Abstract
Purpose:
The purpose of this study was to evaluate whether transporting insulin aspart FlexPens via a pneumatic tube system affects the dosing accuracy of the pens.
Methods:
A total of 115 Novo Nordisk FlexPens containing insulin aspart were randomly assigned to be transported via a pneumatic tube system (n = 92) or to serve as the control (n = 23). Each pen was then randomized to 10 international unit (IU) doses (n = 25) or 30 IU doses (n = 67), providing 600 and 603 doses, respectively, for the pneumatic tube group. The control group also received random assignment to 10 IU doses (n = 6) or 30 IU doses (n = 17), providing 144 and 153 doses, respectively. Each dose was expelled using manufacturer instructions. Weights were recorded, corrected for specific gravity, and evaluated based on acceptable International Organization for Standardization (ISO) dosing limits.
Results:
In the group of pens transported through the pneumatic tube system, none of the 600 doses of 10 IU (0.0%; 95% CI, 0.0 to 0.6) and none of the 603 doses of 30 IU (0.0%; 95% CI, 0.0 to 0.6) fell outside of the range of acceptable weights. Correspondingly, in the control group, none of the 144 doses at 10 IU (0.0%; 95% CI, 0.0 to 2.5) and none of the 153 doses at 30 IU (0.0%; 95% CI, 0.0 to 2.4) were outside of acceptable ISO limits.
Conclusion:
Transportation via pneumatic tube system does not appear to compromise dosing accuracy. Hospital pharmacies may rely on the pneumatic tube system for timely and accurate transport of insulin aspart FlexPens.
Keywords: dosing accuracy, insulin delivery device
Pneumatic tube systems are commonly used in hospitals for transporting medications to patient care areas. However, limited information is published regarding the appropriateness of transporting medications via pneumatic tube. The transport of insulin pens via pneumatic tube has not been studied.
A pneumatic tube system contains a series of tubing that travels behind walls and between floors to allow timely delivery of materials without the use of a courier. At the sending station, medications or other items are placed inside reusable polycarbonate carriers, which are then inserted into the opening of the tubing. Blowers provide air-cushioned transport via vacuums and air pressure, allowing the containers to travel at consistent speeds throughout the tubing. Controlled but rapid deceleration occurs when the containers approach the receiving station to lessen the impact of the container exiting the tubing.1-4
Because shaking and dropping of the carrier may occur within the system, the potential for alterations in the medications has been documented as a reason not to transport insulin via pneumatic tube system.5-7 The insulin products currently marketed are biosynthetic proteins primarily of recombinant DNA origin, and protein denaturation may occur if insulin products are shaken.5-8
Insulin is available in glass vials and pens. An insulin pen is an injection device that is prefilled with insulin; it has been promoted as a more cost-effective delivery device than vial and syringe delivery forms in hospital settings.9-13 Furthermore, insulin pens have demonstrated increased dosing accuracy over vial and syringe.13-17
Pharmaceutical companies, including Novo Nordisk, state that insulin vials may be transported once via pneumatic tube system with appropriate padding to deter breakage of the glass vial.5-7 However, Novo Nordisk does not recommend transportation of the FlexPen via pneumatic tube due to the many physical and mechanical variables that may impact the functionality of the device (Medical Information, Novo Nordisk, Inc, written communication, December 21, 2009).
Timely and accurate delivery of insulin is critical to patient care. The purpose of this study was to evaluate the dosing accuracy of insulin pens after transport via pneumatic tube system. The International Organization for Standardization (ISO) provides an acceptable range of variability for dosing accuracy of insulin pens. The primary outcome of this study was to estimate the proportion of measured doses outside of acceptable ISO limits for the insulin pens transported via pneumatic tube.
Methods
Novo Nordisk FlexPens containing 3 mL insulin aspart were obtained from pharmacy stock. For this study, 115 pens from 23 total boxes with 8 different lots were used. Each box consisted of 5 pens. Using the manufacturer’s recommendations,18 this would allow for 24 measurements for a 10 international unit (IU) dose and 9 measurements for a 30 IU dose. Randomization was prespecified by the statistician. Four pens from each box (n = 92) were randomly selected to be transported via pneumatic tube from the institution’s hospital pharmacy to the outpatient pharmacy; 25 of these pens were used for 10 IU doses (n = 600), and 67 of these pens were used for 30 IU doses (n = 603). One pen from each box (n = 23) was selected to be a control (6 pens at 10 IU [n = 144] and 17 pens at 30 IU [n = 153]) to allow for a confirmatory assessment of dosing accuracy without use of the pneumatic tube system. Based on previous literature, dosing accuracy was assumed to be satisfactory.13-17 The control group also served to assess the unlikely scenario in which the pens included in the study were flawed.
