Abstract
Geographic variation in stroke rates is well established in the general population, with higher rates in the South than in other areas of the United States. ESRD is a potent risk factor for stroke, but whether regional variations in stroke risk exist among dialysis patients is unknown. Medicare claims from 2000 to 2005 were used to ascertain ischemic stroke events in a large cohort of 265,685 incident dialysis patients. A Poisson generalized linear mixed model was generated to determine factors associated with stroke and to ascertain state-by-state geographic variability in stroke rates by generating observed-to-expected (O/E) adjusted rate ratios for stroke. Older age, female sex, African American race and Hispanic ethnicity, unemployed status, diabetes, hypertension, history of stroke, and permanent atrial fibrillation were positively associated with ischemic stroke, whereas body mass index >30 kg/m2 was inversely associated with stroke (P<0.001 for each). After full multivariable adjustment, the three states with O/E rate ratios >1.0 were all in the South: North Carolina, Mississippi, and Oklahoma. Regional efforts to increase primary prevention in the “stroke belt” or to better educate dialysis patients on the signs of stroke so that they may promptly seek care may improve stroke care and outcomes in dialysis patients.
Stroke is a catastrophic health event and a leading cause of disability. It represents a particularly heavy burden for the long-term dialysis population, in whom stroke rates are substantially higher than in the general population.1 In the general population, there is substantial geographic variability in stroke rates, with the southeastern United States having long been recognized as a “stroke belt” of higher stroke mortality rates.2–4 However, whether a stroke belt of increased ischemic stroke incidence exists in dialysis patients has not been formally studied.
Although one might suspect that the same factors contributing to ischemic stroke risk in the general population also apply to dialysis patients, there are several reasons to posit that this might not be the case. First, unlike the general population, dialysis patients across the United States have consistent access to insurance and frequent contact with health care providers, who routinely measure their BP, irrespective of geographic location. Second, the nature of vascular disease differs between dialysis and nondialysis patients, so different pathophysiologic mechanisms may be operative in the two populations.5 Third, dialysis patients fundamentally represent a “survivor cohort” relative to individuals with (predialysis) CKD and its attendant cardiovascular disorders, suggesting that epidemiologic trends evident in one population might not be found in the other.6 Accordingly, it is uncertain whether there is substantial geographic variation in stroke risk among dialysis patients and what factors might, in part, explain such a finding.
