Abstract
Background:
Surgical site infections (SSIs) are the leading cause of hospital-acquired infections and are associated with substantial health care costs, with increased morbidity and death. The Surgical Care Improvement Project (SCIP) contains standards that are nationally reported with the aim of improving patient outcomes after surgery. Our institution’s standards for antimicrobial prophylaxis in the perioperative period are more stringent than these measures and may be considered “beyond SCIP.” The 4 elements of appropriate antimicrobial prophylaxis are timing, antibiotic selection, dosing, and intraoperative redosing.
Objective:
To quantify antimicrobial SSI prophylaxis compliance in accordance with institutional standards and to identify potential opportunities for improvement.
Methods:
Patients aged 18 years or older were included if they had an SSI between January 1, 2009, and June 30, 2010, according to the database maintained prospectively by the Infection Prevention and Control Unit. Adherence to our institution’s practice standards was assessed through analysis of antibiotics administered—timing in relation to the incision, closure, and tourniquet inflation times for the procedure and antibiotic selection, dose, and redosing.
Results:
Overall noncompliance with all 4 elements of antimicrobial prophylaxis was 75.4% among the 760 cases. Repeat dosing had the greatest noncompliance (45.1%); antibiotic selection had the lowest incidence of noncompliance (10.8%).
Conclusions:
Noncompliance existed in each element of antimicrobial SSI prophylaxis, with antibiotic redosing leading in noncompliance. With the implementation of tools to assist the surgical team in following institutional standards, noncompliance will likely decline and additional research opportunities will exist.
Keywords: antibiotic prophylaxis, perioperative care, surgical wound infection
Surgical site infections (SSIs) are the second leading cause of hospital-acquired infections, with approximately 500,000 infections per year in the United States.1,2 A 1999 analysis estimated that SSIs are linked to approximately 20,000 in-hospital deaths each year and may account for $1 billion to $10 billion in US health care costs.3,4 In addition, considerable patient morbidity can occur, including declines in mental health, functional status, productivity, and quality of life.4 A bundle of interventions has been shown to be the most effective means of reducing surgical complications, and antibiotic prophylaxis is an important component in the reduction of SSIs.5 Studies conducted as early as 1961 have shown that wounds contaminated with Staphylococcus aureus were indistinguishable from those without microbial contamination after antibiotic administration before surgical incision.6 In response to the substantial impact these infections may have on patient morbidity, death, and health care costs, national quality measures have been implemented to improve patient outcomes.
The Centers for Medicare and Medicaid Services has implemented the Surgical Infection Prevention (SIP) Project and the Surgical Care Improvement Project (SCIP) to focus on patient outcomes after surgery. The SIP Project began in 2002 with the goal of decreasing morbidity and death associated with postoperative SSI. The project measures included appropriate selection of prophylactic antibiotics, administration of antibiotics within 1 hour before incision, and discontinuation of prophylactic antibiotics within 24 hours postoperatively.7 These 3 measures have since been adopted by The Joint Commission and have been reported on by the Hospital Quality Alliance.8,9
SCIP is an expansion of SIP Project efforts to improve patient outcomes postoperatively. It includes not only prevention of SSIs, but also prevention of venous thromboembolism, adverse cardiac events, and urinary and respiratory complications. The original 3 measures in SSI prevention of the SIP Project are included in SCIP measures with the exception of prophylactic antibiotics postoperatively in cardiac surgery, which may be used for up to 48 hours after surgery. Although these 2 national projects make progress in SSI prevention, the additional factors of appropriate dosing, specific timing of dosing based on the drug, and appropriate redosing of prophylactic antibiotics could have an effect on SSI development.
Our institution’s standards exceed the SCIP recommendations and are based on 4 principles for antibiotic prophylaxis—antibiotic selection, timing of the dose received before incision, dosing, and redosing. This medication use evaluation measured compliance on the basis of these principles for a subset of patients identified as having SSIs. We aimed to identify factors of antimicrobial SSI prophylaxis that had high incidence of noncompliance compared with the institution’s standards in order to focus on potential areas for improvement.