Prior to transport and measurement, the pens were allowed to reach room temperature for 2 hours then padded within the carrier prior to transport. Each dose was expelled by an investigator (L.W., A.W., K.T.) using the technique described within the product labeling.18 Doses were deposited into a polystyrene container and weighed using an analytical balance (Mettler-Toledo, Inc, AE-50, Columbus, Ohio) which has an accuracy of 0.0001 g.
Dosing accuracy was measured by weight and corrected for specific gravity (insulin aspart = 1.005 g/mL at 20°C). Acceptable ISO limits are considered to be ±1 IU for 10 IU doses and ±1.5 IU for 30 IU doses. Measured in grams, this translates into measures between 0.0905 g to 0.1105 g for 10 IU doses and between 0.2865 g to 0.3165 g for 30 IU doses13,14 (Medical Information, Novo Nordisk, Inc, written communication, March 7, 2011).
The proportion of measured doses outside of acceptable ISO limits was estimated, along with an exact binomial 95% confidence interval (CI), separately for 10 IU and 30 IU doses and for pens transported via pneumatic tube system and control pens not transported via pneumatic tube system. Although the aim of this study was not to compare measured weights between pens transported via pneumatic tube system and control pens, we made these comparisons in a secondary analysis, separately for 10 IU and 30 IU doses, and used mixed effects linear regression models including a random effect for insulin pen. Statistical analyses were performed using R Statistical Software (version 2.11.0; R Foundation for Statistical Computing, Vienna, Austria).
Under the assumption that the proportion of measured doses outside acceptable ISO limits for pens transported via pneumatic tube is 0.5%, we included 92 total insulin pens transported via pneumatic tube in this study: 25 pens used for 10 IU doses (n = 600) and 67 pens used for 30 IU doses (n = 603). Our intent was to rule out as much as possible that this proportion is any higher than 1.5%, and therefore the upper limit of the 95% CI for this proportion is of primary interest. Given the study’s assumptions, these sample sizes would result in an upper limit of a 95% CI that will fall below 1.5%.
Results
A summary of the measured weights for the 10 IU and 30 IU doses that were used for pens transported with and without use of the pneumatic tube system is shown in Table 1. Measured weights of insulin aspart doses were recorded for both the pneumatic tube pens and control pens (Figures 1-4). For pens transported through the pneumatic tube system, none of the 600 doses at 10 IU (0.0%; 95% CI, 0.0 to 0.6) and none of the 603 doses at 30 IU (0.0%; 95% CI, 0.0 to 0.6) were outside of acceptable ISO limits. Similarly, for the smaller group of pens not transported through the pneumatic tube system, none of the 144 doses at 10 IU (0.0%; 95% CI, 0.0 to 2.5) and none of the 153 doses at 30 IU (0.0%; 95% CI, 0.0 to 2.4) were outside of acceptable ISO limits. When comparing measured weight between pneumatic tube and control pens, there was no significant evidence of a difference at 10 IU insulin aspart dose, where the mean measured weight was 0.0006 g lower for pneumatic tube pens (95% CI, 0.0017 g lower to 0.0005 g higher; P = .30). This lack of difference was also observed at 30 IU, where the mean measured weight was 0.0007 g lower for pneumatic tube pens (95% CI, 0.0018 g lower to 0.0004 g higher; P = .19).
Table 1.
Comparison of measured weight of insulin aspart doses administered via FlexPens to acceptable ISO limits
| Group |
Measured weight (g) |
Measured doses outside of acceptable ISO limitsa |
|||||
| Median | 25th – 75th percentiles | Min – Max | Mean | SD | n (%) | 95% CI | |
| Pneumatic tube | |||||||
| 10 IU (n=600 doses) | 0.1006 | 0.0994 – 0.1017 | 0.0921 – 0.1086 | 0.1006 | 0.0021 | 0 (0.0%) | 0.0 −0.6 |
| 30 IU (n=603 doses) | 0.2999 | 0.2980 – 0.3014 | 0.2894 – 0.3082 | 0.2997 | 0.0027 | 0 (0.0%) | 0.0 −0.6 |
| Control | |||||||
| 10 IU (n=144 doses) | 0.1010 | 0.1000 – 0.1024 | 0.0962 – 0.1065 | 0.1012 | 0.0018 | 0 (0.0%) | 0.0 −2.5 |
| 30 IU (n=153 doses) | 0.3004 | 0.2992 – 0.3020 | 0.2931 – 0.3060 | 0.3004 | 0.0022 | 0 (0.0%) | 0.0 −2.4 |
Acceptable ISO limits are 0.0905 g and 0.1105 g for 10 IU doses, and 0.2865 g and 0.3165 g for 30 IU doses.
