Abstract
A direct comparison of two strategies for designing antimicrobial polymers is presented. Previously, we published several reports on the use of facially amphiphilic (FA) monomers which led to polynorbornenes with excellent antimicrobial activities and selectivities. Our polymers obtained by copolymerization of structurally similar segregated monomers, in which cationic and non-polar moieties reside on separate repeat units, led to polymers with less pronounced activities. A wide range of polymer amphiphilicities was surveyed by pairing a cationic oxanor-bornene with eleven different non-polar monomers and varying the comonomer feed ratios. Their properties were tested using antimicrobial assays and copolymers possessing intermediate hydrophobicities were the most active. Polymer-induced leakage of dye-filled liposomes and microscopy of polymer-treated bacteria support a membrane-based mode of action. From these results there appears to be profound differences in how a polymer made from FA monomers interacts with the phospholipid bilayer compared with copolymers from segregated monomers. We conclude that a well-defined spatial relationship of the whole polymer is crucial to obtain synthetic mimics of antimicrobial peptides (SMAMPs): charged and non-polar moieties need to be balanced locally, for example, at the monomer level, and not just globally. We advocate the use of FA monomers for better control of biological properties. It is expected that this principle will be usefully applied to other backbones such as the polyacrylates, polystyrenes, and non-natural polyamides.
Keywords: amphiphiles, antimicrobial polymers, peptides, polynorbornene, ring-opening polymerization
Introduction
There is increasing interest in selective antimicrobial polymers whose potency against bacteria and non-toxicity towards mammalian cells distinguishes them from most polymeric biocides that are broadly poisonous.[1-6] This selectivity is especially important for both the development of new antibiotics, as well as for the incorporation of polymers into materials that will be in intimate contact with human tissue such as cardiovascular and orthopedic implants.[7,8] Thus, the number of antimicrobial polymers and peptidomimetics,[9-25] most of which draw structural and functional inspiration from a class of natural cationic macromolecules called antimicrobial peptides (AMPs),[26,27] has grown in recent years. In this vein, the physicochemical properties of a range of potent small molecules and polymers, collectively known as synthetic mimics of antimicrobial peptides (SMAMPs), have been extensively studied.[2,9-25,28,29]
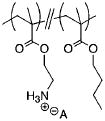
In examining the structural and biological activities of SMAMPs, synthetic polymer chemists have focused on tuning the hydrophobic/hydrophilic balance of their polymers. Altering the amphiphilicity of antimicrobial polymers has been accomplished most commonly through two methods (Table 1). First, in what we have termed the “segregated” route, relatively non-polar monomers are polymerized with a masked cationic monomer to produce positively charged, amphiphilic random copolymers. Using structurally different non-polar monomers that have a range of hydrophobicities or by adjusting feed ratios, the amphiphilicities of the copolymers can be straightforwardly varied. This strategy is exemplified by DeGrado[17] and Gellman,[12] independently, and by us in this paper (structures A–C respectively, Table 1).
Table 1.
Representative structures of antimicrobial polymers obtained by using “segregated” monomers (A, B, and this work, C) and “facially amphiphilic” monomers (D–F).[a]
| Schematic representation | General structure | Base selectivity | MIC [μg mL−1] | ||
|---|---|---|---|---|---|

|
A | ref. [17] |
|
3 | 300 (Ec) |
| B | ref. [12] |

|
32 | 3.1 (Bs) | |
| C |

|
20 | 75 (Sa) | ||
|
D | ref. [9] |

|
34 | 50 (Bs) |
| E | ref. [20] |

|
> 100 | 40 (Ec, Sa) | |
| F | ref. [25] |
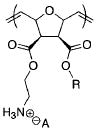
|
> 533 | <3.8 (Sa) | |
For each example, a series of polymers were synthesized and the best selectivities as defined by HC/MIC are noted above (HC=hemolytic concentration, MIC=minimum inhibitory concentration). All hemolytic values are based on an HC50 standard except for B which used a MHC (minimum hemolytic concentration).[12] The bacterial types tested giving these values are also shown (Ec=Escherichia coli, Bs=Bacillus subtilis, Sa= Staphylococcus aureus).