To address this gap in knowledge, we constructed a large cohort of incident dialysis patients to determine whether ischemic stroke rates vary by geography and how differences in stroke rates might be explained by patient characteristics. We reasoned that uncovering the existence of geographic variability in the stroke rates of dialysis patients might provide direction for focused health care efforts in regions at elevated risk, such as screening new dialysis patients for symptoms that might be referable to old strokes, lowering the threshold for investigating cerebrovascular disease, or educating dialysis patients on the importance of seeking immediate care for stroke-type symptoms.
Results
Cohort Characteristics
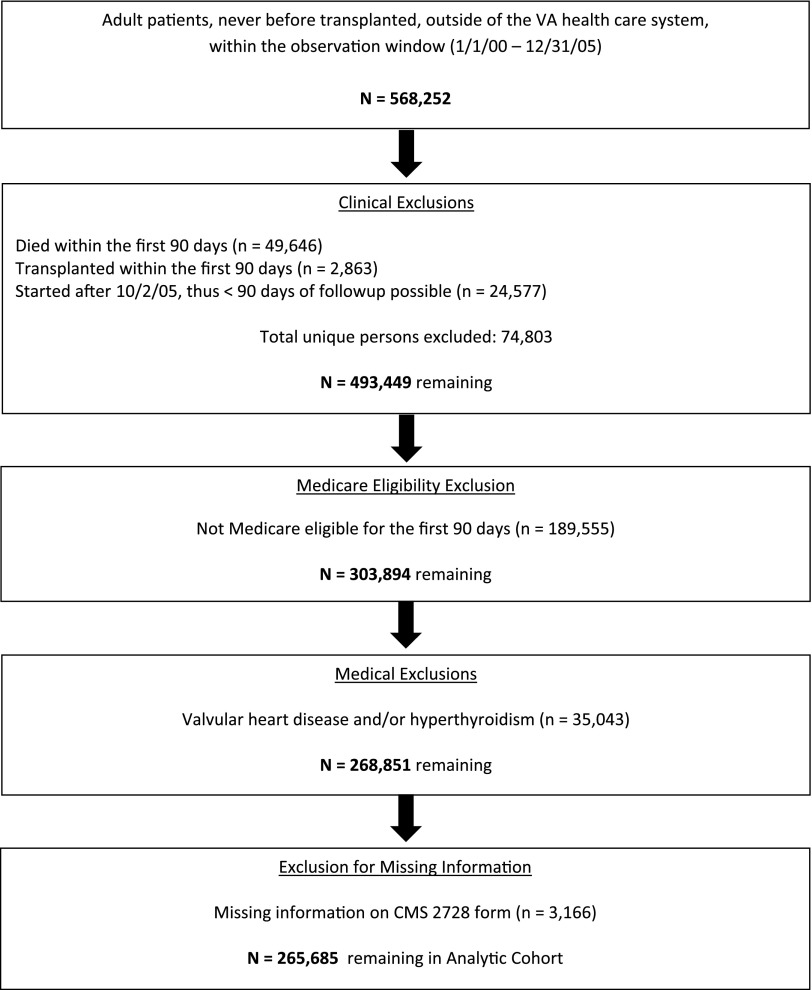
Figure 1 shows the construction of the Medicare-eligible cohort. There were a total of 265,685 Medicare-eligible individuals who initiated dialysis between January 1, 2000, and October 2, 2005, and survived at least 90 days before our final date of December 31, 2005.
Figure 1.
The study cohort as derived from the master sample. Exclusion flowchart demonstrating the creation of the study cohort. VA, Veterans Affairs.
The characteristics of the Medicare-eligible cohort are shown in Table 1. Mean age ± SD was 64.7±15 years; 52.9% of the patients were male; and whites made up the largest group at 54.9%, followed by African Americans at 30.0%. Diabetes, at 47.1%, was the leading cause of ESRD. In terms of comorbid conditions, 84.4% of patients had hypertension, 33.2% had heart failure, and 10.3% had a history of a cerebrovascular accident upon dialysis initiation. More than 93% were undergoing in-center hemodialysis. The cohort was followed for a mean of 2.0 years. Bivariate analyses between individuals who did and did not have strokes during the observation period revealed significant differences (P<0.01) for all covariates examined with the exception of hemoglobin (P=0.05).
Table 1.
Descriptive characteristics of the Medicare-eligible cohort
| Characteristic | All | Stroke | No Stroke | P Valuea |
|---|---|---|---|---|
| Cases (n) | 265,685 | 13,073 | 252,612 | |
| Age (yr) | 64.7±15.1 | 69.4±11.9 | 64.5±15.2 | <0.0001 |
| Male, n (%) | 140,599 (52.9) | 5406 (41.4) | 135,193 (53.5) | <0.0001 |
| Race/ethnicity, n (%) | <0.0001 | |||
| African American | 79,693 (30.0) | 4336 (33.2) | 75,357 (29.8) | |
| White | 145,768 (54.9) | 7030 (53.8) | 138,738 (54.9) | |
| Hispanic | 27,738 (10.4) | 1248 (9.6) | 26,490 (10.5) | |
| Other | 12,486 (4.7) | 459 (3.5) | 12,027 (4.8) | |
| BMI category, n (%) | <0.0001 | |||
| < 20 kg/m2 | 25,710 (9.7) | 1321 (10.1) | 24,389 (9.7) | |
| 20–24.9 kg/m2 | 84,154 (31.7) | 4330 (33.1) | 79,824 (31.6) | |
| 25–29.9 kg/m2 | 76,043 (28.6) | 3840 (29.4) | 72,203 (28.6) | |
| 30+kg/m2 | 79,778 (30.0) | 3582 (27.4) | 76,196 (30.2) | |
| Smoker, n (%) | 14,893 (5.6) | 608 (4.7) | 14,285 (5.7) | <0.0001 |
| Substance abuser, n (%) | 5,370 (2.2) | 157 (1.2) | 5573 (2.2) | <0.0001 |
| Unemployed, n (%) | 252,749 (95.1) | 12,846 (98.3) | 239,903 (95.0) | <0.0001 |