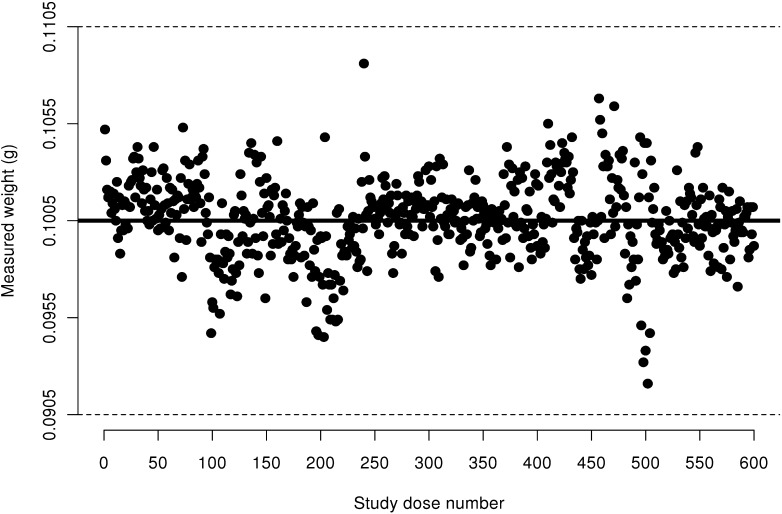
Figure 1.
Measured weight of 10 IU insulin aspart doses from FlexPens transported via pneumatic tube system. Acceptable ISO limits are between 0.0905 g and 0.1105 g; these limits are represented by dashed horizontal lines.
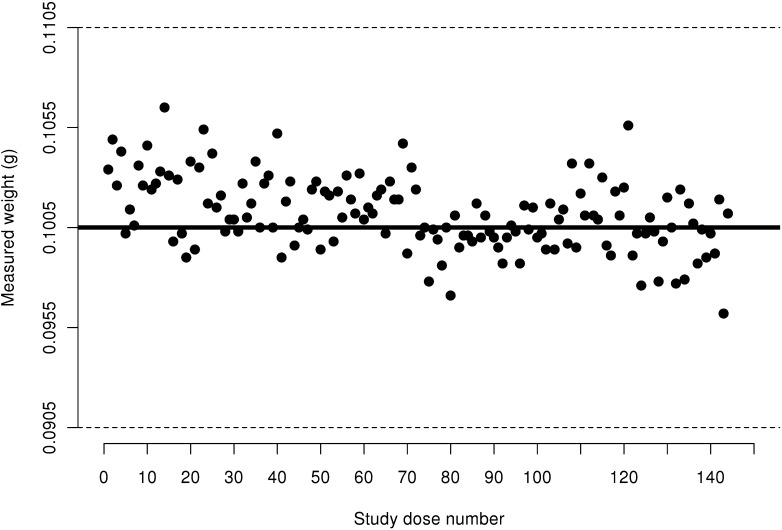
Figure 4.
Measured weight of 30 IU insulin aspart doses from FlexPens not transported via pneumatic tube system. Acceptable ISO limits are between 0.2865 g and 0.3165 g; these limits are represented by dashed horizontal lines.
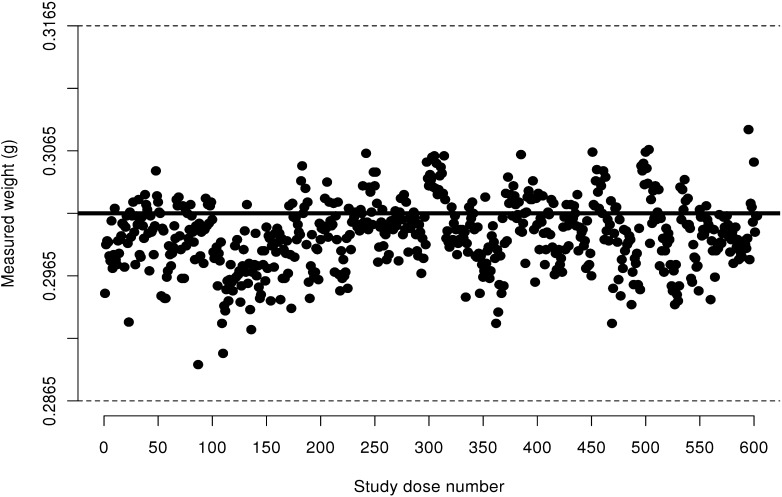
Figure 2.