The second method, using “facially amphiphilic” (FA) repeat units has been a focus of our laboratory for some time.[15,20,24,25,32,33,35,36] In this design, every repeat unit carries a charged and non-polar moiety. Most commonly, it involves the synthesis of monomers with both a masked cationic and a tunable non-polar group. The spatial arrangement of polymer E (Table 1), or the linker flexibilities of polymer F, allows the charged and non-polar moieties to be positioned on opposite sides of the polymer backbone as it rearranges to interact with the phospholipid bilayer. This design has led to the discovery of several very selective polymers in which the molecules preferentially target specific bacteria over mammalian red blood cells (Table 1, polymers E and F).[20,25]
Within this definition, alkylated pyridinium homopolymers would be included since each repeat unit carries a cationic charge and non-polar alkylating side chain. Interestingly Tiller et al. have extensively used alkylated pyridinium homopolymers, to successfully endow surfaces with antimicrobial activity, in which every unit is FA and therefore the charge density was essentially equal for all chains.[6] Recently, Sen and co-workers (polymer D in Table 1)[9] synthesized two series of alkylated pyridinium copolymers. What they termed “same-centered” copolymers, with FA units, carried both a cationic charge and a non-polar alkyl chain while “different-centered” copolymers separated the charged pyridinium group and the alkyl group. Notably, the most selective copolymers were in the “same-centered” set although these are statistical random copolymers using both FA and neutral repeat units meaning that the charge density and spatial arrangement cannot be as carefully controlled compared to when all units are FA. In addition, this also does not allow them to be strictly placed within this second category but since they are based on alkylated polypyridines, which can be considered FA, we have placed them here. Similarly, Young-blood and co-workers used non-ionic hydrophilic groups to modify the hemolytic activity of alkylated pyridium polymers.[23]
Thus, with two successful studies on the use of FA monomers (Table 1, polymers E and F),[20,25] it was speculated that this method may be a more effective way to produce selective antibacterial polymers than the commonly employed segregated monomer route. In this report, we put this speculation to the test with a set of copolymers synthesized from masked cationic and non-polar alkyl substituted norbornene monomers (polymer C, Table 1). Most importantly, we believe this work presents a fair comparison between the two strategies (polymers C versus polymers E, Table 1) in which both methods use a norbornene backbone, the charged groups are always primary amines, and the non-polar alkyl groups are structurally similar. By convention, in the field of antimicrobial polymers, selectivity has been defined in this paper as the ratio between HC and MIC (hemolytic concentration defined as HC50 and minimum inhibitory concentration defined as MIC90, both typically reported in μg mL−1). Within the polynorbornene framework, this study provides a direct comparison between polymers composed of FA repeat units and polymers in which the charged and non-polar moieties are segregated on different monomers (Figure 1). Starting with the polyamine oxanorbornene homopolymer (PAON), little antibacterial and hemolytic activity was discovered. To test these ideas, new copolymers from segregated monomers were designed to survey a wide range of amphiphilicities. The strategy of copolymerizing segregated monomers converted PAON into active polymers though none were found with selectivities superior to those constructed of very comparable FA monomers. This implies that the FA monomer design is superior to the segregated monomer approach within the norbornene framework.
Figure 1.

Two methods to endow polynorbornenes, based on polyamine oxanorbornene, PAON, with antibacterial properties. The center structure was previously reported.[20] The right structure, A5′, synthesized for this study, shows a five-carbon branched chain as the non-polar moiety (one of eleven alkyl chains tested). Presumably, rotation about the single bonds (red curved arrows) in the polymer backbone allows for the orientation of these copolymers into a globally FA conformation.
Results and Discussion
Design and synthesis
With previous reports illustrating how antibacterial and hemolytic activities can be tuned by varying the amphiphilicity of membrane-disruptive polynorbornenes, we set forth to synthesize random copolymers separating monomers splitting the charged and non-polar groups (Figure 2). Instead of installing hydrophobicity early in the synthesis as was done in the previous system using FA monomers, a universal oxanorbornene imide precursor was used and derivatized with different alkyl chains (Figure S1).[30] One Boc-protected amine oxanorbornene and eleven other monomers carrying various alkyl chains were synthesized. As a starting point, 50:50 mol% random copolymers were targeted at two molecular weights (number average molecular weight, Mn) using ring-opening metathesis polymerization with Grubbs’ 3rd generation catalyst (low Mn = 2.7–4.2 kDa and high Mn = 12.2–15.9 kDa of the Boc-protected polymers).[30,31] Gel-permeation chromatography gave monomodal signals and narrow molecular weight indices (1.05–1.15). Using this strategy, copolymers having a broad range of amphiphilicities, but with approximately the same number of amines, and thus charges, could be easily obtained just by varying the alkyl comonomers (Figure 2).
