Abstract
Leukocyte activation and endothelial damage both contribute to cardiovascular disease, a major cause of morbidity and mortality in CKD. Experimental in vitro data link several protein-bound uremic retention solutes to the modulation of inflammatory stimuli, including endothelium and leukocyte responses and cardiovascular damage, corroborating observational in vivo data. However, the impact of these uremic toxins on the crosstalk between endothelium and leukocytes has not been assessed. This study evaluated the effects of acute and continuous exposure to uremic levels of indoxylsulfate (IS), p-cresylsulfate (pCS), and p-cresylglucuronide (pCG) on the recruitment of circulating leukocytes in the rat peritoneal vascular bed using intravital microscopy. Superfusion with IS induced strong leukocyte adhesion, enhanced extravasation, and interrupted blood flow, whereas pCS caused a rapid increase in leukocyte rolling. Superfusion with pCS and pCG combined caused impaired blood flow and vascular leakage but did not further enhance leukocyte rolling over pCS alone. Intravenous infusion with IS confirmed the superfusion results and caused shedding of heparan sulfate, pointing to disruption of the glycocalyx as the mechanism likely mediating IS-induced flow stagnation. These results provide the first clear in vivo evidence that IS, pCS, and pCG exert proinflammatory effects that contribute to vascular damage by stimulating crosstalk between leukocytes and vessels.
Cardiovascular disease remains the most important cause of death among patients with CKD,1 and it is associated with a baseline inflammatory status.2,3 Atherosclerosis is highly prevalent and advances more rapidly in individuals with renal dysfunction compared with the general population.4–6 A key role in the development of atherosclerosis is played by leukocyte–endothelial interactions.2
CKD is characterized by the progressive retention of a host of solutes. A substantial number of these compounds is protein-bound.7,8 The indole indoxylsulfate (IS) and the phenolic conjugates p-cresylsulfate (pCS) and p-cresylglucuronide (pCG) are prototype members of this group. Observational data associate these solutes with enhanced cardiovascular damage and progression of kidney failure, and in in vitro experiments, several underlying isolated molecular mechanisms support the link with these observational data.8–12 Although for all three compounds, one of two key mechanisms of vascular damage (i.e., leukocyte activation or endothelial dysfunction) have been shown in separate in vitro models, they were, to the best of our knowledge, never directly assessed by evaluating the complicated crosstalk between endothelium and leukocytes in an in vivo situation. Intravital microscopy permits in vivo visualization of leukocyte recruitment in translucent tissues in real time. This technique was already applied successfully by our group to study harmful effects of peritoneal dialysis solutions on peritoneal membrane physiology.13
The present study evaluates the effects of an acute peritoneal superfusion of the protein-bound uremic compounds IS, pCS, and pCG and also, continuous intravenous infusion of IS on the recruitment of circulating leukocytes in the rat peritoneal vascular bed using this intravital microscopic method.
Results
Superfusion Experiments
Lipopolysaccharide Induces Substantial Leukocyte Recruitment
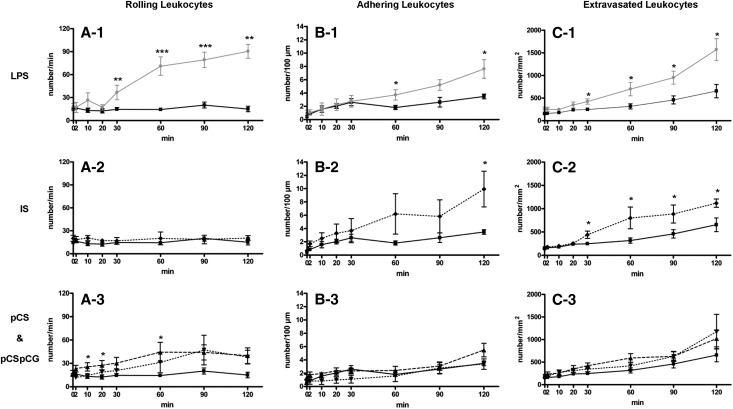
Before assessing leukocyte recruitment in response to an acute exposure to uremic solutes, we first evaluated the effect of lipopolysaccharide (LPS) as a positive control. As represented in Figure 1, there were no differences in baseline leukocyte recruitment values between the control and the LPS group. Stimulation with LPS dramatically and progressively increased the number of rolling leukocytes (number/min; A-1) compared with control from 30 minutes on (36.6±9.4 versus 14.6±2.3, P<0.01), reaching a number of 90.4±9.0 rolling leukocytes after 120 minutes exposure to LPS (P<0.01 versus control). The number of adhering leukocytes (number/100 µm; B-1) was significantly higher versus control after 60 minutes (3.7±0.8 versus 1.8±0.3, P<0.05), reaching a number of 7.6±1.4 after 120 minutes exposure to LPS (P<0.05 versus control). The number of extravasating leukocytes (number/mm2; C-1) increased progressively from 30 minutes on (425±59 versus 247±36, P<0.05), resulting in a total number of 1574±237 at 120 minutes (P<0.05 versus control). Figure 2 illustrates the increasing number of rolling, adhering, and extravasated leukocytes after different time periods of exposure to LPS in a representative experiment.
Figure 1.
Peritoneal leukocyte recruitment in response to superfusion with LPS, IS, pCS, and pCSpCG. The number of (A) rolling, (B) adhering, and (C) extravasated leukocytes at different time points during superfusion by HBSS (▪, full black line, n=12), HBSS with LPS (●, full grey line, n=8), HBSS with IS (◆, dotted line, n=9), HBSS with pCS (▲, broken line, n=8), and HBSS with pCS & pCG (▼, dotted line, n=6). *P<0.05; **P<0.01; ***P<0.001 versus HBSS.
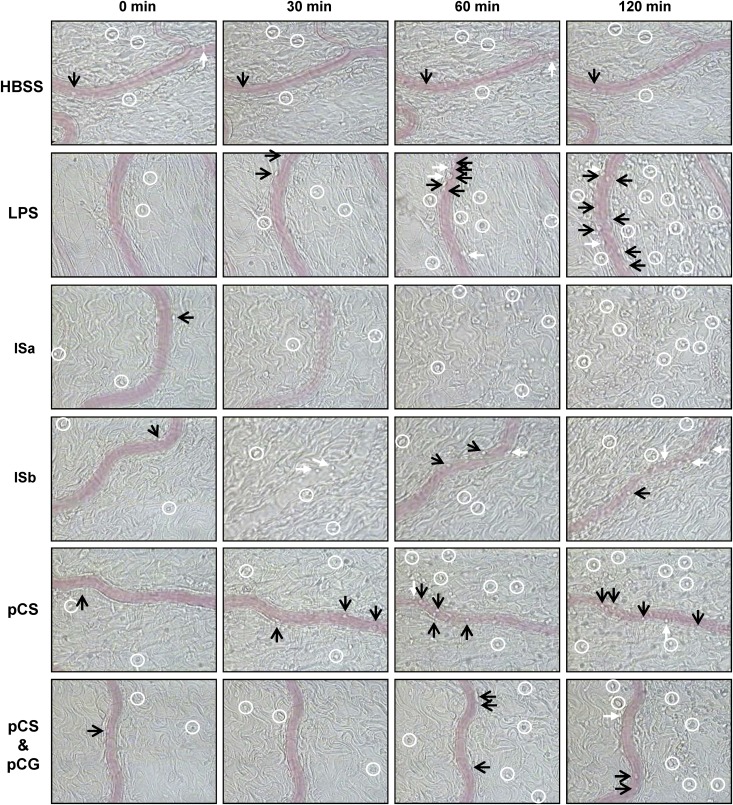
Figure 2.
Images of intravital microscopy during superfusion. Leukocyte rolling (black arrows), adhesion (white arrows), and extravasation (white circles) in response to LPS, IS, pCS, or the combination of pCS and pCG at baseline (t=0 min) and after 30, 60, and 120 min of superfusion. Row ISa is representative for four rats with permanent stop of VRBC after 30–60 min. Row ISb is representative for two rats with transient stop of VRBC, and return 30 min later. The disappearance of a visible signal for the vessel corresponds to the disappearance of moving erythrocytes and thus for a defective (ISa) or temporary (ISb) stop of flow. The number of symbols is not representative of the exact number of leukocytes with corresponding characteristics present in the panels. See online Supplemental Material for a short movie illustrating the difference between rolling and adhering leukocytes.
