Abstract
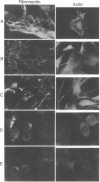
The biological and biochemical properties of Rous sarcoma virus-transformed and revertant field vole cells were investigated. Revertant vole cells appear morphologically similar to normal, uninfected cells, yet, like transformed vole cells, they are fully capable of growing in agar suspension and producing tumors in athymic nude mice. These highly tumorigenic, yet morphologically normal appearing, vole cells express viral-specific antigens such as the gag gene product (Pr76) but lack the env gene protein (gp85). Moreover, they contain the src gene protein, pp60src. These results support the concept of the pleiotropic nature of the src gene product and in addition suggest that pp60src may have multiply mechanisms of action. With this revertant cell system it may be feasible to distinguish between those biochemical functions of the src gene product that are important for tumorigenicity in vivo and those that are related to in vitro morphological transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Erikson E., Collett M. S., Erikson R. I. Peptide analysis of the transformation-specific antigen from avian sarcoma virus-transformed cells. J Virol. 1978 Jun;26(3):773–782. doi: 10.1128/jvi.26.3.773-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Stephenson J. R. Biochemical and immunological characterization of the major envelope glycoprotein of bovine leukemia virus. J Virol. 1977 Aug;23(2):443–447. doi: 10.1128/jvi.23.2.443-447.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R., Vogt V. M., Diggelmann H. Synthesis of avian RNA tumor virus structural proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1067–1075. doi: 10.1101/sqb.1974.039.01.122. [DOI] [PubMed] [Google Scholar]
- Erikson E., Brugge J. S., Erikson R. L. Phosphorylated and nonphosphorylated forms of avian sarcoma virus polypeptide p19. Virology. 1977 Jul 1;80(1):177–185. doi: 10.1016/0042-6822(77)90390-7. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P. Isolation of proteins by gel filtration in 6M guanidinium chloride: application to RNA tumor viruses. Anal Biochem. 1974 Jan;57(1):108–117. doi: 10.1016/0003-2697(74)90057-8. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J. Nature of Rous sarcoma virus-specific RNA in transformed and revertant field vole cells. J Virol. 1979 Feb;29(2):507–515. doi: 10.1128/jvi.29.2.507-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J., Spector D. H. Post-transcriptional control of avian oncornavirus transforming gene sequences in mammalian cells. Nature. 1977 Sep 8;269(5624):175–179. doi: 10.1038/269175a0. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Vogt P. K., Faras A. J. Quantitation and localization of Rous sarcoma virus-specific RNA in transformed and revertant field vole cells. J Virol. 1978 Feb;25(2):518–526. doi: 10.1128/jvi.25.2.518-526.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Panet A., Baltimore D., Hanafusa T. Quantitation of avian RNA tumor virus reverse transcriptase by radioimmunoassay. J Virol. 1975 Jul;16(1):146–152. doi: 10.1128/jvi.16.1.146-152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Hanson C. A., Aaronson S. A., Stephenson J. R. Type C viral gag gene expression in chicken embryo fibroblasts and avian sarcoma virus-transformed mammalian cells. J Virol. 1977 Jul;23(1):74–79. doi: 10.1128/jvi.23.1.74-79.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Yamada K. M., Yamada S. S., Pouysségur J., Pastan I. Microfilament bundles and cell shape are related to adhesiveness to substratum and are dissociable from growth control in cultured fibroblasts. Cell. 1977 Mar;10(3):375–380. doi: 10.1016/0092-8674(77)90024-1. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Goldberg A. R. Lack of correlation between tumorigenicity and level of plasminogen activator in fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4967–4971. doi: 10.1073/pnas.75.10.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Faras A., Varmus H., Vogt P. K., Yunis J. J. Integration of avian sarcoma virus specific DNA in mammalian chromatin. Exp Cell Res. 1975 Jul;93(2):484–486. doi: 10.1016/0014-4827(75)90477-2. [DOI] [PubMed] [Google Scholar]