Figure 2.

50:50 random copolymers synthesized at two different molecular weights. PAON and several non-50:50 compositions were also synthesized for a total of thirty-two polymers. Notation examples: PAON = the homo-amine polymer, A5′=50:50 random copolymer of the amine and the 5′ non-polar monomer.
Biological activities
MIC assays were used to evaluate the antibacterial properties of these polymers against Gram-negative E. coli and Gram-positive S. aureus (Table 2). Low Mn PAON was found to be inactive with an MIC of 400 μg mL−1 against both bacteria while the MIC values of the copolymers against E. coli showed a clear trend with the polymers of intermediate hydrophobicity (A4, A4′, A5, and A5′) having the most potent activities with MICs of 50 μg mL−1 (Table 2 and Figure 3). The most hydrophilic copolymer A1 and the most hydrophobic copolymer A12 were inactive similar to PAON. A similar trend was observed with S. aureus, although these copolymers performed slightly better against Gram-positive bacteria in general. Higher Mn copolymers were fairly inactive compared to their shorter analogues. In comparison, the previous study on polynorbornenes constructed from FA monomers identified a polymer with an MIC as low as 3.8 μg mL−1.[25] Therefore, while the route using segregated monomers was successful in endowing the inactive PAON with modest antibacterial properties by adding hydrophobicity, the MIC values were not as potent in general as the previously reported polynorbornenes.[20,25] Antimicrobial activity was also not as efficient as the AMP, Magainin, which was shown to possess an MIC of 12 μg mL−1 against E. coli.[24]
Table 2.
Antibacterial and hemolytic activities.
| Low Mn polymers[a] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAON | A1 | A2 | A3 | A4 | A4′ | A5 | A5′ | A6 | A6′ | A9 | A12 | |
| MIC (Ec) | 400 | 400 | 200 | 75 | 50 | 50 | 50 | 50 | 100 | 100 | 200 | 400 |
| MIC (Sa) | 400 | 100 | 50 | 25 | 50 | 50 | 75 | 50 | 100 | 50 | 125 | 100 |
| HC | 2000 | 2000 | 250 | 500 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| selectivity (Ec) | – | – | 1.3 | 6.7 | <1.0 | <1.0 | <1.0 | <1.0 | <05 | <05 | <0.3 | <0.1 |
| selectivity (Sa) | – | 20.0 | 5.0 | 20.0 | <1.0 | <1.0 | <0.7 | <1.0 | <0.5 | <1.0 | <0.4 | <0.5 |
|
| ||||||||||||
| High Mn polymers | ||||||||||||
| PAON | A1 | A2 | A3 | A4 | A4′ | A5 | A5′ | A6 | A6′ | A9 | A12 | |
|
| ||||||||||||
| MIC (Ec) | ≥ 400 | ≥ 400 | 200 | 50 | 100 | 200 | 400 | 400 | ≥ 400 | ≥ 400 | ≥ 400 | ≥ 400 |
| MIC (Sa) | 400 | 200 | 200 | 200 | 100 | 200 | 200 | 400 | 200 | 400 | 200 | 400 |
| HC | 2000 | 250 | 250 | 50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
| selectivity (Ec) | – | – | 1.3 | 1.0 | <0.5 | <0.3 | – | – | – | – | – | – |
| selectivity (Sa) | – | 1.3 | 1.3 | 0.3 | <0.5 | <0.3 | <0.3 | – | <0.3 | – | <0.3 | – |
MIC and HC values are reported in μg mL−1. Ec = Escherichia coli, Sa = Staphylococcus aureus, Selectivity = HC/MIC. Selectivity values are not given for inactive polymers (MIC ≥ 400 μg mL−1).
Figure 3.

MICs for E. coli of selected polymers illustrating optimal hydrophobicity for the most active ones A4, A5 (low Mn) and A3 (high Mn).
