Abstract
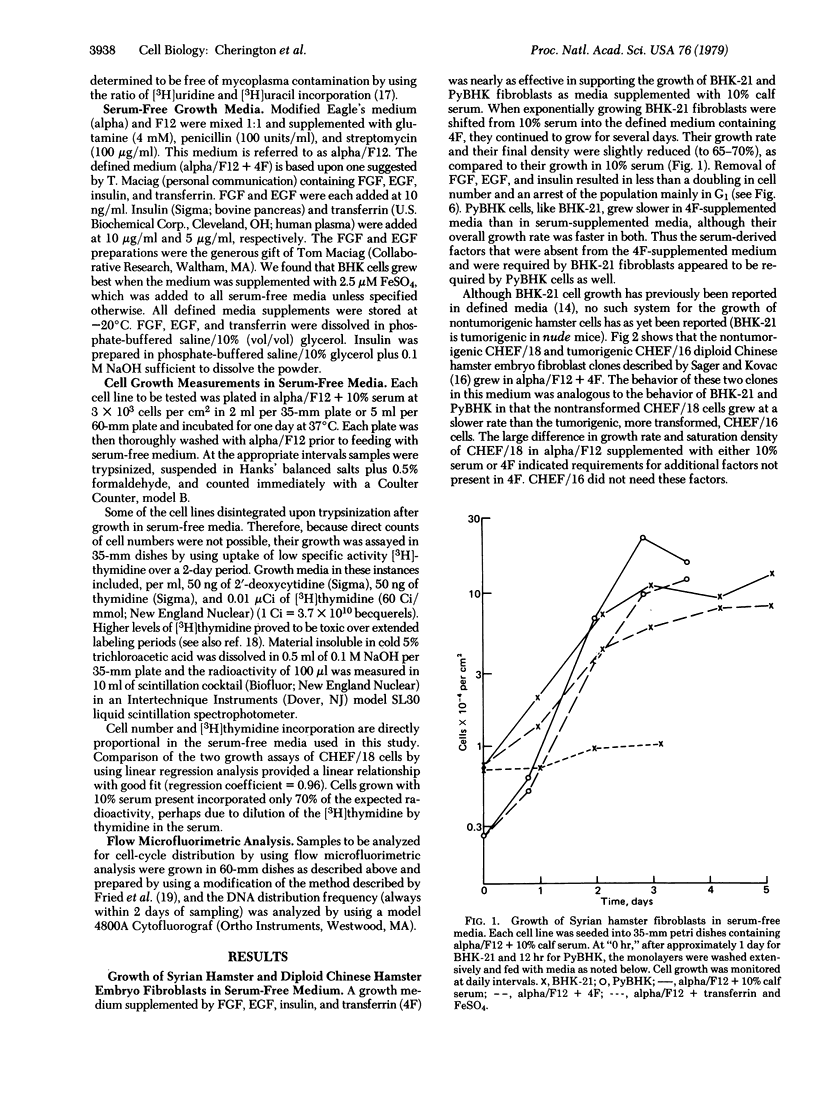
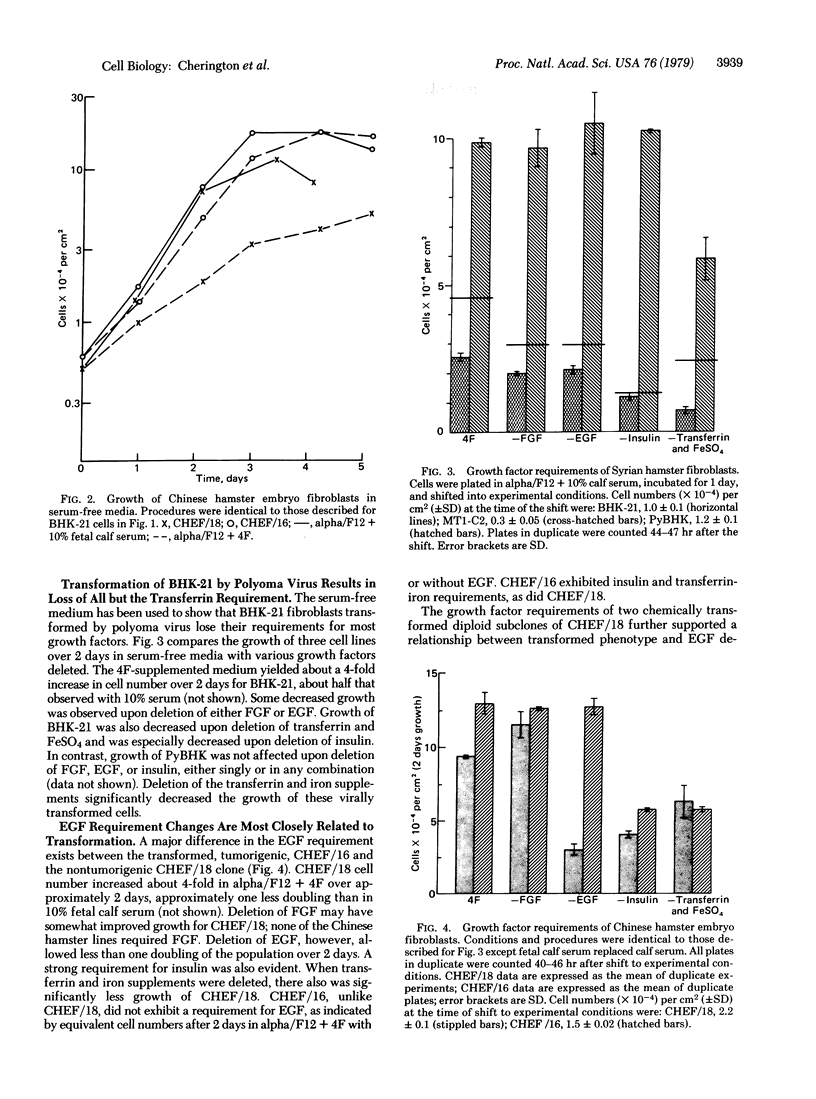
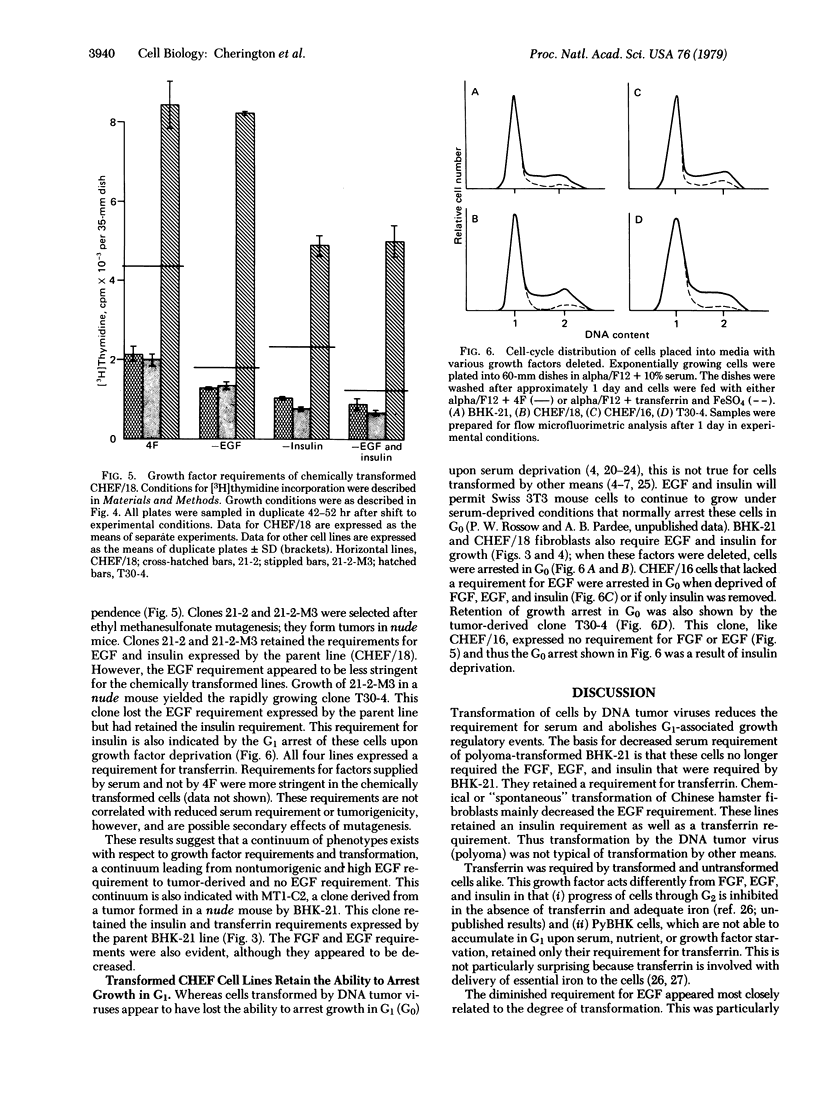
Serum provides growth factors that regulate and limit the growth of normal cells in tissue culture. Animal cells that are malignantly transformed usually exhibit diminished serum requirements for growth in culture. We have used a defined, serum-free medium to determine which of these growth factors becomes dispensable for the growth of transformed Syrian and Chinese hamster fibroblast cells. The medium's four growth factors—epidermal growth factor (EGF), insulin, fibroblast growth factor, and transferrin—were added or omitted as desired. A decreased requirement for EGF was most closely related to tumorigenicity of chemically (ethyl methanesulfonate) transformed cells in nude mice. All lines examined retained their requirement for transferrin, which is needed throughout the growth cycle, in contrast to the other factors, which are needed primarily in G1 phase. Lines that had lost their EGF requirement but had retained their insulin requirement were arrested in G1 by insulin deficiency, indicating that their growth control system remained. Mutagenesis with ethyl methanesulfonate can also create requirements of the transformed cells for unknown factors in serum. We conclude that an initial step that reduces the serum requirement in culture, and in tumorigenesis, is relaxation of the growth-regulatory function of EGF.