Methods
Patient Selection
From the internal database of patients who had SSIs, prospectively maintained by the Infection Prevention and Control (IPAC) Subcommittee of the Institutional Clinical Practice Committee, we included patients (inpatient, ambulatory, or short-stay, or a combination) aged 18 years and older who had undergone surgery between January 1, 2009, and June 30, 2010. This known subset of patients provided the opportunity for assessment of antibiotic prophylaxis utilization. Patients included in the IPAC database had an SSI in accordance with the Centers for Disease Control and Prevention criteria after a Type I or Type II surgical procedure. Cases are identified during surveillance of microbiology reports, re-admission diagnoses, surgical listings, consultation with infectious diseases services, and notification by other hospitals. Surgical specialties in the IPAC database for Type I procedures are cardiac, neurosurgery, orthopedics, general, and vascular surgery; specialties for Type II procedures are colorectal, general, gynecology, thoracic, and solid organ (liver, kidney, and pancreas) transplantation. The data of patients undergoing ear-nose-and-throat, urology, or plastic surgery procedures are not captured in the database prospectively and therefore are not included in this review. Patients were excluded from review if they did not give research authorization consent for chart review. Approximately 90% of surgical patients at this institution consent for chart review.
This retrospective review was approved by The Mayo Clinic Institutional Review Board. Collected data included patient age, height, weight, sex, and body mass index; wound classification (types I-VI); prophylactic antibiotics given (including drug name, dose, and time administered) and repeat doses after incision; incision and closure times; tourniquet inflation and deflation times when applicable; and the infectious isolate if available. Potential missing data led to the assumption that the event or activity did not occur or the medication was not administered. Adherence to institutional practice standards for SSI prophylaxis was then assessed.
Assessment
Compliance with institutional practice standards included meeting the 4 criteria. Antibiotic selection was considered appropriate if the antibiotic combination corresponded with Table 1 or a suitable alternative that had comparable microbial coverage.5,7,10-16 Antibiotic dosing was evaluated using the doses listed in Table 1, which were developed on the basis of guideline statements and institutional consensus for weight-based dosing of cephalosporins.5,10,11,13,15,16 Antibiotic timing compliance was met when the medication infusion was started within 1 hour of the incision time and completed before the incision. Exceptions included vancomycin and fluoroquinolones, which should be started between 60 and 120 minutes before the incision, depending on the necessary infusion time. Charting on antibiotic administration included antibiotic start times only. Therefore, infusion completion times were assumed a priori on the basis of start time charted and institutional intravenous administration guidelines. If a tourniquet was to be used in the procedure, the antibiotic infusion must have been complete before tourniquet inflation.
Table 1.
| Surgical procedure | Antibiotic choicea | Dosea |
| Cardiac | Cefazolin | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| OR vancomycin | 15 mg/kg | |
| Colorectal and appendectomy (nonperforated) | Cefoxitin or cefotetanc | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| OR cefazolinc | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb | |
| PLUS metronidazole | 500 mg | |
| OR ampicillin/sulbactamc | 3 g | |
| OR (metronidazole or clindamycin) PLUS (ciprofloxacinor levofloxacin) | 500 mg | |
| 600-900 mg | ||
| 400 mg | ||
| 500 mg or 750 mg | ||
| GI tract: esophageal or gastroduodenald | Cefazolinc | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| GI tract: biliarye | Cefazolinc | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| Gynecologic: hysterectomy | Cefoxitin, cefotetan, or cefazolinc | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| OR ampicillin/sulbactamc | 3 g | |
| OR (ciprofloxacin or levofloxacin) | 400 mg | |
| 500 mg or 750 mg | ||
| With colon involvement or high risk (ie, debulking) | PLUS (metronidazole or clindamycin) | 500 mg |
| 600-900 mg | ||
| Gynecologic: cesarean | Cefazolinc | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| Neurosurgery | Cefazolin | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| OR vancomycin | 15 mg/kg | |
| Orthopedic | Cefazolin or cefuroxime | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| 1.5 g | ||
| OR vancomycin | 15 mg/kg | |
| Thoracic (noncardiac) | Cefazolin or cefuroxime | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| 1.5 g | ||
| OR vancomycin | 15 mg/kg | |
| Vascular | Cefazolin | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| OR vancomycin | 15 mg/kg | |
| Kidney transplant | Cefazolin | <80 kg: 1 g; 80-120 kg: 2 g; >120 kg: 3 gb |
| Pancreas transplant | Piperacillin/tazobactam | 3.375 g |
| Liver transplant | Cefotaxime | 1 g |
Note: GI = gastrointestinal.
