been correlated with loss of chondrogenic potential and cartilage destruction [36, 38]. In fact this is exactly what we see in lead-treated articular chondrocytes. During this stage of caspase activation, as evidenced by simultaneously decreased Col2 levels, articular chondrocytes, though still viable, fail to maintain normal production of joint matrix components.
The reliance of articular chondrocytes on TGF-β signaling to maintain normal function is evidenced by both in vitro studies and in transgenic animal models [39]. Our lab had previously shown TGF-β signaling in growth plate chondrocytes to be vulnerable to lead [40]; interestingly our study observed effects in ACs even at a 10 fold lower dose. It is for these reasons that we hypothesized that lead alters articular chondrocyte phenotype through disruption of TGF-β signaling. Importantly, lead treatment was found to suppress TGF-β signaling, as measured by P3TP-lux reporter activity. This suppression was achieved by altering components of the signaling milieu, suppressing transducers phosphoSmad2 and 3, and elevating the endogenous negative regulator Smurf2. Exogenous TGF-β was unable to restore normal signaling; however expression of constitutively active Smad3 did restore signaling (supplemental data). Taken in concert, these experiments point to the TGF-β receptor as a putative lead target.
The actual role of TGF-β signaling in OA pathology is quite complex and is not fully understood; however, cartilage from OA patients has been shown to contain elevated levels of Smurf2, and in this regard lead treatment is similar [17]. Complexity arises because TGF-β has also been shown to signal through non-canonical pathways [41, 42] and disruption of TGF-β can lead to up-regulation of BMP signaling [31]; however, we observed no compensatory increase in BMP signaling upon lead treatment (data not shown). Furthermore, TGF-β signaling is subject to modulation via co-receptors such as endoglin [43]. Further studies are needed to sort out points of interaction between TGF-β and BMP signaling and downstream effects on MAPK, ERKs, and other kinases, but the current study shows that lead treatment inhibits canonical TGF-β signaling in articular chondrocytes, and vivo this inhibition correlates with fibrillation of the articular surface.
In the expression profile of matrix components, degrading enzymes, viability and caspase activation, and disruption of TGF-β signaling, lead treated articular chondrocytes mirror the cellular changes seen in osteoarthritis, as does damage to the articular surface in vivo. However, these findings also underscore the need for further study of in vitro and in vivo lead effects. Due to the novelty of lead inhibition of TGF-β signaling in articular chondrocytes, the mechanism by which lead is exhibiting these effects is yet to be adequately delineated. Therefore, continued in vitro work is needed. Bone lead levels and blood lead levels strongly correlate [44], however the correlation of lead dose to actual biological effect, extrapolations of cellular damage to morphologically significant changes in function, and the contribution of lead to the genesis and progression of OA require further investigation. The experiments conducted within this manuscript have begun to describe a model and potential mechanism of the generation and progression of osteoarthritis. This is of critical importance, as the generation of osteoarthritis is itself not well understood. In light of this disparity of knowledge, understanding the mechanism by which lead exerts its disruption of articular chondrocyte function, especially if found to act through similar channels as other causes of OA, is the first step towards the ultimate goal of improved preventative measures and clinical therapies for joint degenerative diseases.
AKNOWLEDGMENTS
Funded by Toxicology Training Grant (T32ES07026), the NIEHS Center Grant (P30ES01247), NIH R01AR045700, NIH P50AR054041, and Arthritis Foundation Arthritis Investigator Award.