Measured weight of 30 IU insulin aspart doses from FlexPens transported via pneumatic tube system. Acceptable ISO limits are between 0.2865 g and 0.3165 g; these limits are represented by dashed horizontal lines.
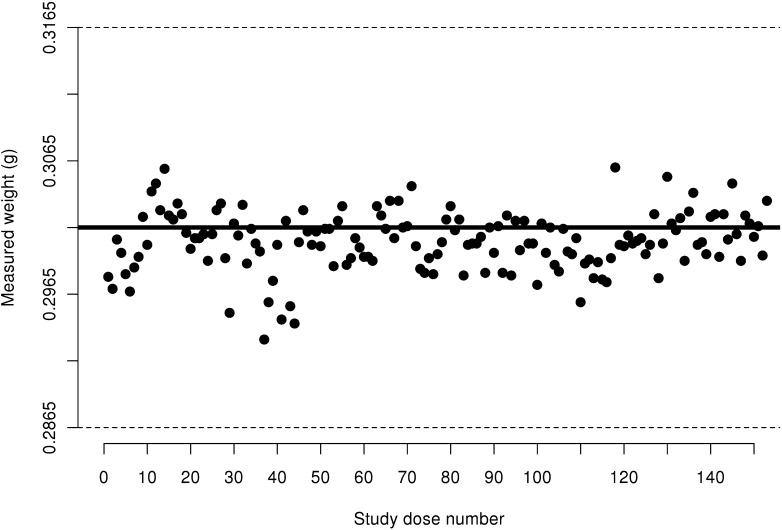
Figure 3.
Measured weight of 10 IU insulin aspart doses from FlexPens not transported via pneumatic tube system. Acceptable ISO limits are between 0.0905 g and 0.1105 g; these limits are represented by dashed horizontal lines.
Discussion
The results of this study provide evidence that the likelihood of the occurrence of a dose outside of acceptable ISO limits when using insulin pens transported via a pneumatic tube system is very small. More specifically, based on 95% confidence limits obtained from our data, we expect that no more than 0.6% of doses would be outside of acceptable ISO limits.
The methodology utilized in this study mirrors that of other studies evaluating dosing accuracy of insulin pens; however, our study only evaluated the insulin apart FlexPen.16,17 Hanel et al16 compared the dosing accuracy of the insulin glargine OptiClik (Sanofi-aventis), insulin glargine SoloStar (Sanofi-aventis), insulin detemir FlexPen (Novo Nordisk), and NPH insulin HumaPen LUXURA (Eli Lilly) based on compliance with the acceptable ISO limits. The investigators used 8 pens for the 2 volumes for each of the 4 pen types evaluated. The investigators used 192 doses per pen at 10 IU and 72 doses per pen at 30 IU (total of 64 pens and 1,056 measurements). Measurements outside of ISO limits were seen at 10 IU and 30 IU with the OptiClik (13 [6.8%] and 10 [13.9%]) and the SoloStar (1 [0.6%] and 3 [4.2%]). No doses were outside of the acceptable ISO limits with the FlexPen at 10 IU and 1 dose (1.4%) was outside of range at 30 IU. No doses were outside of the acceptable ISO limits for the HumaPen LUXURA. All doses outside of the acceptable ISO limit were underdoses. The investigators concluded that the FlexPen and HumaPen LUXURA were more accurate than the OptiClik and SoloStar.
In addition, Weise et al17 compared the dosing accuracy of the SoloStar filled with insulin glargine and the FlexPen filled with insulin detemir by investigating whether the pens complied with the acceptable ISO limits per dose. Eighteen pens were used for the 2 volumes for each pen type evaluated. The investigators used 432 doses per pen at 10 IU and 162 doses per pen at 30 IU (total of 72 pens and 1,188 doses). Measurements outside of ISO limits were seen at 10 IU and 30 IU with the FlexPen (1 [0.2%] and 1 [0.6%]) and SoloStar (2 [0.4%] and 3 [1.8%]). Similar to the study conducted by Hanel et al, all doses outside of the acceptable ISO limit were underdoses. The investigators concluded that the FlexPen was more accurate than the SoloStar.