Antibiotic choice and dosing are in accordance with guideline recommendations and institutional consensus.5,7,10-16
For a patient weighing >120 kg, 2 g of cephalosporin should be used for repeat doses after the initial 3-g dose.
When the patient has allergy to penicillins or cephalosporins, reasonable treatment alternatives are clindamycin PLUS gentamicin, ciprofloxacin, levofloxacin, or aztreonam.
Only when morbid obesity, obstruction, or decreased acidity or motility is present.
When the patient is older than 70 years or has acute cholecystitis, nonfunctioning gallbladder, jaundice, or common duct stones.
Finally, compliance with antibiotic redosing was achieved when, in procedures longer than 3 hours, the same preprocedure antibiotics were given at the appropriate dose and within the time frames specified in Table 2. The redosing interval is 1 or 2 times the half-life for the specified antibiotic as listed in the package insert.5,10,12,15-25 For example, for cefazolin used preoperatively for antimicrobial prophylaxis, an additional dose should be administered every 2 to 5 hours intraoperatively until incision closure when the procedure lasts longer than 3 hours. There were 3 potential sources of noncompliance with respect to antibiotic redosing: (1) no redosing when redosing was indicated; (2) when antibiotics were redosed, the timing was noncompliant; and (3) the dosing of the antibiotic was noncompliant. If multiple repeat doses were given during the course of a procedure, only the initial repeat doses were assessed for appropriateness. Assessment of postoperative duration of antibiotic therapy was not completed because this evaluation was limited to the pre- and intraoperative periods. Descriptive statistical analysis was performed using Microsoft Excel (Microsoft Corp, Redmond, Washington).
Table 2.
Institutional criteria for intraoperative antibiotic redosing intervals for prevention of surgical site infections
Results
Between January 1, 2009, and June 30, 2010, 760 patients who had SSIs were captured in the IPAC database, from a total of 33,447 surgeries evaluated by IPAC at Mayo Clinic in Rochester, Minnesota. This number represents an overall SSI rate of 2.3% within these surgical specialties. Patient demographic characteristics, as well as the number of SSIs by surgical service, are described in Table 3. The average age of patients who had an SSI during this time was 57.6 years, and men comprised approximately half of the population. Most frequently, SSIs occurred in general surgery and orthopedic surgery; however, these cases represent only 4.4% of the 3,983 general surgery cases and 1.6% of the 10,377 orthopedic surgery cases in this time period. Noncompliance with 1 or more of the 4 elements of antibiotic administration for prophylaxis was 75.4% or 573 of the 760 patients reviewed.
Table 3.
Patient demographic characteristics
| Characteristic | Prevalencea (N=760) |
| Mean (SD) age, years | 57.6 (16.9) |
| Male | 369 (48.2) |
| Mean (SD) weight, kg | 81.9 (30.7) |
| No culture obtained | 118 (15.5) |
| Surgical specialty | |
| General | 174 (22.9) |
| Orthopedic | 165 (21.7) |
| Colon/rectal | 92 (12.1) |
| Gynecologic | 78 (10.3) |
| Neurologic | 71 (9.3) |
| Cardiac | 56 (7.4) |
| Thoracic | 54 (7.1) |
| Vascular | 42 (5.5) |
| Transplantation | 28 (3.7) |
Values are presented as number and percentage of patients unless specified otherwise.