REFERENCES
- 1.Khalil N, et al. Association of cumulative lead and neurocognitive function in an occupational cohort. Neuropsychology. 2009;23(1):10–19. doi: 10.1037/a0013757. [DOI] [PubMed] [Google Scholar]
- 2.Symanski E, Hertz-Picciotto I. Blood lead levels in relation to menopause, smoking, and pregnancy history. Am J Epidemiol. 1995;141(11):1047–1058. doi: 10.1093/oxfordjournals.aje.a117369. [DOI] [PubMed] [Google Scholar]
- 3.Patrick L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev. 2006;11(1):2–22. [PubMed] [Google Scholar]
- 4.Lilis R, et al. Lead effects among secondary lead smelter workers with blood lead levels below 80 microgram/100 ml. Arch Environ Health. 1977;32(6):256–266. doi: 10.1080/00039896.1977.10667292. [DOI] [PubMed] [Google Scholar]
- 5.Peh WC, Reinus WR. Lead arthropathy: a cause of delayed onset lead poisoning. Skeletal Radiol. 1995;24(5):357–360. doi: 10.1007/BF00197067. [DOI] [PubMed] [Google Scholar]
- 6.Crabill MR, et al. Lead foreign body arthropathy in a horse. J Am Vet Med Assoc. 1994;205(6):864–866. [PubMed] [Google Scholar]
- 7.Bolanos AA, et al. Intra-articular histopathologic changes secondary to local lead intoxication in rabbit knee joints. J Trauma. 1995;38(4):668–671. doi: 10.1097/00005373-199504000-00038. [DOI] [PubMed] [Google Scholar]
- 8.Harding NR, et al. Experimental lead arthropathy: an animal model. J Trauma. 1999;47(5):951–955. doi: 10.1097/00005373-199911000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Kasten-Jolly J, Heo Y, Lawrence DA. Central nervous system cytokine gene expression: modulation by lead. Journal of biochemical and molecular toxicology. 2011;25(1):41–54. doi: 10.1002/jbt.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goebel C, et al. The gut cytokine balance as a target of lead toxicity. Life sciences. 1999;64(24):2207–2214. doi: 10.1016/s0024-3205(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 11.Ballock RT, et al. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158(2):414–429. doi: 10.1006/dbio.1993.1200. [DOI] [PubMed] [Google Scholar]
- 12.Kupcsik L, et al. Improving chondrogenesis: potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Eng Part A. 2010;16(6):1845–1855. doi: 10.1089/ten.TEA.2009.0531. [DOI] [PubMed] [Google Scholar]
- 13.Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthodontics & craniofacial research. 2005;8(1):11–17. doi: 10.1111/j.1601-6343.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi HY, Ricci G, Zafarullah M. Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes. Biochim Biophys Acta. 2008;1783(9):1605–1612. doi: 10.1016/j.bbamcr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2001;98(3):974–979. doi: 10.1073/pnas.98.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X, Liang M, Feng XH. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. J Biol Chem. 2000;275(47):36818–36822. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 17.Wu Q, et al. Induction of an osteoarthritis-like phenotype and degradation of phosphorylated Smad3 by Smurf2 in transgenic mice. Arthritis Rheum. 2008;58(10):3132–3144. doi: 10.1002/art.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavsak P, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6(6):1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 19.Serra R, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li TF, et al. Aberrant hypertrophy in Smad3-deficient murine chondrocytes is rescued by restoring transforming growth factor beta-activated kinase 1/activating transcription factor 2 signaling: a potential clinical implication for osteoarthritis. Arthritis Rheum. 2010;62(8):2359–2369. doi: 10.1002/art.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villegas-Navarro A, et al. Determination of lead in paired samples of blood and synovial fluid of bovines. Exp Toxicol Pathol. 1993;45(1):47–49. doi: 10.1016/s0940-2993(11)80454-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang CY, Lin YW, Yang JL. Activation of protein kinase Calpha signaling prevents cytotoxicity and mutagenicity following lead acetate in CL3 human lung cancer cells. Toxicology. 2008;250(1):55–61. doi: 10.1016/j.tox.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Dabrowska-Bouta B, Struzynska L, Rafalowska U. Effect of acute and chronic lead exposure on the level of sulfhydryl groups in rat brain. Acta Neurobiol Exp (Wars) 1996;56(1):233–236. doi: 10.55782/ane-1996-1125. [DOI] [PubMed] [Google Scholar]