Keywords: serum-free medium, transformed phenotypes, growth control, diploid Chinese hamster embryo fibroblasts
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Identifying environmental chemicals causing mutations and cancer. Science. 1979 May 11;204(4393):587–593. doi: 10.1126/science.373122. [DOI] [PubMed] [Google Scholar]
- Bartholomew J. C., Yokota H., Ross P. Effect of serum on the growth of Balb oT3 A31 mouse fibroblasts and an SV40-transformed derivative. J Cell Physiol. 1976 Jul;88(3):277–286. doi: 10.1002/jcp.1040880303. [DOI] [PubMed] [Google Scholar]
- Bush H., Shodell M. Cell cycle changes in transformed cells growing under serum-free conditions. J Cell Physiol. 1977 Mar;90(3):573–583. doi: 10.1002/jcp.1040900319. [DOI] [PubMed] [Google Scholar]
- Clarke G. D., Stoker M. G., Ludlow A., Thornton M. Requirement of serum for DNA synthesis in BHK 21 cells: effects of density, suspension and virus transformation. Nature. 1970 Aug 22;227(5260):798–801. doi: 10.1038/227798a0. [DOI] [PubMed] [Google Scholar]
- Dubrow R., Riddle V. G., Pardee A. B. Different responses to drugs and serum of cells transformed by various means. Cancer Res. 1979 Jul;39(7 Pt 1):2718–2726. [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pol J. A., Bono V. H., Jr, Johnson G. S. Control of growth by picolinic acid: differential response of normal and transformed cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2889–2893. doi: 10.1073/pnas.74.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried J., Perez A. G., Clarkson B. D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J Cell Biol. 1976 Oct;71(1):172–181. doi: 10.1083/jcb.71.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A., Messmer T. O. Control of growth of benzo(a)pyrene-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3229–3232. doi: 10.1073/pnas.73.9.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Sager R. Noncoordinate expression of SV40-induced transformation and tumorigenicity in mouse cell hybrids. Somatic Cell Genet. 1979 Jan;5(1):129–143. doi: 10.1007/BF01538791. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Stein S. Resting state in normal and simian virus 40 transformed Chinese hamster lung cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1655–1659. doi: 10.1073/pnas.73.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer T. O. Nature of the iron requirement for Chinese hamster V79 cells in tissue culture medium. Exp Cell Res. 1973 Mar 15;77(1):404–408. doi: 10.1016/0014-4827(73)90594-6. [DOI] [PubMed] [Google Scholar]
- Mierzejewski K., Rozengurt E. Stimulation of DNA synthesis and cell division in a chemically defined medium: effect of epidermal growth factor, insulin and vitamin B12 on resting cultures of 3T6 cells. Biochem Biophys Res Commun. 1976 Nov 22;73(2):271–278. doi: 10.1016/0006-291x(76)90703-8. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Proper J. A., Volkenant M. E., Wells D. J., Getz M. J. Mechanism of growth arrest of chemically transformed cells in culture. Cancer Res. 1978 Sep;38(9):2807–2812. [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., James L. J. Selective killing of transformed baby hamster kidney (BHK) cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4994–4998. doi: 10.1073/pnas.72.12.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A., Bagwell C. B., Irvin G. L., 3rd Radiation from tritiated thymidine perturbs the cell cycle progression of stimulated lymphocytes. Science. 1979 Mar 9;203(4384):1025–1027. doi: 10.1126/science.424727. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Durbin H., Clingan D., de Asua L. J. Iron salts and transferrin are specifically required for cell division of cultured 3T6 cells. Biochem Biophys Res Commun. 1977 Apr 11;75(3):556–562. doi: 10.1016/0006-291x(77)91508-x. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Pledger W. J., Martin P., Antoniades H., Stiles C. D. Transforming viruses directly reduce the cellular growth requirement for a platelet derived growth factor. J Cell Physiol. 1978 Dec;97(3 Pt 1):371–380. doi: 10.1002/jcp.1040970312. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]