IS Induces Adhesion and Extravasation of Leukocytes Similar to LPS
Baseline leukocyte recruitment values were not different between the control and the IS group, which are represented in Figure 1 and illustrated in a representative experiment in Figure 2. Exposure to IS provoked similar effects as with the LPS-induced recruitment, except for the number of rolling leukocytes (A-2). A progressive increase in the number of adhering leukocytes (B-2) was observed (9.9±2.7 versus 3.5±0.3 at 120 minutes, P<0.05). A pronounced and progressive effect of IS on the number of extravasating leukocytes (C-2) was observed, reaching significance after 30 minutes of exposure (440±79 versus 247±36, P<0.05) and further increasing until 120 minutes (1120±89 versus 655±147, P<0.05).
pCS and the Combination of pCS and pCG Cause Moderate Leukocyte Recruitment
No difference in baseline values between the control and experimental groups was observed, which is shown in Figure 1 and illustrated in a representative experiment in Figure 2. Compared with control, superfusion with pCS caused an almost immediate but moderate increase in the number of rolling leukocytes (A-3) after 10 minutes (25.5±5.4 versus 13.2±2.6, P<0.05), with a maximal effect after 60 minutes of exposure (44.3±12.7 versus 14.3±2.2, P<0.01); subsequently, rolling stagnated. The combination of pCS and pCG induced a similar effect on rolling, but no additive effect of combining the two compounds was observed. No effect was seen on the number of adhering leukocytes (B-3) in the presence of pCS or the presence of the combination of both pCS and pCG together. Although a trend to an increase in the number of extravasating leukocytes (C-3) in the presence of pCS and pCSpCG after 120 minutes was observed, the increase was not significant.
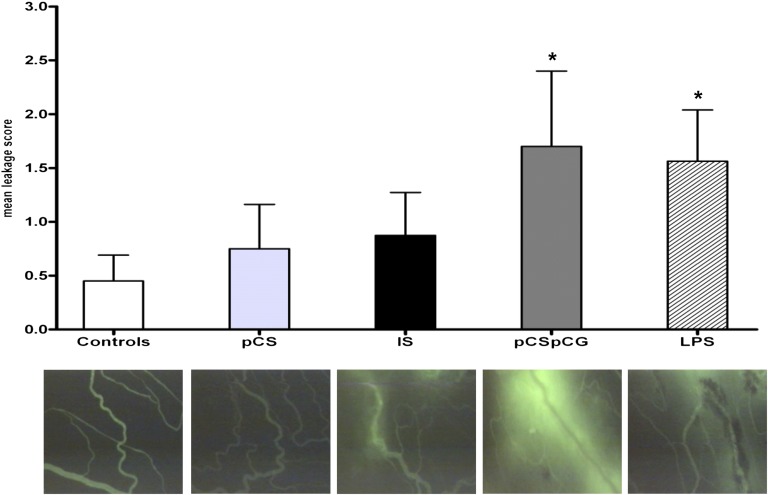
LPS and pCSpCG Induce Capillary Leakage
Capillary leakage was significantly higher compared with control in both the LPS and pCSpCG groups (P<0.05) (Figure 3).
Figure 3.
Capillary leakage. Mean leakage of the fluorescent isothiocyanate labeled albumin is significantly higher in the LPS and in the pCSpCG group, compared to controls. Representative pictures are shown (magnification ×100). *P<0.05 versus controls.
IS and to a Lesser Extent, the Combination of pCS and pCG Have Negative Effects on Blood Flow
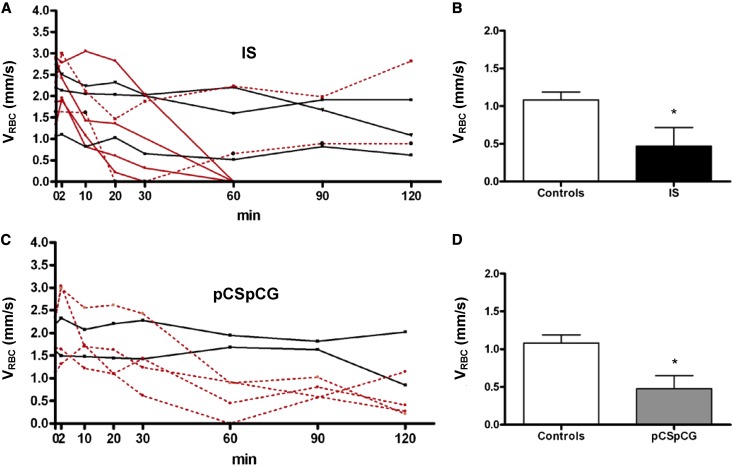
In six of nine IS-treated rats, an interruption of blood flow was observed, which is illustrated in Figures 2 and 4A. In four rats, flow stopped permanently after 30–60 minutes (Figure 4A). Furthermore, a strong decrease in red blood cell velocity (VRBC) was detected after 10–20 minutes in the other two rats. In one of these two rats, the blood flow disappeared completely but returned 30 minutes later (Figure 4A). The increased number of adhering and extravasating leukocytes with IS was not the result of a cessation of blood flow, because this increase was most pronounced in the experiments where blood flow remained stable (data not shown).
Figure 4.
Impaired red blood cell velocity subsequent to IS and pCSpCG superfusion. In six out of nine IS- treated rats, an interruption of flow was observed in the period between the 30th and the 60th minute of exposure (A, red lines). In four rats, flow stopped permanently after 30–60 min (A, full red lines). A strong decrease in red blood cell velocity (VRBC) was detected after 10–20 min in two other rats (A, dotted red lines). The remaining three animals showed less dramatic decreases of flow pattern (A, black lines). A strong blood flow decrease was observed in four out of six pCSpCG rats (C, dotted red lines). The combination of pCS and pCG caused a flow-stop in one rat after 60 min, but flow was restored. The remaining two animals showed only minor declines of VRBC (C, black lines). Comparing the lowest detected values, both for IS (B) and pCSpCG (D), lower values were found compared to control lowest values. *P<0.05 versus controls.
A strong blood flow decrease was also observed in four of six pCSpCG rats (Figure 4C). The combination of pCS and pCG caused a flow stop in one of four rats after approximately 60 minutes, but flow was restored again 30 minutes later. The flow in the three other rats showed a clear decline but remained present. pCS alone did not cause flow problems.
Hemodynamic Parameters
As shown in Table 1, no changes occurred in BP. Although baseline venular diameters (VDs) of the pCSpCG group were significantly lower compared with the pCS group, they remained stable throughout the evaluation period. No difference was observed with the control group. Unexpectedly, the flow problems observed after exposure to IS and pCSpCG were not reflected by significant changes in VRBC if the analysis was performed per time point. However, comparing the lowest detected values per animal for VRBC independently of time for both IS (Figure 4B) and pCSpCG (Figure 4D), lower values were found than for the nadir of controls (0.5±0.25 and 0.5±0.17 versus 1.1±0.11, respectively, P<0.05). Like for VRBC, a decreasing trend in venular wall shear rate (VWSR) was observed in the IS and pCSpCG rats, but the effect was not significant.
Table 1.