Timing of Antibiotic Administration Prior to Incision
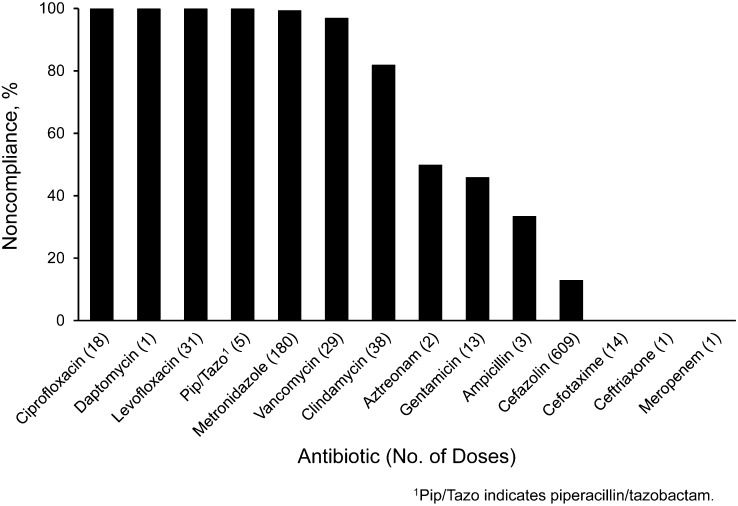
Many antibiotic doses failed both to start within 1 hour before incision (exceptions including fluoroquinolones and vancomycin) and to complete infusing before incision time (Figure 1). At our institution, cephalosporins are administered by intravenous push over 3 minutes; all other antibiotics assessed are administered as an intravenous piggyback over a longer period. Of the 3 cephalosporins used in antimicrobial prophylaxis in this patient group, there were no cases of noncompliance with timing before incision for cefotaxime and ceftriaxone (14 doses and 1 dose, respectively, observed), and there was 13% noncompliance for cefazolin (79 noncompliant of 609 doses observed). Metronidazole, cefazolin, levofloxacin, and clindamycin comprised 321 (84.5%) of the 380 doses with noncompliant timing before incision. Assessment of antibiotic administration timing compliance in relation to tourniquet inflation showed that only the orthopedic surgery and vascular surgery divisions used tourniquets. The vascular surgery division had no instances of antibiotic timing noncompliance in relation to tourniquet inflation; however, orthopedic surgery had a noncompliance rate of 11.1% (11 of 99 patients with tourniquets).
Figure 1.
Percent noncompliance in timing of antibiotic administration before surgical incision.
Antibiotic Selection
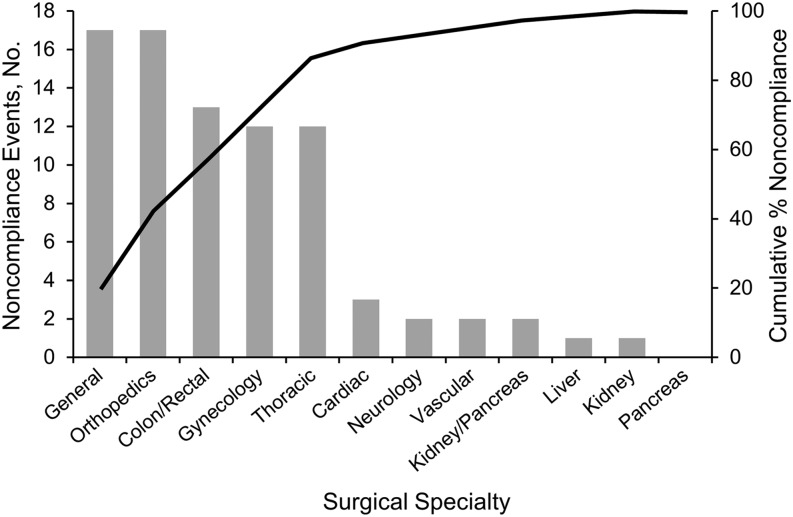
Antibiotic selection had the lowest incidence of noncompliance at 10.8%. The kidney and pancreas division demonstrated the highest percentage of noncompliance; however, this division had the fewest procedures assessed in the 18 months (2 noncompliance cases of 5 cases). Thoracic surgery had the next highest percentage of noncompliance in antibiotic selection (12 of 54 cases). The percentage of cases in which no antibiotics were charted was highest in orthopedic surgery, where 7.1% of patients with SSI did not receive antibiotics (12 of 168 patients). Figure 2 shows the surgical specialties included in the top 80% of antibiotic selection noncompliance. Of the 82 cases of antibiotic selection noncompliance, 86.5% occurred in the general, orthopedic, colon and rectal, gynecology, and thoracic surgery divisions.