Given that none of the insulin pens that were transported via a pneumatic tube system in our study resulted in doses outside of acceptable ISO limits, future studies involving even higher numbers of insulin pens are required to further understand how often doses outside of ISO limits occur when a pneumatic tube system is used and to better compare dosing accuracy between pens transported with and without use of the pneumatic tube system.
Limitations to this study include in vitro measurement of insulin aspart dose accuracy by a pharmacist and pharmacy interns. Insulin administration in a health system would likely be completed by a nurse or possibly self-administered by the patient. Although package insert instructions may be followed by these individuals, technique could vary between clinical administration to the patient and dose expelled into a container. Considering the potential for varied technique and the previously reported underdosing,16,17 the investigators question whether applied force or speed when pressing the push-button has an influence on the dose expelled. This may also warrant further investigation. Future research should consider dosing accuracy of the other insulin pens currently on the market if transported via pneumatic tube.
In conclusion, timely and accurate delivery of insulin is critical to providing patient care; therefore, hospital pharmacies may rely on the pneumatic tube system for transport of insulin products. No insulin aspart doses evaluated in the current study fell outside of the acceptable ISO limits. Transportation via pneumatic tube system does not appear to compromise dosing accuracy.
Acknowledgments
No author has any conflicts of interest to disclose or received funding related to this project or manuscript development.
References
- 1.Swisslog www.swisslog.com Accessed August 15, 2011.
- 2.Pneumatic Eagle. http://www.eaglepneumatic.com/ Accessed July 2, 2012.
- 3.Kelly Tube Systems. July 2, 2012 http://www.kellytubesystems.com/products.htm Accessed.
- 4.Pevco July 2, 2012 http://www.pevco.com/about_pneumatic.html Accessed.
- 5.Considerations for delivering meds via pneumatic tube Pharmacist’s Lett/Prescriber’s Lett. 2010;26(1):260116 [Google Scholar]
- 6.Peak A. Delivering medications via pneumatic tube systems. Hosp Pharm. 2003;38:287–290 [Google Scholar]
- 7.Peak A. Delivering medications via pneumatic tube system. Am J Health Syst Pharm. 2002;59:1376. [DOI] [PubMed] [Google Scholar]
- 8.McEvoy GK, ed. Insulins General Statement. American Hospital Formulary Service drug information (no. 68:20.08). Bethesda, MD: American Society; of Health-System Pharmacists; 2011:3175–3186 [Google Scholar]
- 9.Institute for Safe Medication Practices. http://www.ismp.org/Newsletters/acutecare/articles/20080508.asp Accessed April 28, 2009.
- 10.Davis EM, Christensen CM, Nystrom KK, et al. Patient satisfaction and costs associated with insulin administered by pen device or syringe during hospitalization. Am J Health Syst Pharm. 2008;65:1347–1357 [DOI] [PubMed] [Google Scholar]
- 11.Korytkowski M, Bell D, Jacobsen C, et al. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–2848 [DOI] [PubMed] [Google Scholar]
- 12.Ward LG, Aton SS. Utilizing an insulin therapeutic interchange to support insulin pen use within a hospital. Am J Health Syst Pharm. 2011;68:1349–1352 [DOI] [PubMed] [Google Scholar]
- 13.Keith K, Nicholson D, Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr (Phil). 2004;43:69–74 [DOI] [PubMed] [Google Scholar]
- 14.Lteif AN, Schwenk WF. Accuracy of pen injectors versus insulin syringes in children with type 1 diabetes. Diabetes Care. 1999;22:137–140 [DOI] [PubMed] [Google Scholar]
- 15.Gnanalingham MG, Newland P, Smith CP. Accuracy and reproducibility of low dose insulin administration using pen-injectors and syringes. Arch Dis Child. 1998;79:59–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanel H, Weise A, Sun W, et al. Differences in the dose accuracy of insulin pens. J Diabetes Sci Technol. 2008;2:478–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weise A, Pfutzner JW, Borig J, et al. Comparison of the dose accuracy of prefilled insulin pens. J Diabetes Sci Technol. 2009;3:149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NovoLog FlexPen [package insert] Princeton, NJ: Novo Nordisk Inc; 2011 [Google Scholar]