Hemodynamic parameters
| Time (min) | BP (mmHg) | VD (µm) | VRBC (mm/s) | VWSR (s−1) |
|---|---|---|---|---|
| Superfusion | ||||
| Controls (n=12) | ||||
| 0 | 132±3 | 18.9±1.1 | 1.7±0.14 | 774±85 |
| 30 | 133±3 | 19.1±1.2 | 1.5±0.11 | 656±53 |
| 60 | 126±3 | 19±1.3 | 1.4±0.15 | 595±73 |
| 90 | 122±3 | 18.9±1.5 | 1.2±0.16 | 535±75 |
| 120 | 117±6 | 17.1±1.1 | 1.5±0.18 | 751±99 |
| IS (n=9) | ||||
| 0 | 136±4 | 21.3±1.3 | 2.1±0.22 | 787±72 |
| 30 | 131±4 | 21.2±1.5 | 1.0±0.32 | 431±124 |
| 60 | 127±6 | 19.7±1.7 | 0.9±0.34 | 422±152 |
| 90 | 127±7 | 19.2±1.5 | 1.0±0.32 | 430±130 |
| 120 | 125±8 | 20.2±2.0 | 1.0±0.52 | 384±290 |
| pCS (n=8) | ||||
| 0 | 126±2 | 19.8±0.9 | 2.3±0.18 | 932±118 |
| 30 | 122±3a | 19.7±0.8 | 2.0±0.31 | 769±152 |
| 60 | 120±2 | 20.2±0.8 | 1.8±0.49 | 805±235 |
| 90 | 112±3a | 19.8±0.6 | 1.8±0.38 | 796±173 |
| 120 | 107±5 | 20.5±0.9 | 1.6±0.41 | 698±175 |
| pCSpCG (n=6) | ||||
| 0 | 129±6 | 15.8±0.8b | 1.9±0.20 | 948±94 |
| 30 | 129±6 | 15.1±1.2b | 1.6±0.30 | 810±110 |
| 60 | 127±6 | 15.3±1.2b | 0.9±0.40 | 513±198 |
| 90 | 119±4 | 15.8±1.1b | 1.2±0.20 | 600±129 |
| 120 | 110±13 | 15.9±1.2b | 0.8±0.30 | 425±151 |
| Infusion | ||||
| Controls (n=8) | ||||
| 0 | 121±4 | |||
| 60 | 113±4 | |||
| 120 | 111±2 | |||
| 150 | 105±6 | 17.4±0.6 | 1.8±0.33 | 847±133 |
| IS (n=6) | ||||
| 0 | 133±3 | |||
| 60 | 111±11 | |||
| 120 | 115±9 | |||
| 150 | 107±7 | 16.6±0.3 | 0.7±0.38a | 343±179a |
BP, VD, VRBC, and VWSR are shown as mean ± SEM.
P<0.05 versus control group.
P<0.05 versus pCS group at 0, 30, 60, 90, and 120 minutes.
Circulating Leukocytes and Hematocrit
Total numbers of leukocytes and hematocrit values were not significantly different between the groups at any of the experimental time points (data not shown).
Continuous Infusion Experiments with IS
Continuous Infusion with IS Elicits Similar Effects as Local IS Superfusion
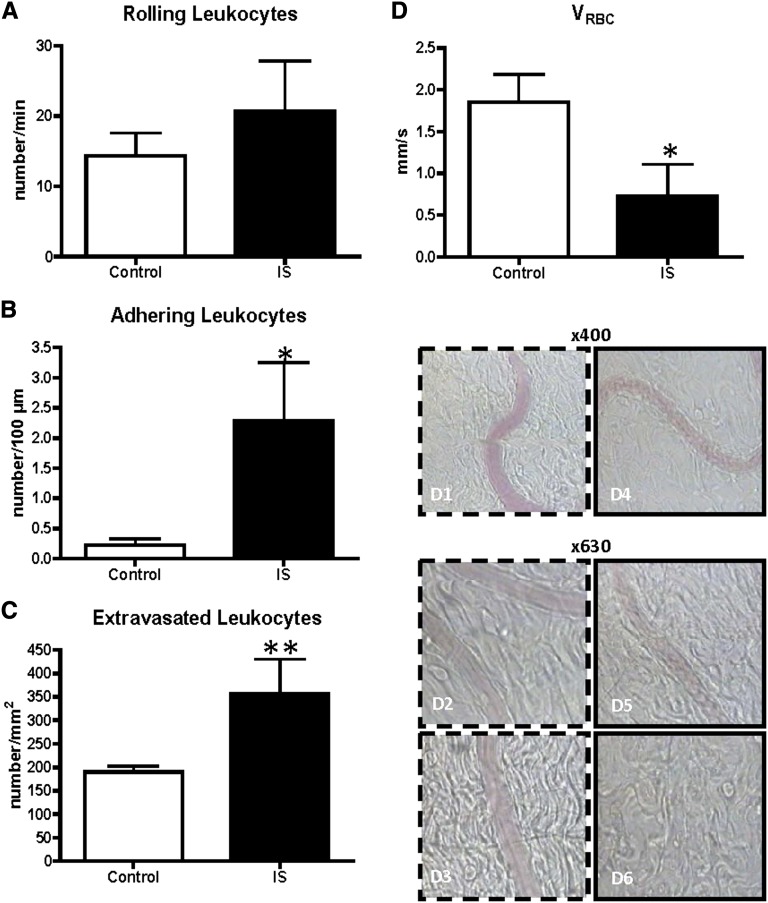
Intravascular infusion with IS (mean concentration=232.7±4.7 mg/L) also induced significantly more leukocyte adhesion (2.28±0.97 versus 0.22±0.11, P<0.05) and extravasation (357.1±73.4 versus 189.8±12.7, P<0.01) compared with controls (Figure 5, A–C).
Figure 5.
Peritoneal leukocyte recruitment (A–C) and effect on flow (D) after intravenous infusion with indoxylsulfate. The number of (A) rolling, (B) adhering, and (C) extravasated leukocytes, after 150 min of continuous infusion with indoxylsulfate (IS) at concentrations in the uremic range. Red blood cell velocity (VRBC) (D) was impaired after infusion with IS (D4–6), compared to the controls (D1–3). Controls (white bars, n=8); IS (black bars, n=6). *P<0.05 versus controls; **P<0.01 versus controls.
Also, five of six rats showed a disturbed blood flow pattern with a significantly lower VRBC compared with controls (0.72±0.38 versus 1.85±0.33, P<0.05), although no complete flow stop occurred (Figure 5D).
Hemodynamic Parameters
Similar to the superfusion experiments, no changes in BP occurred. The measured VD was not significantly different between the two groups. Flow problems after intravascular exposure to IS were reflected by significant changes in VRBC and VWSR (Table 1).
Disruption of the Glycocalyx
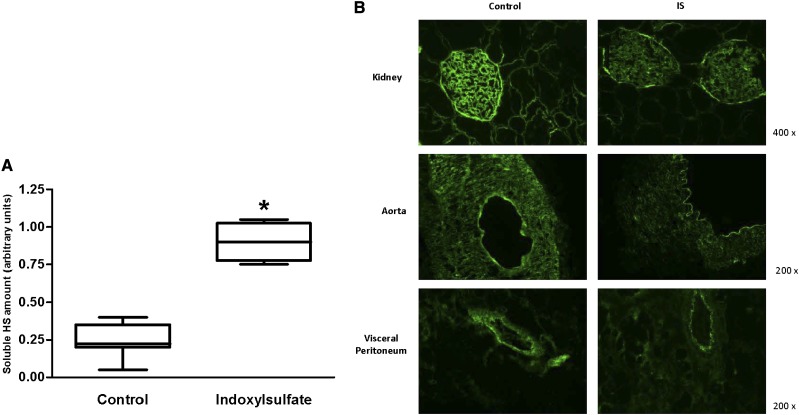
Significantly higher levels of soluble heparan sulfate (HS; 0.9±0.07 versus 0.25±0.04, P<0.01) were detected in the serum (Figure 6A), and consistently less staining was observed in the tissues of IS-treated animals (Figure 6B), which points to degradation of the endothelial glycocalyx.
Figure 6.
Disruption of the glycocalyx. (A) The amount of soluble heparan sulfate (HS) in the serum was significantly higher in the IS rats (n=6) versus the control rats (n=8) (P<0.01). (B) Staining of heparan sulfate structures in the glycocalyx was consistenly less pronounced in the tissue exposed to IS compared to control.