As expected, incorporating more hydrophobic groups onto the polyamine resulted in copolymers with increased ability to lyse human red blood cells.[20] A high hemolytic concentration (HC) is one measure of non-toxicity against mammalian cells and is essential for accurately capturing AMP activity. For the low Mn copolymers, undesirable hemolytic activities (HC ≤ 50 μg mL−1) were measured for A4 and all copolymers that were more hydrophobic. The higher Mn copolymers displayed undesirable hemolytic activities starting even sooner at A3. Selectivity values (HC/MIC) of 20 were observed for low Mn A1 and A3; selectivity for S. aureus was 20 for both copolymers. These values are better than those of Magainin, which has a selectivity of 10 for both E. coli and S. aureus.[24] Notably, these values are far below those observed for the most selective polymers in the previous studies utilizing FA monomers which were >100 and >533 (Table 1).[20,25]
Nonetheless, the ability to adjust biological activities using segregated monomers that split the charged and non-polar moieties was encouraging and thus feed ratios deviating from 50:50 were also explored. Non 50:50 copolymer analogues of low Mn A1, which was inactive, and A5′ which was active but not selective were tested (Table 3). Unexpectedly, the biological activities did not improve dramatically. In particular, incorporating more hydrophobicity into A1 hardly improved the activity as much as anticipated while increasing the charge density of A5′ did not eliminate the copolymer’s high hemolytic activity.
Table 3.
Biological data for non-50:50 copolymers (low Mn).
| A1 Copolymers | A5′ Copolymers | |||||||
|---|---|---|---|---|---|---|---|---|
| A0.810.2 | A0.610.4 | A0.410.6 | A0.210.8 | A0.85′0.2 | A0.65′0.4 | A0.45′0.6 | A0.25′0.8 | |
| MIC (Ec) | 400 | 400 | 250 | 200 | 50 | 25 | 100 | 250 |
| MIC (Sa) | 200 | 100 | 200 | 250 | 50 | 50 | 100 | 250 |
| HC | 250 | 2000 | 2000 | 500 | <50 | <50 | <50 | <50 |
| selectivity (Ec) | – | – | 8.0 | 2.5 | <1.0 | <2.0 | <0.5 | 0.6 |
| selectivity (Sa) | 1.3 | 8.0 | 10.0 | 2.0 | <1.0 | <1.0 | <0.5 | 0.6 |
A0.810.2 = a random copolymer with ≈ 80:20 molar ratio of monomers.
The inability to tune the biological properties was surprising given earlier findings.[20,25] It was thought that adding hydrophobicity to A1 via increasing the ratio of the non-polar unit should theoretically produce a copolymer with a similar amphiphilicity as the more hydrophobic copolymers A4 and A5, both of which were active (Table 2). In other words, if global amphiphilicity was a key determinant of activity then an A1 copolymer with a high enough ratio of non-polar groups (> 50%) should approach a hydrophobicity, and therefore activity, of A4 or A5 both of which were modestly antibacterial. Similarly, making A5′ more hydrophilic through feed ratio adjustments unpredictably did not decrease its hemolytic activity even though it was expected that a higher amine to non-polar monomer ratio could improve the HC value of A5′ (HC ≤ 50 μg mL−1) closer to that of the relatively less hydrophobic A1 (HC = 2000 μg mL−1).
It has been previously demonstrated that preformed secondary structures are not necessary for AMP-like activity.[13,17,24,32-34] Collectively, these studies imply that globally amphiphilic conformations, induced by the membrane bilayer, are able to effectively capture AMP activity which was recently supported by Gellman’s observations.[12] At the same time, Sen recently demonstrated that “same centered,” FA repeat units were superior to “different centered,” segregated ones.[9] All of our previous designs were based on the supposition that local amphiphilicity was critical to generating highly potent yet non-toxic SMAMPs. For the first time, we have directly compared our FA design to segregated ones and the inability to tune the biological activity of the copolymers argues that the spatial arrangement of charge and non-polar groups is important and not just the overall global amphiphilicity. In fact there may be profound advantages in how a polymer made from FA monomers interacts with the phospholipid bilayer compared to copolymerized segregated monomers.
The imperfect statistical nature of random copolymerization can lead to runs of polar and non-polar units, at least for some percentage of the polymer population (Figure 4A, shows a run of three cationic units as well as sections of adjacent non-polar units). This arrangement may not be optimal for disrupting the lipid membrane bilayer especially if the polymer loops in and out of the membrane rather than having close contacts with the membrane as postulated here for the polymers containing FA units (Figure 4B). With polymers from FA monomers, 100% of the polymer population has every monomer containing the identical ratio of cationic to hydrophobic character. The local charge density and the consistency of that density throughout the polymer are extremely important in the polynorbornene systems it appears. More structural studies of these polymers in the presence of lipid bilayers, such as in our previous work with antimicrobial oligomers (AMOs), are underway.[35,36]
Figure 4.