Figure 2.
Antibiotic selection noncompliance: pareto chart.
Antibiotic Dosing
Vancomycin had the greatest incidence of noncompliant dosing, with 16 (55.2%) of the 29 doses not following practice recommendations. Cefazolin followed vancomycin in noncompliance (23.3%, or 142 of 609 doses). Gentamicin, clindamycin, and piperacillin/tazobactam had a low incidence of dosing noncompliance, and all other antibiotics had 0% noncompliance for dosing. Of the total 163 noncompliant doses of antibiotic for SSI prophylaxis, 87.1% (142 doses) were given as cefazolin. Although a large percentage of vancomycin doses were noncompliant, the doses account for only 9.8% of noncompliant antibiotic doses overall.
Antibiotic Redosing
Among the patients with surgical procedures exceeding 3 hours and who received preoperative antibiotics with a half-life of 1 to 2 hours, and therefore who had an indication for antibiotic redosing, 40.8% of patients (180 of 441 patients) did not receive a repeat antibiotic dose. Of patients who did receive repeat antibiotic doses intraoperatively, 34.1% (133 of 390 doses) were not appropriately dosed as per dosing guidelines. Among doses, 14.1% were noncompliant in the timing of redosing administration (55 of 390 doses). Noncompliance with 1 or more of these 3 elements of redosing resulted in an overall noncompliance rate with antibiotic redosing intraoperatively of 45.1% (343 of 760 patients).
Discussion
Many practices surrounding prophylaxis of SSIs are supported by decades of literature. Yet, elements of the antibiotic administration process still have an unknown impact on the success of SSI prophylaxis. On examining the elements required for successful SSI prophylaxis, we found many opportunities for noncompliance. This review showed that overall compliance to the 4 factors of antimicrobial prophylaxis for SSIs was 24.4%. These factors are appropriate antibiotic timing, selection, dosing, and redosing.
The question of optimal timing of prophylactic antibiotics for SSI has been in the medical literature for more than 40 years.5 A study in 1961 showed that wound contamination with Staphylococcus aureus was eliminated with the administration of antibiotics before incision.6 Further research, in 1969, showed a reduced frequency of wound infection with either preoperative or early postoperative antibiotic administration.25 Stone et al26 published a study in 1976 that showed a significant reduction in wound infection rates when antibiotics were given preoperatively instead of postoperatively or not at all. The administration of prophylactic antibiotics within the 60 minutes before incision was established in 1989.27 This practice was further supported in 1992 when a reduced risk of wound infection was found with preoperative antibiotics given within 2 hours of incision.28 Currently, no consensus exists on completing prophylactic antibiotic infusions before incision.
When considering the principle that adequate drug concentrations should be present at the site of potential wound infection for its prevention, some clinicians may argue that adequate concentration may not be achieved with only partial doses administered by the time of incision. The institutional practice standard is that the antibiotic dose should be completed before incision or tourniquet inflation. This standard ensures adequate drug concentrations at the intended site of action prior to incision or before restricting blood flow with the tourniquet. This standard was met in 57.2% of cases reviewed.