Quantification of Uremic Solute Concentrations in HBSS Solutions and Sera by HPLC
The concentrations of the uremic toxins in the HBSS solutions conformed with those concentrations observed in CKD patients. IS-HBSS solutions had a mean IS level of 232±4.4 mg/L; the pCS-HBSS solutions contained a mean pCS level of 100±2.3 mg/L, and the pCSpCG-HBSS solutions contained a pCS level of 85±1.5 mg/L and a pCG level of 26±0.33 mg/L. The serum levels of the superfusion rats, which were low (total) to undetectable (free) at the start of the experiment, steadily increased but never exceeded 15% of the levels in the HBSS superfusion solutions (Table 2). Serum levels of the intravenously infused rats were low at baseline but increased rapidly after a bolus injection with IS combined with continuous infusion to reach stable concentrations in the uremic range after 2 and 3 hours of continuous infusion (Table 2).
Table 2.
Uremic solute concentrations in sera
| Experimental Set-Up | Time (min) | Total Solute Concentration (mg/L) | Free Solute Concentration (mg/L) |
|---|---|---|---|
| Superfusion | |||
| IS group | |||
| IS | 0 | 2.1±0.23 | 0.4±0.05 |
| IS | 60 | 23.1±1.38a | 2.9±0.78a |
| IS | 120 | 28.5±2.60a | 6.6±2.07a |
| pCS group | |||
| pCS | 0 | 0.7±0.17 | <0.25b |
| pCS | 60 | 9.9±0.10a | 1.2±0.13 |
| pCS | 120 | 11.3±1.04a | 2.9±0.95 |
| pCS/pCG group | |||
| pCS | 0 | 0.5±0.16 | <0.25b |
| pCS | 60 | 9.9±1.47a | 0.9±0.34 |
| pCS | 120 | 12.7±2.24a | 1.1±0.40 |
| pCG | 0 | 1.5±0.39 | 1.3±0.36 |
| pCG | 60 | 1.8±0.33 | 1.6±0.31 |
| pCG | 120 | 1.9±0.40 | 1.9±0.41 |
| Infusion IS group | |||
| IS | 0 | 1.4±0.14 | 0.06±0.005 |
| IS | 120 | 230.8±18.9c | 77.2±20.30c |
| IS | 180 | 234.6±7.52c | 66.1±9.52c |
Uremic solute concentrations are expressed as mean ± SEM.
P<0.05 versus baseline (0 minutes).
Below detection limit.
P<0.01 versus baseline.
Effects on Leukocyte Recruitment Were Not Caused by Chemotaxis
Compared with control, pCS and pCG (alone or combined) and IS had no effect on the formyl-methyl-leucyl-phenylalanine (fMLP) -induced chemotaxis of polymorphonuclear leukocytes (PMNLs); also, they did not have a chemotactic potential on their own in this assay.
Interrupted Blood Flow Is Not Caused by Vasoactive Properties, Formation of Fibrin Plugs, or Capillary Plugging
Because detectable changes in VD were absent throughout the experiments with IS, vasoconstrictive and vasodilating properties of IS were tested in vitro by a wire myograph in segments of both aorta and femoral artery. IS did not elicit a vasoactive effect in vitro, even when present for 2 hours. Fibrin deposition was not observed by histologic staining in any of the tissue samples from the superfusion animals with flow problems in the peritoneal microcirculation. In addition, capillary plugging of leukocytes could not be shown during the infusion experiments.
IS Induces No Oxidative Burst Activity in Leukocytes
There was no direct in vitro effect of IS on leukocyte oxidative burst after 10 minutes of incubation (standard protocol burst test) or 120 minutes of incubation (data not shown).
Discussion
In this study, the effects of the protein-bound uremic solutes IS, pCS, and pCG on leukocyte recruitment were evaluated for the first time in an in vivo rat model using intravital microscopy.
Superfusion with IS caused an immediate firm adhesion and extravasation of the circulating leukocytes (Figures 1 and 2). In addition, IS had a dramatic effect on blood flow, resulting in an interruption or even a complete flow stop (Figures 2 and 4). Intravenous infusion with IS to mimic more closely the in vivo situation in uremic patients confirmed the superfusion results (Figure 5), albeit in a more moderate way, and caused degradation of the endothelial glycocalyx (Figure 6). However, superfusion with pCS induced a moderate activation, only resulting in an increased number of rolling leukocytes (Figures 1 and 2). The addition of pCG to pCS caused leakage of albumin and an impaired blood flow (Figures 3 and 4).
Until now, experimental effects of IS, pCS, and pCG have mainly been evaluated in an in vitro setting. IS plays a role in inflammation-related processes by inducing production of reactive oxygen species in endothelial cells,14,15 increasing endothelial microparticle release,16 enhancing the proliferation of vascular smooth muscle cells,17 and inhibiting endothelial cell repair.18 IS increases cardiac fibrosis19 and osteoblastic resistance to parathyroid hormone,20 which might contribute to vascular calcification. Also, pCS was shown to have an immune stimulating effect caused by the increase of leukocyte free radical production.21 In addition, a synergistic effect of pCS and pCG on leukocytes was shown.22 Vascular damage by pCS is suggested by demonstration of endothelial microparticle release.23
Clinical studies already linked IS to mortality and aortic calcification.24 In addition, a correlation between IS and plasma levels of IL-6 was shown.25 p-Cresol (acting as a surrogate for pCS) has been shown to be related to clinical outcomes.26,27 Recent studies confirmed that free pCS levels were predictive for mortality at different stages of CKD28 and cardiovascular and all-cause mortality in hemodialysis patients.29 The relation between pCS and coronary artery disease in patients with no or only moderate degrees of CKD30 extends the association of this compound with cardiovascular outcomes beyond the scope of pronounced uremia.31
In clinical studies, it is difficult to determine whether a given uremic toxin intrinsically has deleterious effects or is merely an inert biomarker for the degree of renal dysfunction. The present in vivo approach enables us to evaluate the effects of specific uremic compounds on the crosstalk between the major cell types involved in vascular damage: the leukocytes and the endothelium. Intravital microscopy permits direct in vivo visualization. Different parameters can be studied concomitantly in the same animal. This technique was already used by our group to study effects of peritoneal dialysis solutions on peritoneal membrane physiology.13
The present in vivo superfusion data show a pronounced effect of IS on leukocyte recruitment, resulting in immediate and strong adhesion and extravasation. Moreover, intravenous administration confirmed these findings. These in vivo data strengthen previous in vitro findings showing that IS influences expression of endothelial adhesion molecules, such as intercellular adhesion molecule 132 and E-selectin.33 In addition, Ito et al.33 showed IS-induced leukocyte adhesion to the femoral artery after administering IS in the drinking water of nephrectomized mice for 10 days, which was significantly reduced by anti–E-selectin antibody treatment. The firm arrest of leukocytes, which was seen in our study after exposure to IS, has already been observed for another tryptophan metabolite, kynurenic acid, in an in vitro model.34
pCS activated the crosstalk between leukocytes and the endothelium, which is in part, attributable to leukocyte activation as already suggested earlier for this compound by in vitro experiments.21,22 Increased production of free radicals by leukocytes can contribute to endothelial damage. The additive effect of pCG and pCS in vitro as shown by Meert et al.22 for leukocytes could not be confirmed in the present recruitment studies. However, addition of pCG to pCS induced leakage through the vessel wall and a drop in blood flow that was not observed in the presence of pCS alone.
An attempt to explain the leukocyte recruitment characteristics of the tested uremic solutes was made by evaluating their chemotactic properties. Because these compounds did not affect the chemotaxis process and were not chemotactic by themselves, a possible direct role on the expression of leukocyte or endothelial surface molecules or an indirect role by inducing the release of substances from other cell types, like the mesothelial cells, because of extravascular superfusion of the uremic toxins, which in their turn, could have an influence on leukocyte recruitment, is suggested. The latter could, however, be excluded, because intravenous infusion engendered similar results, pointing to a direct effect on leukocytes and/or the endothelium. Because neither chemotaxis nor leukocyte oxidative burst was influenced by IS, it is likely that the observed effect of IS is essentially related to direct effects on endothelial function.
The present study also showed a substantial impact on blood flow during exposure to IS and to a lesser extent, superfusion with pCSpCG. The underlying mechanism of this disturbed blood flow was, however, not clear.