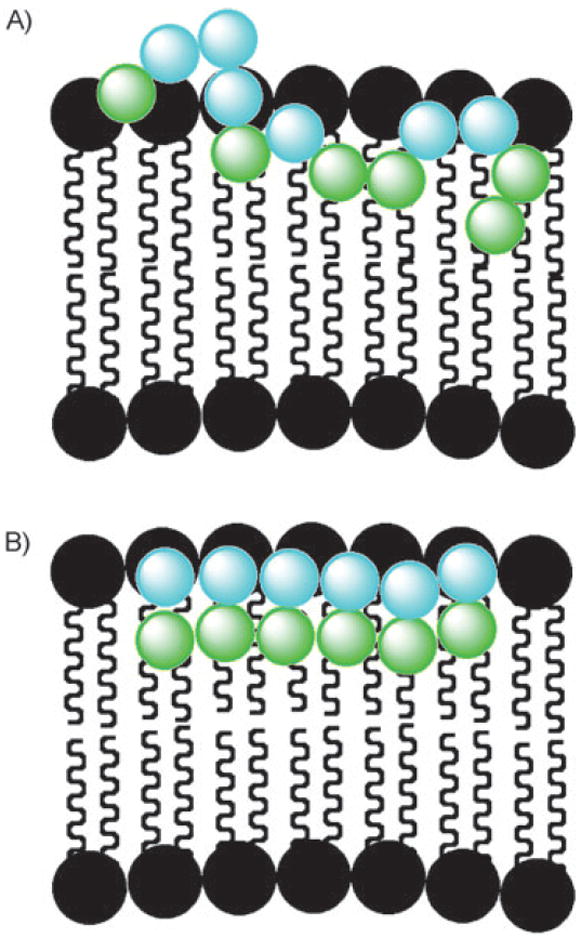
Cartoon proposing that polymer interactions with the polar head group (black circles) and the non-polar lipid tails (squiggly lines) of the membrane are significantly different for polymers from segregated monomers (A) versus that of polymers from FA monomers (B).
Membrane studies
Two experiments were used to probe the membrane-disruption activity of these copolymers; vesicle dye leakage and fluorescence microscopy. Polymer-induced leakage studies on dye-filled liposomes composed of E. coli lipid extracts were performed and showed that the most active copolymers caused the most dye release (Figure 5). These E. coli vesicles seemed to model membranes quite well with the dye-leakage results tracking approximately with the MIC values. Still, they were not perfect mimics of E. coli as was noticed when comparing the induced-leakage abilities of PAON and A12. Both PAON and A12 had an MIC of 400 μg mL−1 against E. coli yet A12 caused much more dye release. Presumably, in the complex interactions between these polymers and bacteria, more hydrophobic polymers can interact better with a liposome than with intact bacteria. Still, A12 did not cause as much leakage as the less hydrophobic but more antibacterial A4 and A5, showing that an optimum balance was critical to capture AMP-like activity.
Figure 5.
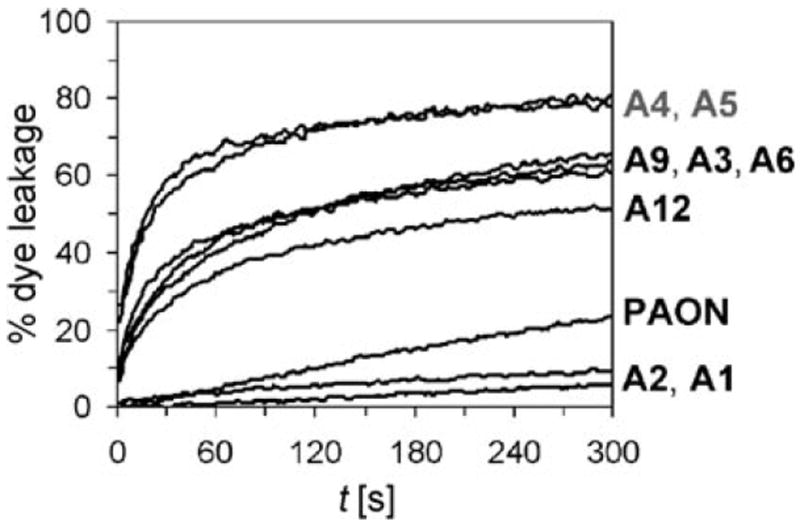
Graph of polymer-induced dye leakage.
