Abstract
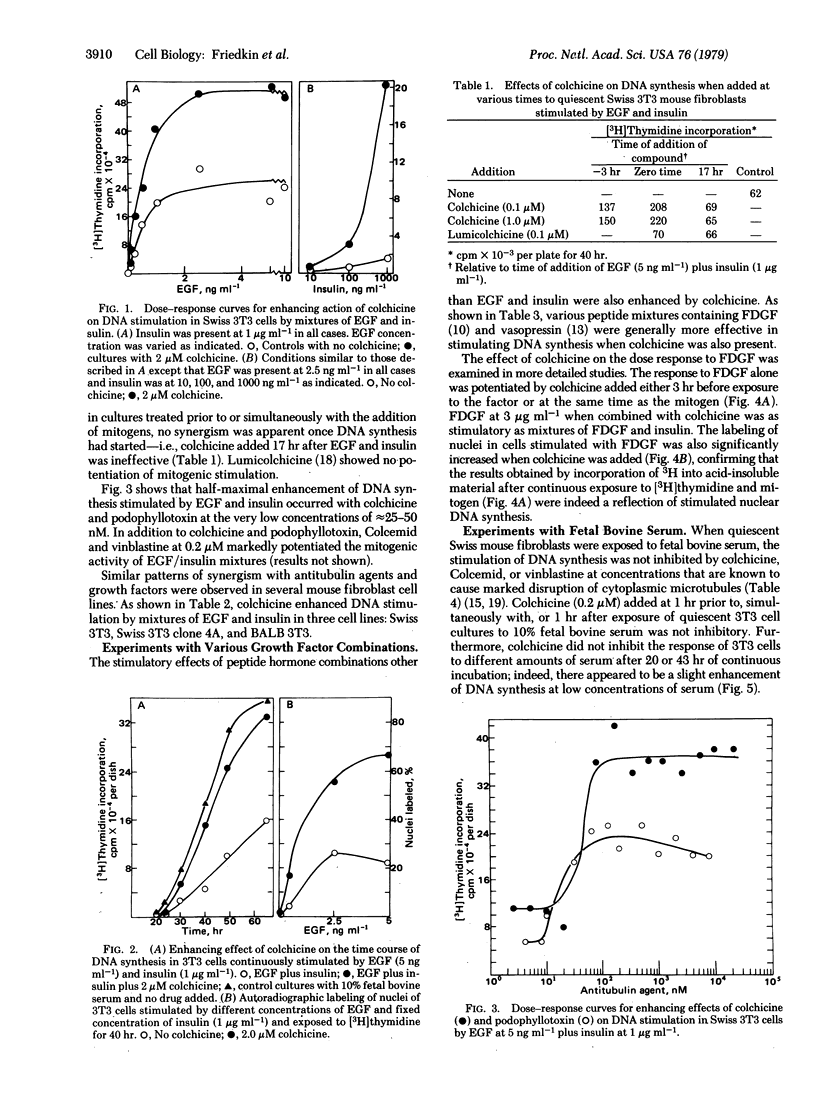
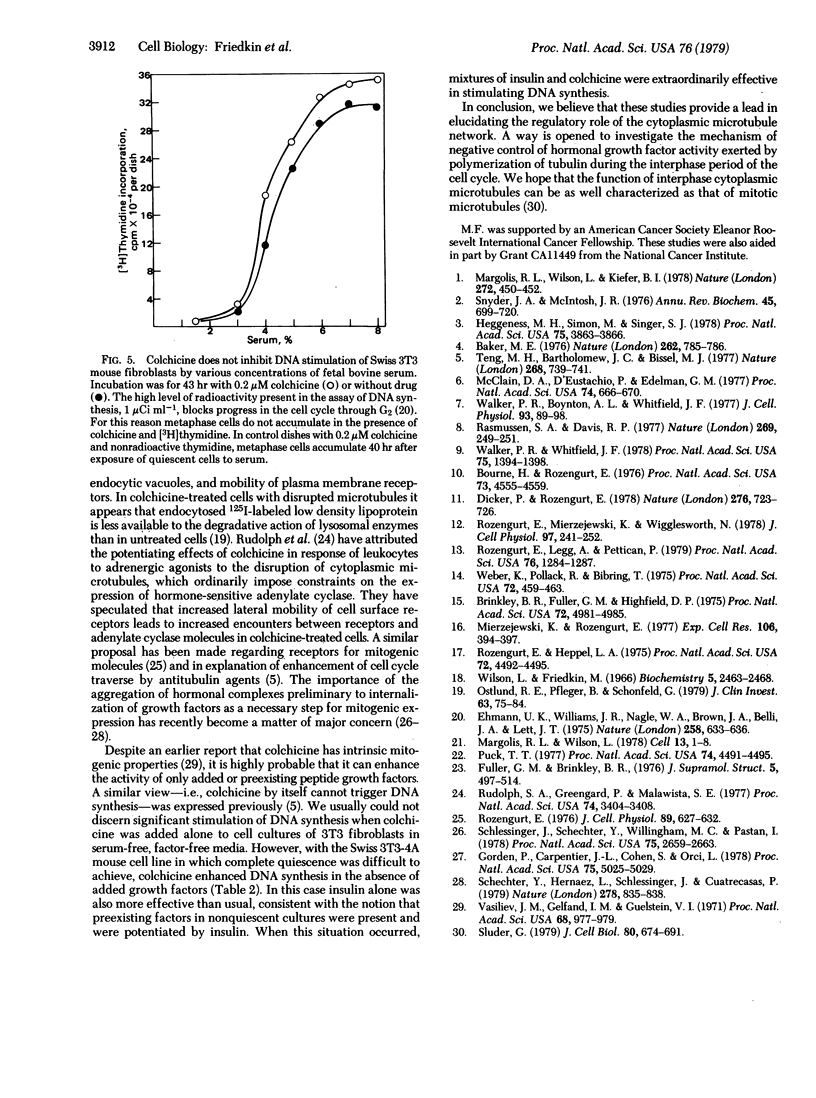
Colchicine and other antitubulin agents markedly enhanced the stimulation of DNA synthesis by combinations of various growth factors such as epidermal growth factor, insulin, fibroblast-derived growth factor, and vasopressin in serum-free cultures of several quiescent 3T3 mouse fibroblast cell lines. Enhancing effects were observed based on continuous incorporation of [3H]thymidine into DNA as well as by autoradiographic labeling of cell nuclei. The concentration of colchicine and podophyllotoxin required to produce half-maximal enhancement of DNA synthesis stimulated by epidermal growth factor and insulin was 25-50 nM. Lumicolchicine did not produce enhancing effects. The disassembly of microtubules resulting from the action of colchicine, Colcemid, and vinblastine did not inhibit the stimulation of DNA synthesis in quiescent Swiss 3T3 fibroblasts by fetal bovine serum. We conclude that the cytoplasmic microtubule network in 3T3 mouse fibroblasts does not exert a positive regulatory function in the initiation of DNA synthesis but rather can produce a constraint on the initial action of the peptide growth factors in serum-free media.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. E. Colchicine inhibits mitogenesis in C1300 neuroblastoma cells that have been arrested in G0. Nature. 1976 Aug 26;262(5571):785–786. doi: 10.1038/262785a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Ehmann U. K., Williams J. R., Nagle W. A., Brown J. A., Belli J. A., Lett J. T. Perturbations in cell cycle progression from radioactive DNA precursors. Nature. 1975 Dec 18;258(5536):633–636. doi: 10.1038/258633a0. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R. Structure and control of assembly of cytoplasmic microtubules in normal and transformed cells. J Supramol Struct. 1976;5(4):497(349)–514(366). doi: 10.1002/jss.400050407. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L., Keifer B. I. Mitotic mechanism based on intrinsic microtubule behaviour. Nature. 1978 Mar 30;272(5652):450–452. doi: 10.1038/272450a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- McClain D. A., D'Eustachio P., Edelman G. M. Role of surface modulating assemblies in growth control of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1977 Feb;74(2):666–670. doi: 10.1073/pnas.74.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierzejewski K., Rozengurt E. Vitamin B12 enhances the stimulation of DNA synthesis by serum in resting cultures of 3T6 cells. Exp Cell Res. 1977 May;106(2):394–397. doi: 10.1016/0014-4827(77)90187-2. [DOI] [PubMed] [Google Scholar]