In view of the significant adhesion induced in the presence of IS, capillary plugging by leukocytes would be the most logical reason of capillary no flow,35 but this could not be observed during the experiments. In the presence of pCSpCG, however, where no significant adhesion was induced, induction of vascular leakage could play a role in the reduced blood flow.
Because no hemodynamic effect of IS could be shown by either direct observation of vessel wall diameters or wire myograph on thoracic and femoral arteries, a possible role of the coagulation cascade in this phenomenon could be an interesting line of thought. Low and oscillatory shear stress is well known to cause endothelial activation,36–38 which can shift the thromboresistant surface of the endothelium to a prothrombotic state.39 This process can result in the expression of tissue factor (TF), the main stimulus for thrombin generation. Gondouin et al.40 showed the ability of IS and indole acetic acid to induce TF production through the aryl hydrocarbon receptor pathway, which was associated with increased procoagulant activity. CKD patients have elevated levels of soluble TF 41 and kynurenin, which like IS, belongs to the large group of the indoles and is associated with increased soluble TF levels and hypercoagulability in CKD patients.42 Furthermore, endothelial microparticles, considered as markers of endothelial dysfunction, are shed on endothelial activation and involved in the regulation of coagulation by activating the TF pathway,43 whereas both IS and pCS have been shown to elicit endothelial microparticle release in vitro.16,23 However, in the present superfusion study, additional staining for fibrin deposition in tissue samples of rats, in which flow stop was observed, did not show presence of any fibrin.
Another hypothesis could be degradation of the endothelial glycocalyx. This protective mesh of polysaccharides coats the luminal side of all blood vessels and functions as a barrier in preventing leakage of serum proteins. Furthermore, the healthy endothelial glycocalyx prevents leukocyte binding and coagulation at the endothelial cell surface, particularly by binding and regulating enzymes involved in the coagulation cascade. Therefore, glycocalyx destruction can have direct effects on coagulation and fibrinolytic responses as well as leukocyte adhesion, and it induces an impairment of shear stress-dependent nitric oxide production in arteries and cultured endothelial cells.44–52 Disruption of this structure can lead to capillary flow impairment and by extrapolation, similar phenomena downstream.
Pathologic loss of the glycocalyx is associated with impaired vascular wall protection throughout the circulatory system. It was shown recently that dialysis patients have an impaired glycocalyx barrier, where its constituents are shed into the blood.53 In the setting of CKD, several factors may contribute to the alteration of the endothelial glycocalyx, but the exact responsible mechanisms have, until now, only partially been elucidated. A chronic inflammatory status and increased oxidative stress, both associated with vascular dysfunction in CKD, contribute to severe atherosclerosis and cardiovascular morbidity and mortality in these patients,3,54–56 but they have also been linked to glycocalyx disruption.57,58 Of note, IS has been shown repeatedly to induce generation of reactive oxygen species in the endothelium.14,32,59 Also, hypervolemia, a common problem in CKD and dialysis patients, can alter the endothelial surface layer, probably through atrial natriuretic peptide, which causes shedding of glycocalyx constituents into the blood.60
The present study showed that infusion of IS quickly elicits increased circulating serum levels of glycocalyx constituents like HS, which coincides with a profound reduction in glycocalyx volume. A similar rapid glycocalyx injury was also observed in mice after injection with LPS in a model of acute lung injury61 and patients after acute myocardial infarction.62 Also, TNF-α is able to induce rapid shedding of the endothelial glycocalyx in the intact coronary vascular system.50
Taking all these elements together, IS-induced loss of glycocalyx could be a logical explanation for the observed disrupted blood flow. The negative charge of blood cells prevents them from adhering to the vessel wall, which itself carries a negative charge imparted by the endothelial glycocalyx.63,64 Proteoglycans, particularly HS, are largely responsible for this anionic charge, and disruption of the glycocalyx thus leads to capillary flow impairment. The exact mechanism causing endothelial glycocalyx degradation in our model should be elucidated in future studies. However, the present study provides for the first time direct in vivo evidence linking IS to structural and functional damage to the vessels.
Both in vivo and in vitro data from several studies show that a change or reduction of endothelial glycocalyx leads to leukocyte adhesion, whereas this effect is partly neutralized by exogenous heparinoids added to replace those heparinoids lost because of damage to the glycocalyx.46,65–67 Of note, our intravital microscopy data are registered in postcapillary venules, which are slightly broader and downstream of the narrowest capillaries, where a few adhering leukocytes can restrain flow pattern in a way as observed directly in conditions of reduced glycocalyx (http://www.youtube.com/watch?feature=player_embedded&v=6wHBninEwHA). All these arguments together suggest that the disturbed leukocyte–endothelium crosstalk as visually observed by us on the one hand and the biochemical and morphologic findings that conform with glycocalyx changes on the other hand are interrelated.
The present in vivo data support the hypothesis that protein-bound uremic toxins are not only involved in the progression of CKD but also the promotion of cardiovascular disease. Although pCS, pCG, and IS are all protein-bound molecules, they seem to exert divergent effects. The large group of protein-bound uremic solutes cannot be considered as a homogenous entity, because pathophysiology, degree and strength of protein binding, protein binding sites, retention pattern, and even the nature of the binding proteins are unlikely to be the same for all of them.
One should be careful in extrapolating the present findings to the arterial vascular bed, because leukocyte recruitment was visualized in the venular peritoneal bed. Although leukocyte recruitment in postcapillary venules is of importance in the development of atherosclerotic lesions,68 arterial and venular endothelium does not respond equally to various inflammatory stimuli.69,70 However, the peritoneal vascular bed is one of the sole systems allowing direct in vivo visualization of the interplay between leukocytes and the endothelium.
In conclusion, the present study provides in vivo evidence that IS, pCS, and pCG exert proinflammatory effects that could contribute to vascular damage by stimulating crosstalk between leukocytes and vessels. In addition, IS and the combination of pCSpCG induced unusual blood flow patterns, which may adversely affect overall organ perfusion. Endothelial glycocalyx damage seems to play a role in the IS-induced impaired blood flow, and the molecular mechanisms involved should be examined further to develop future therapeutic strategies.
Concise Methods
Laboratory Animals
The study was performed in healthy female Wistar rats (Charles River Laboratories, L'Abresle, France). They were handled in accordance to the National Institutes of Health guide for the care and use of laboratory animals, avoiding the influence of environmental stress. The ethical committee for animal experiments at the Faculty of Medicine and Health Sciences, Ghent University, Belgium, approved the protocol.
Acute Peritoneal Superfusion and Continuous Intravenous Infusion Experiments with Uremic Toxins
Rats were anesthetized subcutaneously in the neck with thiobutabarbital (100 mg/kg Inactin; Sigma, St. Louis, MO). After intubation of the trachea (polyethylene [PE] 240 catheter; Becton Dickinson, Erembodegem, Belgium) to ensure open airways throughout the experiment, a jugular vein was cannulated (PE50; Becton Dickinson) for continuous infusion of isotonic saline or uremic toxin solution, and a carotid artery was cannulated (PE50; Becton Dickinson) for continuous monitoring of arterial BP and blood sampling.
Rats undergoing superfusion were immediately prepared for intravital microscopy. Rats undergoing intravenous infusion were first loaded with a uremic toxin.
Intravital Microscopy
Cromoglycate (10 mg/kg intravenous Cromolyn; Sigma, St. Louis, MO) was administered 15 minutes before surgery to block degranulation of mast cells induced by surgical manipulation. Body temperature was kept at 37°C during the whole experiment by a heating pad with feedback control. A small midline abdominal incision was made, and a short segment of the small bowel was exteriorized. Carefully avoiding stretching, the visceral peritoneum was spread over a glass plate and superfused continuously with HBSS (Gibco; Life Technologies Europe B.V., Gent, Belgium) maintained at 37°C.
In each animal, a single unbranched venule with a diameter of 15–25 μm and a length of 150–200 μm was selected for investigation. After completion of surgery and exposure of the venule, the preparation was allowed to stabilize for 30 minutes. The exposed intestine was moistened with sterile soaked gauze to prevent tissue dehydration.