Lastly, live E. coli bacteria were incubated with a two-component stain and treated with and without polymer (Figure 6). The stain was made up of a green-emitting dye (SYTO9 from Invitrogen) which has the ability to stain all cells and a red-emitting propidium iodide dye that can only enter cells with compromised membranes. Fluorescence microscopy showed that PAON essentially had the same effect on E. coli as the negative control. It was unclear though whether there was no significant interaction between the cationic PAON and the net anionic membrane of E. coli, or if there are interactions just not destructive ones. In contrast, antibacterial A4 not only displayed more intense red-emission but also agglutination of the cells possibly due to the aggregation of patches of torn membrane.
Figure 6.

Fluorescence microscopy of stained E. coli in the absence and presence of polymer. Each sample consisted of 108 bacteria cells incubated for 30 min with polymer (75 μg mL−1 final concentration) or buffer. Left panels were visualized with a green filter to display SYTO9 emission and the right panels show the same fields visualized with a red filter to display propidium iodide emission.
Conclusion
In this report we have a direct comparison between two strategies to make antimicrobial polynorbornenes. Using a common copolymerization strategy, several antibacterial polyamine oxanorbornenes were identified. On the other hand, a previous Scheme using designed “facially amphiphilic” monomers was reported to give polymers with better activities and superior selectivities. There exists tracts of charged and non-polar units (in other words not perfectly alternating) along the polymer backbone when the “segregated” monomer route is followed. This arrangement of repeat units was shown to play a significant role in the copolymer’s activity, especially for the low Mn copolymers which have an average degree of polymerization of 8. Important trends for the segregated copolymers were identified. In particular an optimum hydrophobicity was observed for the most active 50:50 copolymers.
Surprisingly, investigation of non-50:50 copolymers did not lead to improved activities or selectivities. This result argues that the balance of hydrophobic/hydrophilic areas at the local monomer level is much more critical to attain highly active and selective polymers rather than just the global amphiphilicity or overall charge density. This report further clarifies the importance of the spatial relationship of the charged and non-polar moieties in antimicrobial polymers and advocates the use of FA monomers for better control of biological properties. It is expected that this principle will be usefully applied to other polymeric backbones such as the polyacrylates, polystyrenes, and non-natural polyamides. A nice model system including strictly alternating copolymers would be an ideal further test.
Supplementary Material
Acknowledgments
We acknowledge the National Institutes of Health (RO1-GM-65803) and the Office of Naval Research (N00014-07-1-0520) for their generous support. We are also grateful for early contributions to the project by Jason Rennie and Courtney McConoghy.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.200801233.
References
- 1.Madkour AE, Tew GN. Polym Int. 2008;57:6–10. [Google Scholar]
- 2.Gabriel GJ, Som A, Madkour AE, Eren T, Tew GN. Mater Sci Eng R: Reports. 2007;57:28–64. doi: 10.1016/j.mser.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenawy ER, Worley SD, Broughton R. Biomacromolecules. 2007;8:1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 4.Klibanov AM. J Mater Chem. 2007;17:2479–2482. [Google Scholar]
- 5.Park D, Wang J, Klibanov AM. Biotechnol Prog. 2006;22:584–589. doi: 10.1021/bp0503383. [DOI] [PubMed] [Google Scholar]