- 24.Massague J, Attisano L, Wrana JL. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994;4(5):172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 25.Jokiranta TS, et al. Extracellular domain of type I receptor for transforming growth factor-beta: molecular modelling using protectin (CD59) as a template. FEBS letters. 1995;376(1–2):31–36. doi: 10.1016/0014-5793(95)01239-7. [DOI] [PubMed] [Google Scholar]
- 26.Crabb ID, et al. Differential effects of parathyroid hormone on chick growth plate and articular chondrocytes. Calcif Tissue Int. 1992;50(1):61–66. doi: 10.1007/BF00297299. [DOI] [PubMed] [Google Scholar]
- 27.Pateder DB, et al. PTHrP expression in chondrocytes, regulation by TGF-beta, and interactions between epiphyseal and growth plate chondrocytes. Exp Cell Res. 2000;256(2):555–562. doi: 10.1006/excr.2000.4860. [DOI] [PubMed] [Google Scholar]
- 28.Dare EV, et al. Differentiation of a fibrin gel encapsulated chondrogenic cell line. The International journal of artificial organs. 2007;30(7):619–627. doi: 10.1177/039139880703000710. [DOI] [PubMed] [Google Scholar]
- 29.Lau WF, Tertinegg I, Heersche JN. Effects of retinoic acid on cartilage differentiation in a chondrogenic cell line. Teratology. 1993;47(6):555–563. doi: 10.1002/tera.1420470607. [DOI] [PubMed] [Google Scholar]
- 30.Grigoriadis AE, Aubin JE, Heersche JN. Effects of dexamethasone and vitamin D3 on cartilage differentiation in a clonal chondrogenic cell population. Endocrinology. 1989;125(4):2103–2110. doi: 10.1210/endo-125-4-2103. [DOI] [PubMed] [Google Scholar]
- 31.Li TF, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. J Bone Miner Res. 2006;21(1):4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrana JL, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 33.Lagna G, et al. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383(6603):832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson CM, et al. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141(12):4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 35.Freije JM, et al. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. The Journal of biological chemistry. 1994;269(24):16766–16773. [PubMed] [Google Scholar]
- 36.Schulze-Tanzil G, et al. Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12(6):448–458. doi: 10.1016/j.joca.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Lires-Dean M, et al. Anti-apoptotic effect of transforming growth factor-beta1 on human articular chondrocytes: role of protein phosphatase 2A. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(11):1370–1378. doi: 10.1016/j.joca.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, et al. Correlation of apoptosis of articular chondrocytes in osteoarthritis with degree of cartilage destruction and expression of apoptosis-related proteins: surviving, caspase-3, and Bcl-xl. Zhonghua yi xue za zhi. 2008;88(19):1339–1341. [PubMed] [Google Scholar]
- 39.Drissi H, et al. Transcriptional regulation of chondrocyte maturation: potential involvement of transcription factors in OA pathogenesis. Mol Aspects Med. 2005;26(3):169–179. doi: 10.1016/j.mam.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Zuscik MJ, et al. Lead alters parathyroid hormone-related peptide and transforming growth factor-beta1 effects and AP-1 and NF-kappaB signaling in chondrocytes. J Orthop Res. 2002;20(4):811–818. doi: 10.1016/S0736-0266(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 41.Blaney Davidson EN, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. Journal of immunology. 2009;182(12):7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 42.Plaas A, et al. The relationship between fibrogenic TGFbeta1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(9):1081–1090. doi: 10.1016/j.joca.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Finnson KW, et al. Endoglin differentially regulates TGF-beta-induced Smad2/3 and Smad1/5 signalling and its expression correlates with extracellular matrix production and cellular differentiation state in human chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(11):1518–1527. doi: 10.1016/j.joca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Carmouche JJ, et al. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspect. 2005;113(6):749–755. doi: 10.1289/ehp.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]