Observations were made with an Axiotech Vario 100 HD microscope (Zeiss, Jena, Germany) using water immersion objectives (Achroplan ×10 and ×40). The microscopic stage was operated by using a joystick (Lang, Hüttenberg, Germany). The tissue was transilluminated by a fiberoptic using a light source (KL 1500; Schott, Wiesbaden, Germany) equipped with a 150 W halogen lamp. The resulting image was displayed on a television monitor by a TK-1281 camera (Victor Company of Japan LTD-JVC, Tokyo, Japan) or a high-speed video camera (Kodak Motioncorder Analyzer; Eastman Kodak Company, San Diego, CA) and video recorded (S-VHS Panasonic AG-7350; Panasonic, Matsushita, Japan) for offline analysis with an image analysis software program (Cap-Image; Ingenieurbüro Zeintl, Heidelberg, Germany) as previously described by De Vriese and Lameire13 and De Vriese et al.71 The number of rolling, adhering, and extravasated leukocytes, leukocyte rolling velocity, VRBC, and VD were measured three times at each time point. VWSR was calculated as VWSR=8×VRBC/D. At the end of the experiment, the animal was euthanized by an overdose of anesthetic. If hemodynamic instability occurred, the experiment was ended prematurely.
Study of Leukocyte Recruitment
Rolling leukocytes were assessed by counting the number of rollers per minute crossing an imaginary line perpendicular to the axis of the vessel. They were defined as those cells moving at lower velocity than red blood cells and being in contact with the endothelial surface. Adhering leukocytes were defined as white cells not moving during a 30-second period; they were counted as the number per 100 µm vessel length. Extravasated leukocytes were determined as the number counted within a predefined area of perivenular tissue. Preactivation of the tissue was minimized by considering only vessels in which baseline leukocyte rolling was <30 cells/min and baseline adhesion was <3 cells/100 µm vascular endothelium for additional analysis.
Acute Superfusion Experiments
Forty-three rats were used to study leukocyte recruitment in the peritoneal microcirculation after acute exposure of the peritoneum with LPS or uremic toxins dissolved in HBSS.
Inflammatory Stimulus
As a positive control for leukocyte stimulation, 1 µg/ml LPS derived from Escherichia coli (E. coli serotype 0127:B8; Phenol extracted, L3129; Sigma, Bornem, Belgium) was used.72–74
Uremic Toxins
The uremic toxins were tested at concentrations in the uremic range as applied in preceding in vitro experiments: 236 mg/L (1.1132 mmol/L) for IS,7 100 mg/L (0.5313 mmol/L) for pCS,21 and 24 mg/L (0.0847 mmol/L) for pCG.22 pCS was synthesized according to the work by Feigenbaum and Neuberg75 as a potassium salt. pCG was synthesized from protected glucuronyl-trichloroacetimidate and p-cresol using a protocol similar to the protocol described by Van der Eycken et al.76 IS was purchased from Sigma-Aldrich (Bornem, Belgium). Because recent in vitro findings pointed out synergistic effects of pCS and the other conjugate of p-cresol, pCG,22 this combination was also tested.
Study Design
After a 30-minute stabilization period, the peritoneal membrane was superfused with the control solution (HBSS alone), LPS (positive control), or one or two uremic solutes dissolved in HBSS. After establishing baseline levels (0 minutes), measurements were repeated after 2, 10, 20, 30, 60, 90, and 120 minutes of exposure to HBSS with or without LPS or uremic solute(s).
Capillary Leakage
FITC-labeled albumin (Sigma-Aldrich) was injected (200 mg/kg intravenously) at the end of each experiment to check for capillary leakage. A visual score was allocated (0, no leakage at all; 1, suggestive of weak leakage at a few spots; 2, evidence of leakage at many spots; 3, heavy leakage covering the whole area).
Tissue Sampling and Histology
Samples of the superfused visceral peritoneum were immediately fixed in a 4% phosphate-buffered formaldehyde solution (pH 7; Klinipath, Olen, Belgium) and embedded in paraffin. From all tissue samples, 5-µm sections were cut with a Leica RM 2145 sliding microtome (Leica Microsystems, Nussloch, Germany) for histology. To evaluate the presence of fibrin plugs, a Masson Trichome staining was used as described before.77 The staining was examined with a Olympus BX41 microscope (Olympus, Aartselaar, Belgium).
Circulating Leukocytes and Hematocrit
Arterial blood samples were taken at baseline and after 1 and 2 hours; 25 µL blood was added to 475 µL 2% orthophosphoric acid (VWR International, Leuven, Belgium) to lyse the red blood cells. Peripheral leukocytes were counted in a Bürker chamber and expressed as number per millimeter3. To assess the hydration status of the rats, microhematocrit capillaries (Brand, Wertheim, Germany) were used.
Continuous Infusion Experiments
IS was further evaluated by intravenous infusion to check whether the substantial effects observed during superfusion were confirmed in a condition better mimicking the in vivo exposure to uremic toxins.
Study Design
Six rats received a bolus injection (13.6 mg) of 0.5 ml combined with continuous intravenous infusion (0.38 mg/min) of IS dissolved in isotonic saline. Preliminary bolus experiments were performed to calculate the amount of continuous infusion necessary to obtain stable intravascular concentrations in the uremic range (data not shown). Eight rats served as a control and were loaded with isotonic saline at the same infusion rate.
After 2 hours of loading, rats were subjected to intravital microscopy. The peritoneal membrane was superfused with HBSS, and after a 30-minute stabilization period, recordings were made. Blood was taken at baseline and after 1, 2, and 3 hours of infusion for determination of IS concentration. Additional blood was taken at the end for measurement of soluble HS (sHS) fragments, the major components of the endothelial glycocalyx.
Quantification of sHS in Serum
To quantify the amount of sHS in serum, a competition ELISA was performed.78 Flat-bottomed 96-well plates (Nunc A/S, Roskilde, Denmark) were coated with 5 μg/well HS from bovine kidney (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) in PBS. Wells were blocked with 1% gelatin/PBS (BD Biosciences, Alphen a/d Rijn, The Netherlands). Serum samples were incubated with the anti-HS antibody JM403 for 1 hour in a separate plate precoated with gelatin/PBS. In addition, different amounts of HS from bovine kidney were incubated with the anti-HS antibody JM403 to prepare a standard curve. The HS-coated plate was washed with PBS/Tween, and the anti-HS antibody preincubated with the serum samples or HS from bovine kidney was added and incubated for 1 hour. Subsequently, wells were washed with PBS/Tween, and anti-HS antibody binding was detected by incubating with the appropriate horseradish peroxidase-conjugated antibody for 1 hour. Finally, the plates were washed with PBS/Tween and incubated with tetramethylbenzidine solution (SFRI Laboratories, Berganton, France). After 15 minutes, the reaction was stopped with 2 M H2SO4, and absorption was measured at 450 nm.
Visualization of the Glycocalyx
Samples of the visceral peritoneum, kidney, and aorta were snap-frozen for analysis of the glycocalyx component HS by fluorescent immunohistochemical staining. Frozen sections (2 µm) were fixed in ice-cold acetone for 10 minutes and incubated with the anti-HS mouse monoclonal antibody JM40379 diluted in PBS containing 1% BSA (Sigma-Aldrich Chemie, Zwijndrecht, The Netherlands) and 0.05% sodium azide (immunofluorescence buffer) for 45 minutes at room temperature. Subsequently, sections were rinsed with PBS and incubated with the appropriate Alexa 488-conjugated secondary antibody (5 μg/ml; Life Technologies Europe BV, Bleiswijk, The Netherlands) in immunofluorescence buffer for 45 minutes at room temperature. Finally, sections were rinsed with PBS, postfixed with 1% paraformaldehyde-PBS, and embedded in VectaShield mounting medium H-1000 (Brunschwig Chemie, Amsterdam, The Netherlands).
Quantification of Uremic Toxins in HBSS Solutions and Sera by HPLC
The solute (pCS, pCG, and IS) concentrations in the experimental solutions and serum at different time points were checked by HPLC analysis as previously described.80 The limit of detection is 0.25 mg/L for pCS and pCG and 0.15 mg/L for IS.