- 6.Tiller JC, Liao C-J, Lewis K, Klibanov AM. Proc Natl Acad Sci USA. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hetrick EM, Schoenfisch MH. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche RO. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 9.Sambhy W, Peterson BR, Sen A. Angew Chem. 2008;120:1270–1274. [Google Scholar]; Angew Chem Int Ed. 2008;47:1250–1254. doi: 10.1002/anie.200702287. [DOI] [PubMed] [Google Scholar]
- 10.Radzishevsky IS, Rotem S, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. Nat Biotechnol. 2007;25:657–659. doi: 10.1038/nbt1309. [DOI] [PubMed] [Google Scholar]
- 11.Radzishevsky IS, Rotem S, Zaknoon F, Gaidukov L, Dagan A, Mor A. Antimicrob Agents Chemother. 2005;49:2412–2420. doi: 10.1128/AAC.49.6.2412-2420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowery BP, Lee SE, Kissounko DA, Epand RF, Epand RM, Weisblum B, Stahl SS, Gellman SH. J Am Chem Soc. 2007;129:15474–15476. doi: 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt MA, Weisblum B, Gellman SH. J Am Chem Soc. 2007;129:417–428. doi: 10.1021/ja0666553. [DOI] [PubMed] [Google Scholar]
- 14.Tang H, Doerksen RJ, Jones TV, Klein ML, Tew GN. Chem Biol. 2006;13:427–435. doi: 10.1016/j.chembiol.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Tew GN, Clements D, Tang H, Arnt L, Scott RW. Biochim Biophys Acta Biomembranes. 2006;1758:1387–1392. doi: 10.1016/j.bbamem.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Tang H, Doerksen RJ, Tew GN. Chem Commun. 2005:1537–1539. doi: 10.1039/b413679a. [DOI] [PubMed] [Google Scholar]
- 17.Kuroda K, DeGrado WF. J Am Chem Soc. 2005;127:4128–4129. doi: 10.1021/ja044205+. [DOI] [PubMed] [Google Scholar]
- 18.Gelman MA, Weisblum B, Lynn DM, Gellman SH. Org Lett. 2004;6:557–560. doi: 10.1021/ol036341+. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Choi S, Chen B, Doerksen RJ, Clements DJ, Winkler JD, Klein ML, DeGrado WF. Angew Chem. 2004;116:1178–1182. doi: 10.1002/anie.200352791. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:1158–1162. doi: 10.1002/anie.200352791. [DOI] [PubMed] [Google Scholar]
- 20.Ilker MF, Nüsslein K, Tew GN, Coughlin EB. J Am Chem Soc. 2004;126:15870–15875. doi: 10.1021/ja045664d. [DOI] [PubMed] [Google Scholar]
- 21.Tew GN, Liu D, Chen B, Doerksen RJ, Kaplan J, Carroll PJ, Klein ML, DeGrado WF. Proc Natl Acad Sci USA. 2002;99:5110–5114. doi: 10.1073/pnas.082046199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter EA, Wang XF, Lee HS, Weisblum B, Gellman SH. Nature. 2000;404:565–565. doi: 10.1038/35007145. [DOI] [PubMed] [Google Scholar]
- 23.Allison BC, Applegate BM, Youngblood JP. Biomacromolecules. 2007;8:2995–2999. doi: 10.1021/bm7004627. [DOI] [PubMed] [Google Scholar]
- 24.Arnt L, Nüsslein K, Tew GN. J Polym Sci Part A Polym Chem. 2004;42:3860–3864. [Google Scholar]
- 25.Lienkamp K, Madkour AE, Musante A, Nelson CF, Nüsslein K, Tew GN. J Am Chem Soc. 2008;130:9836–9843. doi: 10.1021/ja801662y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brogden KA. Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 27.Zasloff M. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 28.Gabriel GJ, Tew GN. Org Biomol Chem. 2008;6:417–423. doi: 10.1039/b714490n. [DOI] [PubMed] [Google Scholar]
- 29.Som A, Vemparala S, Ivanov I, Tew GN. Biopolymers. 2008;90:83–93. doi: 10.1002/bip.20970. [DOI] [PubMed] [Google Scholar]
- 30.See Supporting Information.
- 31.Love JA, Morgan JP, Trnka TM, Grubbs RH. Angew Chem. 2002;114:4207–4209. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:4035–4037. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 32.Arnt L, Tew GN. Langmuir. 2003;19:2404–2408. [Google Scholar]
- 33.Arnt L, Tew GN. J Am Chem Soc. 2002;124:7664–7665. doi: 10.1021/ja026607s. [DOI] [PubMed] [Google Scholar]
- 34.Oren Z, Shai Y. Biochemistry. 1997;36:1826–1835. doi: 10.1021/bi962507l. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Gordon VD, Mishra A, Som A, Purdy KR, Davis MA, Tew GN, Wong GCL. J Am Chem Soc. 2007;129:12141–12147. doi: 10.1021/ja072310o. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Tang H, Even MA, Wang J, Tew GN, Chen Z. J Am Chem Soc. 2006;128:2711–2714. doi: 10.1021/ja057029t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