Assessment of compliance with antibiotic administration timing found the greatest overall noncompliance for antibiotics when the infusion time was longer than 5 minutes. Despite this overall trend, cefazolin had the second highest incidence of administration timing noncompliance. This degree of noncompliance is likely due to the commonality of cefazolin use, with only 13% of all administered doses being noncompliant. The frequent use of cefazolin would cause an expectation that it would have a greater incidence of noncompliance; however, the percentage of doses found to have timing noncompliance is low in comparison with other antibiotics, including metronidazole, fluoroquinolones, clindamycin, and vancomycin.
Quality measures currently in place could be affecting the administration time of antibiotics. For instance, the SCIP measures include administration of prophylactic antibiotics within 1 hour of incision time, yet they do not specify how much of the dose has to be administered before incision. Thus, in accordance with SCIP standards, a dose of antibiotics could be started only 1 minute before incision time and the measure would still be met. Although this administration may meet national quality metrics, it is not optimal practice for preventing SSIs if a guiding principle is optimal concentrations from incision to closure or end of procedure. Greater attention to completion of antibiotic administration before incision or tourniquet inflation may improve the incidence of SSI.
Given that the goal of SSI prophylaxis is to prevent infection of the wound by organisms potentially present at or near the area of incision or manipulation, investigators have theorized that the antibiotic should be dosed to allow adequate levels at the incision site in order to prevent microbial growth.10 Studies in obese patients receiving antimicrobial prophylaxis for SSI have shown that a 1-g dose of cefazolin consistently had blood and tissue levels lower than the minimum inhibitory concentration for both gram-positive and gram-negative organisms. When this dose was increased to 2 g preoperatively, the incidence of SSI decreased.11 This finding supports the idea that antibiotics must be dosed appropriately to allow for drug concentrations above the minimum inhibitory concentration for organisms likely present at the site of the procedure from incision through closure.
When examining noncompliance with antibiotic dosing in the present group of patients, we found that dosing according to guidelines or package insert occurred successfully for most antibiotics. Antibiotics commonly found to have usage noncompliance with dosing were cefazolin, vancomycin, and gentamicin—antibiotics with recommendations for weight-based dosing. During this study period, standardized dosing for cefazolin in the perioperative period was established at our institution. Emphasis on weight-based dosing had not been accomplished before 2009. Before this implementation, other dosing schemes may have been used, thus causing a higher rate of dosing noncompliance in the use of cefazolin to prevent SSIs. This guideline for weight-based dosing of cefazolin has since been implemented to improve this area of noncompliance.
Gentamicin and vancomycin have unique challenges for accurate weight-based dosing. These 2 medications are dosed on a milligram-per-kilogram basis, which provides greater room for error because the dosing body weight (an additional calculation for many institutions) is needed for aminoglycosides while actual body weight is used for vancomycin. In each of these instances, providers need to calculate a correct dose rather than use a dosing table. Thus, a systematic method with which to more accurately address weight-based dosing of antibiotics is necessary to ensure accuracy of dosing. Current quality measures implemented on a national level do not include attention to accurate dosing or redosing of antibiotics, and, thus, this aspect may be a neglected portion of the treatment bundle required for SSI prophylaxis.
In terms of appropriate antibiotic selection, a number of guidelines recommend prophylactic antibiotics on the basis of surgical site and procedure.10-16 Although small variations exist in these guideline documents, the choice of prophylactic antibiotic should accomplish one portion of the goal of preventing SSIs. Our institution’s practice standards are intended to include SCIP elements and to cover “beyond SCIP” elements.
The divisions of kidney and pancreas surgery and thoracic surgery had the greatest percentage of noncompliance in surgical cases that involved SSIs; however, these cases of noncompliance represent a small percentage of the overall number of cases with antibiotic selection noncompliance. More than 80% of antibiotic selection noncompliance occurs in the thoracic, gynecology, general, orthopedics, and colon and rectal surgery divisions (Figure 2), and therefore it would be the most prudent to address these areas.