- Ostlund R. E., Jr, Pfleger B., Schonfeld G. Role of microtubules in low density lipoprotein processing by cultured cells. J Clin Invest. 1979 Jan;63(1):75–84. doi: 10.1172/JCI109281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck T. T. Cyclic AMP, the microtubule-microfilament system, and cancer. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4491–4495. doi: 10.1073/pnas.74.10.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S. A., Davis R. P. Effect of microtubular antagonists on lymphocyte mitogenesis. Nature. 1977 Sep 15;269(5625):249–251. doi: 10.1038/269249a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Coordination of early membrane changes in growth stimulation. J Cell Physiol. 1976 Dec;89(4):627–631. doi: 10.1002/jcp.1040890419. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Mierzejewski K., Wigglesworth N. Uridine transport and phosphorylation in mouse cells in culture: effect of growth-promoting factors, cell cycle transit and oncogenic transformation. J Cell Physiol. 1978 Nov;97(2):241–251. doi: 10.1002/jcp.1040970213. [DOI] [PubMed] [Google Scholar]
- Rudolph S. A., Greengard P., Malawista S. E. Effects of colchicine on cyclic AMP levels in human leukocytes. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3404–3408. doi: 10.1073/pnas.74.8.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G. Role of spindle microtubules in the control of cell cycle timing. J Cell Biol. 1979 Mar;80(3):674–691. doi: 10.1083/jcb.80.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. doi: 10.1146/annurev.bi.45.070176.003411. [DOI] [PubMed] [Google Scholar]
- Teng M. H., Bartholomew J. C., Bissell M. J. Synergism between anti-microtubule agents and growth stimulants in enhancement of cell cycle traverse. Nature. 1977 Aug 25;268(5622):739–741. doi: 10.1038/268739a0. [DOI] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Guelstein V. I. Initiation of DNA synthesis in cell cultures by colcemid. Proc Natl Acad Sci U S A. 1971 May;68(5):977–979. doi: 10.1073/pnas.68.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. R., Boynton A. L., Whitfield J. F. The inhibition by colchicine of the initiation of DNA synthesis by hepatocytes in regenerating rat liver and by cultivated WI-38 and C3H10T1/2 cells. J Cell Physiol. 1977 Oct;93(1):89–97. doi: 10.1002/jcp.1040930112. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Whitfield J. F. Inhibition by colchicine of changes in amino acid transport and initiation of DNA synthesis in regenerating rat liver. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1394–1398. doi: 10.1073/pnas.75.3.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]