Endotoxin Concentration
All experimental solutions were checked for endotoxin contamination by means of the Limulus Amebocyte Lysate QCL-1000 Test, a quantitative kinetic and chromogenic assay (Cambrex Bio Science, Walkersville, MD). All HBSS and isotonic saline solutions with and without uremic toxins were endotoxin-free (<0.003 EU/ml).
Chemotaxis
PMNL chemotaxis was determined by the underagarose method.81 fMLP (Sigma Aldrich) dissolved in PBS at a final concentration of 4×10−7 M was used as a chemoattractant; 5×105 PMNLs were suspended in 10 µl control or an experimental solution (containing pCS, pCSpCG, or IS at the above-mentioned concentrations). After a 2-hour incubation period at 37°C, the cells were fixed with methanol and paraformaldehyde and stained with Giemsa (Merck, Darmstadt, Germany). The distance migrated under the agarose was measured under the microscope. In addition, the chemotactic capacity of the uremic solutes by themselves was also tested by using them in the assay instead of fMLP.
Studies of Vasoactive Effect of IS In Vitro
Because of the unusual flow patterns seen during the IS superfusion experiments, vasocontractile effect of IS on the vessel was studied using a wire myograph as described by Nimmegeers et al.82 Briefly, three female Wistar rats were used. After cervical dislocation, the thoracic aorta and femoral artery were carefully removed from each animal and transferred to cooled Krebs-Ringer bicarbonate (KRB) solution. Ring segments of the isolated arteries were mounted in a small vessel myograph with a tissue chamber filled with 10 ml KRB solution and cleansed from adhering tissue. Two stainless steel wires were guided through the lumen of the segments. One was fixed to a force transducer, and the other was connected to a micrometer. After mounting, the preparations were allowed to equilibrate for 30 minutes in the KRB solution bubbled with 95% O2-5% CO2 (pH 7.4) at 37°C. After applying the optimal resting tension, preparations were contracted three times with a KRB solution containing 30 mmol/L K+ and 10−6 M norepinephrine, washed, and allowed to relax to basal tension. To evaluate endothelial integrity, precontraction was elicited with 10−6 M norepinephrine, and when a stable contraction plateau was obtained, the relaxing influence of adding 10−6 M acetylcholine was evaluated. After washing the ring segments so that they were again at optimal basal tension, cumulative concentrations of IS were added to the KRB solution (10−7–10−3 M). The latter concentration (concentration tested in the intravital experiment) was left in contact with the preparations for 2 hours.
Oxidative Burst Activity
Heparinized whole blood of healthy volunteers was incubated for 10 minutes (n=7) at 37°C with saline (control), IS at four different concentrations (236, 118, 59, and 29.5 mg/L), or the salt control KCl (83 mg/L) equivalent with the highest concentration of IS. To evaluate the effect of the solutes on the oxidative burst activity of the leukocytes, the Bursttest (Phagoburst; Orpegen Pharma, Heidelberg, Germany) was applied as described previously.21 Oxidative burst activity was measured by flow cytometry. To mimic the 2-hour exposure of the in vivo experiments, the experiments were repeated with a 120-minute incubation time with saline (control), KCl (83 mg/L), or IS (236 mg/L) at baseline conditions. Monocytes, granulocytes, and lymphocytes were gated separately in the light scatter dot plot according to size (forward scatter) and granularity (side scatter). The percentage of rhodamine-positive cells was assessed. A marker was placed to exclude background fluorescence.
Statistical Analyses
Data analysis was performed with SPSS Statistics version 19 (IBM, Armonk, NY) using the nonparametric Mann Whitney U test or Kruskall Wallis test for comparison between two or more groups. Post hoc analysis was performed using Dunn multiple comparison test. The results are expressed as mean ± SEM. A P value<0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
The authors thank Julien Dupont and Marie-Anne Waterloos for their expert technical assistance.
Support was given by Dutch Kidney Foundation Grant KJPB 09.01 and Consortium Grant CP09.03 (GLYCOREN; to A.R. and J.v.d.V.).
The results presented in this paper have not been published previously in whole or part, except in abstract form.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012030281/-/DCSupplemental.
References
- 1.Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N, European Uremic Toxin Work Group : Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Ross R: Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Stenvinkel P: Malnutrition and chronic inflammation as risk factors for cardiovascular disease in chronic renal failure. Blood Purif 19: 143–151, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Amann K, Gross ML, Ritz E: Pathophysiology underlying accelerated atherogenesis in renal disease: Closing in on the target. J Am Soc Nephrol 15: 1664–1666, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS: Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 58: 353–362, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J: Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Vanholder R, De Smet R, Glorieux G, Argilés A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jörres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox) : Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Glorieux G, Vanholder R: New uremic toxins - which solutes should be removed? Contrib Nephrol 168: 117–128, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Brunet P, Gondouin B, Duval-Sabatier A, Dou L, Cerini C, Dignat-George F, Jourde-Chiche N, Argiles A, Burtey S: Does uremia cause vascular dysfunction? Kidney Blood Press Res 34: 284–290, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Vanholder R, Brunet P: Protein-bound toxins—update 2009. Semin Dial 22: 334–339, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Liabeuf S, Drüeke TB, Massy ZA: Protein-bound uremic toxins: New insight from clinical studies. Toxins (Basel) 3: 911–919, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanholder R, Schepers E, Pletinck A, Neirynck N, Glorieux G: An update on protein-bound uremic retention solutes. J Ren Nutr 22: 90–94, 2012 [DOI] [PubMed] [Google Scholar]
- 13.De Vriese AS, Lameire NH: Intravital microscopy: An integrated evaluation of peritoneal function and structure. Nephrol Dial Transplant 16: 657–660, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P: The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5: 1302–1308, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Masai N, Tatebe J, Yoshino G, Morita T: Indoxyl sulfate stimulates monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells by inducing oxidative stress through activation of the NADPH oxidase-nuclear factor-κB pathway. Circ J 74: 2216–2224, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F: Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost 4: 566–573, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Tsuruoka S, Ioka T, Ando H, Ito C, Akimoto T, Fujimura A, Asano Y, Kusano E: Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int 69: 1780–1785, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P: The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442–451, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H: Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 31: 1771–1779, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Nii-Kono T, Iwasaki Y, Uchida M, Fujieda A, Hosokawa A, Motojima M, Yamato H, Kurokawa K, Fukagawa M: Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int 71: 738–743, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R: P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 22: 592–596, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Meert N, Schepers E, Glorieux G, Van Landschoot M, Goeman JL, Waterloos MA, Dhondt A, Van der Eycken J, Vanholder R: Novel method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: Clinical data and pathophysiological implications. Nephrol Dial Transplant 27: 2388–2396, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) : Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CT, Kuo CC, Chen YM, Hsu CY, Lee WC, Tsai YC, Ng HY, Kuo LC, Chiou TT, Yang YK, Cheng BC, Chen JB: Factors associated with blood concentrations of indoxyl sulfate and p-cresol in patients undergoing peritoneal dialysis. Perit Dial Int 30: 456–463, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 27.De Smet R, Van Kaer J, Van Vlem B, De Cubber A, Brunet P, Lameire N, Vanholder R: Toxicity of free p-cresol: A prospective and cross-sectional analysis. Clin Chem 49: 470–478, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Wu IW, Hsu KH, Hsu HJ, Lee CC, Sun CY, Tsai CJ, Wu MS: Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrol Dial Transplant 27: 1169–1175, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Wang CP, Lu LF, Yu TH, Hung WC, Chiu CA, Chung FM, Yeh LR, Chen HJ, Lee YJ, Houng JY: Serum levels of total p-cresylsulphate are associated with angiographic coronary atherosclerosis severity in stable angina patients with early stage of renal failure. Atherosclerosis 211: 579–583, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Massy ZA, Barreto DV, Barreto FC, Vanholder R: Uraemic toxins for consideration by the cardiologist-Beyond traditional and non-traditional cardiovascular risk factors. Atherosclerosis 211: 381–383, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T: Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol 31: 435–441, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Ito S, Osaka M, Higuchi Y, Nishijima F, Ishii H, Yoshida M: Indoxyl sulfate induces leukocyte-endothelial interactions through upregulation of E-selectin. J Biol Chem 285: 38869–38875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barth MC, Ahluwalia N, Anderson TJ, Hardy GJ, Sinha S, Alvarez-Cardona JA, Pruitt IE, Rhee EP, Colvin RA, Gerszten RE: Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J Biol Chem 284: 19189–19195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid-Schönbein GW: Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc 46: 2397–2401, 1987 [PubMed] [Google Scholar]