The final factor involved in antibiotic prophylaxis for SSIs is redosing for longer surgical procedures. An analysis of cefazolin concentrations showed drug concentrations dropping below the target concentrations of free cefazolin approximately 3.5 hours after a single dose of antibiotics preoperatively.29 A study by Zanetti et al30 showed reduced risk of SSIs overall in patients who had antibiotics redosed intraoperatively in procedures lasting more than 400 minutes and reduced risk in patients with cardiac surgery procedures exceeding 240 minutes. Ohge et al31 found that cefazolin concentrations had dropped below 80% of the minimum inhibitory concentration in the adipose tissue and peritoneum for several organisms 3 hours after administration of the preoperative dose, thus recommending redosing at 3 hours. Scher32 confirmed the benefit of redosing cefazolin after 3 hours when he saw reduced rates of wound infection in patients who received a repeat dose of cefazolin for procedures that exceeded 3 hours compared with patients who received only a preoperative dose. Extrapolation from the published data on cefazolin redosing intraoperatively indicates that antibiotic redosing should occur within 1 or 2 half-lives for the duration of the procedure.
Antibiotic redosing was found to have the greatest overall noncompliance in SSI prophylaxis. The lack of repeat doses of antibiotics being administered was the largest source of noncompliance. This lack could have been due to oversight because of the many other activities occurring in the operating room. Among patients who received repeat antibiotic doses, many did not receive dosing in accordance with practice guidelines or the package insert. Such noncompliance could reflect a lack of understanding in dosing practices for repeat antibiotic dosing. For example, in several instances when a 2-g dose of cefazolin was appropriately given preoperatively, repeat doses of 1 g of cefazolin were given instead of repeating the initial 2-g dose. Finally, because the appropriate time frame for administering repeat doses of antibiotics differs on the basis of the antibiotic half-life, it is important to create a reference guide to improve the means for compliance in this area. This medication use evaluation validated implementation of steps to improve compliance in the 4 areas related to SSI prophylaxis.
It became apparent with this review that despite previous implementation of institutional standards for administration of prophylactic antibiotics for SSIs, full compliance with these 4 elements had not been achieved. Opportunities to simplify administration and integrate the practice standards into clinical work flow were identified.
The institution used this gained knowledge and work flow information to make improvements in compliance with the studied factors. These measures include a procedure-driven prompt in documentation software for antibiotic selection, a reminder from the documentation software in the operating room to redose antibiotics at a specific time, a change in the institutional intravenous administration guidelines to allow for metronidazole infusion over 30 minutes instead of 1 hour, and more specific institutional standardization of cefazolin dosing intraoperatively. Future research will analyze the effects of the implementation of these measures on compliance with institutional practice standards and on SSI rate.
Conclusion
Antibiotic prophylaxis is 1 aspect of the multitude of efforts necessary to minimize SSI. This assessment quantified the institution’s noncompliance in all 4 elements of antibiotic administration for SSI prophylaxis based on institutional standards. Several opportunities for improved compliance were identified and addressed within the institution. Further research will determine whether these changes will improve noncompliance.
Acknowledgments
The authors thank Mayo Clinic Pharmacy Services Informatics and Technology for continued support and assistance in retrieving patient data.
References
- 1.Burke JP. Infection control: a problem for patient safety. N Engl J Med. 2003;348(7):651–656 [DOI] [PubMed] [Google Scholar]
- 2.National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996 A report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control. 1996;24(5):380–388 [PubMed] [Google Scholar]
- 3.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–730 [DOI] [PubMed] [Google Scholar]
- 4.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9(2):196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg. 2011;253(6):1082–1093 [DOI] [PubMed] [Google Scholar]
- 6.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168 [PubMed] [Google Scholar]
- 7.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43(3):322–30 [DOI] [PubMed] [Google Scholar]
- 8. The Joint Commission. Accountability measures [core measure sets]. August 2011. http://www.jointcommission.org/assets/1/6/ACCOUNTABILITY_MEASURES_August_2011_rev.pdf. Accessed October 18, 2011.
- 9. U.S. Department of Health and Human Services Medicare hospital compare quality of care. http://www.hospitalcompare.hhs.gov. Updated May 17, 2012. Accessed October 18, 2011.