- 36.Alom-Ruiz SP, Anilkumar N, Shah AM: Reactive oxygen species and endothelial activation. Antioxid Redox Signal 10: 1089–1100, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Davies PF, Barbee KA, Lal R, Robotewskyj A, Griem ML: Hemodynamics and atherogenesis. Endothelial surface dynamics in flow signal transduction. Ann N Y Acad Sci 748: 86–102, 1995 [PubMed] [Google Scholar]
- 38.Malek AM, Alper SL, Izumo S: Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Gross PL, Aird WC: The endothelium and thrombosis. Semin Thromb Hemost 26: 463–478, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde-Chiche N, Poitevin S, Arnaud L, Vanholder R, Brunet P, Dignat-George F, Burtey S: Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway [published online ahead of print May 1, 2013]. Kidney Int 10.1038/ki.2013.133 [DOI] [PubMed] [Google Scholar]
- 41.Mercier E, Branger B, Vecina F, Al-Sabadani B, Berlan J, Dauzat M, Fourcade J, Gris JC: Tissue factor coagulation pathway and blood cells activation state in renal insufficiency. Hematol J 2: 18–25, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Pawlak K, Mysliwiec M, Pawlak D: Hypercoagulability is independently associated with kynurenine pathway activation in dialysed uraemic patients. Thromb Haemost 102: 49–55, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J, Dignat-George F: Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 99: 3962–3970, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H: Vasculoprotective properties of the endothelial glycocalyx: Effects of fluid shear stress. J Intern Med 259: 393–400, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Nieuwdorp M, Meuwese MC, Vink H, Hoekstra JB, Kastelein JJ, Stroes ES: The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr Opin Lipidol 16: 507–511, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Constantinescu AA, Vink H, Spaan JA: Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 23: 1541–1547, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM: Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F: Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 285: H722–H726, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG: The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch 454: 345–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, Conzen P, Becker BF: TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol 104: 78–89, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Vink H, Constantinescu AA, Spaan JA: Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 101: 1500–1502, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Lipowsky HH: The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann Biomed Eng 40: 840–848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H: Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol 23: 1900–1908, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z: Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol 3: 505–521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schepers E, Barreto DV, Liabeuf S, Glorieux G, Eloot S, Barreto FC, Massy Z, Vanholder R, European Uremic Toxin Work Group (EUTox) : Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clin J Am Soc Nephrol 6: 2374–2383, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stenvinkel P: Endothelial dysfunction and inflammation-is there a link? Nephrol Dial Transplant 16: 1968–1971, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Singh A, Ramnath RD, Foster RR, Wylie EC, Fridén V, Dasgupta I, Haraldsson B, Welsh GI, Mathieson PW, Satchell SC: Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS One 8: e55852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Golen RF, van Gulik TM, Heger M: Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med 52: 1382–1402, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y, Niwa T: Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem 403: 1841–1850, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF: Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol 289: H1993–H1999, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM: The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18: 1217–1225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostrowski SR, Pedersen SH, Jensen JS, Mogelvang R, Johansson PI: Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit Care 17: R32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desjardins C, Duling BR: Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol 258: H647–H654, 1990 [DOI] [PubMed] [Google Scholar]
- 64.Vink H, Wieringa PA, Spaan JA: Evidence that cell surface charge reduction modifes capillary red cell velocity-flux relationships in hamster cremaster muscle. J Physiol 489: 193–201, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulivor AW, Lipowsky HH: Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol 283: H1282–H1291, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Mulivor AW, Lipowsky HH: Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation 16: 657–666, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Rops AL, Jacobs CW, Linssen PC, Boezeman JB, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, van der Vlag J, Berden JH: Heparan sulfate on activated glomerular endothelial cells and exogenous heparinoids influence the rolling and adhesion of leucocytes. Nephrol Dial Transplant 22: 1070–1077, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Eriksson EE: Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation 124: 2129–2138, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Ley K, Gaehtgens P: Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res 69: 1034–1041, 1991 [DOI] [PubMed] [Google Scholar]
- 70.Eriksson EE, Karlof E, Lundmark K, Rotzius P, Hedin U, Xie X: Powerful inflammatory properties of large vein endothelium in vivo. Arterioscler Thromb Vasc Biol 25: 723–728, 2005 [DOI] [PubMed] [Google Scholar]
- 71.De Vriese AS, Verbeuren TJ, Vallez MO, Lameire NH, De Buyzere M, Vanhoutte PM: Off-line analysis of red blood cell velocity in renal arterioles. J Vasc Res 37: 26–31, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Geerts AM, Cheung KJ, Van Vlierberghe H, De Vriese AS, Mortier S, Vanheule E, Lameire N, De Vos M, Colle I: Decreased leukocyte recruitment in the mesenteric microcirculation of rats with cirrhosis is partially restored by treatment with peginterferon: An in vivo study. J Hepatol 46: 804–815, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Woodman RC, Teoh D, Payne D, Kubes P: Thrombin and leukocyte recruitment in endotoxemia. Am J Physiol Heart Circ Physiol 279: H1338–H1345, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Hickey MJ, Granger DN, Kubes P: Inducible nitric oxide synthase (iNOS) and regulation of leucocyte/endothelial cell interactions: Studies in iNOS-deficient mice. Acta Physiol Scand 173: 119–126, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Feigenbaum J, Neuberg CA: Simplified method for the preparation of aromatic sulfuric acid esters. J Am Chem Soc 63: 3529–3530, 1941 [Google Scholar]
- 76.Van der Eycken E, Terryn N, Goeman JL, Carlens G, Nerinckx W, Claeyssens M, Van der Eycken J, Van Montagu M, Brito-Arias M, Engler G: Sudan-beta-D-glucuronides and their use for the histochemical localization of beta-glucuronidase activity in transgenic plants. Plant Cell Rep 19: 966–970, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Pletinck A, Consoli C, Van Landschoot M, Steppan S, Topley N, Passlick-Deetjen J, Vanholder R, Van Biesen W: Salt intake induces epithelial-to-mesenchymal transition of the peritoneal membrane in rats. Nephrol Dial Transplant 25: 1688–1696, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Rops AL, van den Hoven MJ, Baselmans MM, Lensen JF, Wijnhoven TJ, van den Heuvel LP, van Kuppevelt TH, Berden JH, van der Vlag J: Heparan sulfate domains on cultured activated glomerular endothelial cells mediate leukocyte trafficking. Kidney Int 73: 52–62, 2008 [DOI] [PubMed] [Google Scholar]
- 79.van den Born J, Gunnarsson K, Bakker MA, Kjellén L, Kusche-Gullberg M, Maccarana M, Berden JH, Lindahl U: Presence of N-unsubstituted glucosamine units in native heparan sulfate revealed by a monoclonal antibody. J Biol Chem 270: 31303–31309, 1995 [DOI] [PubMed] [Google Scholar]
- 80.Meert N, Eloot S, Waterloos MA, Van Landschoot M, Dhondt A, Glorieux G, Ledebo I, Vanholder R: Effective removal of protein-bound uraemic solutes by different convective strategies: A prospective trial. Nephrol Dial Transplant 24: 562–570, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Cohen G, Hörl WH: Retinol binding protein isolated from acute renal failure patients inhibits polymorphonuclear leucocyte functions. Eur J Clin Invest 34: 774–781, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Nimmegeers S, Sips P, Buys E, Brouckaert P, Van de Voorde J: Functional role of the soluble guanylyl cyclase alpha(1) subunit in vascular smooth muscle relaxation. Cardiovasc Res 76: 149–159, 2007 [DOI] [PubMed] [Google Scholar]