- 10.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR; Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20(4):250–278 [DOI] [PubMed] [Google Scholar]
- 11.Forse RA, Karam B, MacLean LD, Christou NV. Antibiotic prophylaxis for surgery in morbidly obese patients. Surgery. 1989;106(4):750–756 [PubMed] [Google Scholar]
- 12.Page CP, Bohnen JM, Fletcher JR, McManus AT, Solomkin JS, Wittmann DH. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg. 1993;128(1):79–88 Erratum in: Arch Surg. 1993;128(4):410 [DOI] [PubMed] [Google Scholar]
- 13.Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE, Jr, et al. ; Infectious Diseases Society of America. Quality standard for antimicrobial prophylaxis in surgical procedures. Clin Infect Dis. 1994;18(3):422–427 [DOI] [PubMed] [Google Scholar]
- 14.American Society of Health-System Pharmacists ASHP therapeutic guidelines on antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 1999;56(18):1839–1888 [DOI] [PubMed] [Google Scholar]
- 15.Antimicrobial prophylaxis for surgery In: Treatment Guidelines from the Medical Letter. 2009;7(82):47–52 [PubMed] [Google Scholar]
- 16.Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189(4):395–404 [DOI] [PubMed] [Google Scholar]
- 17.Cefazolin (cefazolin sodium) injection, powder, for solution. Schaumburg, IL: Sagent Pharmaceuticals; 2011. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 18.Claforan (cefotaxime sodium) injection. Bridgewater, NJ: Sanofi-Aventis; 2009. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 19.Unasyn (ampicillin sodium and sulbactam sodium) injection, powder, for solution. New York: Roerig; 2007. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 20.Zinacef (cefuroxime) injection, solution. Research Triangle Park, NC: GlaxoSmithKline; 2010. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 21.Mefoxin (cefoxitin sodium) injection, solution. Lake Forest, IL: Bioniche Pharma USA; 2009. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 22.Cleocin phosphate (clindamycin phosphate) injection, solution. New York: Pharmacia and Upjohn Company; 2011. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 23.Vancomycin hydrochloride injection, powder, lyophilized, for solution. Schaumburg, IL: APP Pharmaceuticals; 2011. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 24.Flagyl (metronidazole) injection, solution. Deerfield, IL: Baxter Healthcare Corporation; 2011. http://dailymed.nlm.nih.gov. Accessed January 11, 2013 [Google Scholar]
- 25.Polk HC, Jr, Lopez-Mayor JF. Postoperative wound infection: a prospective study of determinant factors and prevention. Surgery. 1969;66(1):97–103 [PubMed] [Google Scholar]
- 26.Stone HH, Hooper CA, Kolb LD, Geheber CE, Dawkins EJ. Antibiotic prophylaxis in gastric, biliary and colonic surgery. Ann Surg. 1976;184(4):443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galandiuk S, Polk HC, Jr, Jagelman DG, Fazio VW. Re-emphasis of priorities in surgical antibiotic prophylaxis. Surg Gynecol Obstet. 1989;169(3):219–222 [PubMed] [Google Scholar]
- 28.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–286 [DOI] [PubMed] [Google Scholar]
- 29.Koopman E, Nix DE, Erstad BL, Demeure MJ, Hayes MM, Ruth JT, et al. End-of-procedure cefazolin concentrations after administration for prevention of surgical-site infection. Am J Health Syst Pharm. 2007;64(18):1927–1934 [DOI] [PubMed] [Google Scholar]
- 30.Zanetti G, Giardina R, Platt R. Intraoperative redosing of cefazolin and risk for surgical site infection in cardiac surgery. Emerg Infect Dis. 2001;7(5):828–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohge H, Takesue Y, Yokoyama T, et al. An additional dose of cefazolin for intraoperative prophylaxis. Surg Today. 1999;29(12):1233–1236 [DOI] [PubMed] [Google Scholar]
- 32.Scher KS. Studies on the duration of antibiotic administration for surgical prophylaxis. Am Surg. 1997;63(1):59–62 [PubMed] [Google Scholar]