| Unable to ambulate, n (%) | 11,443 (4.3) | 745 (5.7) | 10,698 (4.2) | <0.0001 |
| Unable to transfer, n (%) | 4075 (1.5) | 295 (2.3) | 3780 (1.5) | <0.0001 |
| In-center HD, n (%)b | 247,584 (93.2) | 12,258 (93.8) | 235,326 (93.2) | 0.007 |
| Hemoglobin < 11.0 g/dl, n (%) | 176,664 (72.9) | 8759 (73.6) | 167,905 (72.83) | 0.05 |
| Comorbid conditions, n (%) | ||||
| AF | 38,619 (14.5) | 2863 (21.9) | 35,756 (14.2) | <0.0001 |
| Hypertension | 224,159 (84.4) | 11,410 (87.3) | 212,749 (84.2) | <0.0001 |
| Diabetes mellitus | 141,067 (53.1) | 8050 (61.6) | 133,017 (52.7) | <0.0001 |
| Congestive heart failure | 88,226 (33.2) | 4990 (38.2) | 83,236 (33.0) | <0.0001 |
| Coronary artery disease | 74,734 (28.1) | 4302 (32.9) | 70,431 (27.9) | <0.0001 |
| Peripheral vascular disease | 40,662 (15.3) | 2431 (18.6) | 38,231 (15.1) | <0.0001 |
| Prior cerebrovascular accident | 27,304 (10.3) | 3055 (23.4) | 24,249 (9.6) | <0.0001 |
| Liu comorbidity index score | 5.1±2.8 | 5.8±2.8 | 5.01±2.8 | <0.0001 |
| Cause of ESRD, n (%) | <0.0001 | |||
| Diabetes | 125,220 (47.2) | 7178 (54.9) | 118,042 (46.7) | |
| Hypertension | 70,117 (26.4) | 3480 (26.6) | 66,637 (26.4) | |
| GN | 22,817 (8.6) | 604 (4.6) | 225,213 (8.8) | |
| Other | 47,531(17.9) | 1811 (13.9) | 465,720 (18.1) |
For the comparison between individuals who did and did not experience a stroke.
In-center hemodialysis is contrasted to self-care dialysis, which consists of peritoneal dialysis and home hemodialysis.
Stroke Events
Of 265,685 individuals, 13,073 (4.9%) experienced at least one stroke. Total ischemic stroke events numbered 14,240: Of individuals with a stroke, 91.9% had one stroke, 7.3% had two, and 0.8% had three or more. Total follow-up time was 431,049 patient-years, resulting in a rate of 33 ischemic strokes per 1000 patient-years. Stroke rates were generally quite stable over time, as shown in Supplemental Table 1.
Person-Level Factors Associated with Stroke
After multivariable adjustment, factors independently associated with ischemic stroke are shown Table 2. Older age, female sex, African American race and Hispanic ethnicity, and being unemployed at the time of dialysis initiation were associated with ischemic stroke (P<0.0001 for all). A body mass index of ≥30 kg/m2 was associated with a significantly lower rate of ischemic strokes. In terms of comorbid conditions, permanent atrial fibrillation (AF) (treated as a time-dependent variable), diabetes, hypertension, and history of a cerebrovascular accident were also associated with ischemic stroke (P<0.0001). State of residence was also associated with ischemic stroke independent of other factors, as described below.
Table 2.
Patient-level factors associated with ischemic stroke
| Variable | ARR (99% CI) | P value |
|---|---|---|
| Age, per year | 1.02 (1.01 to 1.02) | <0.0001 |
| Female sex | 1.50 (1.44 to 1.57) | <0.0001 |
| Race/ethnicity | ||
| White | 1 (reference) | |
| African American | 1.28 (1.22 to 1.35) | <0.0001 |
| Hispanic | 1.15 (1.06 to 1.24) | <0.0001 |
| Other | 0.86 (0.77 to 0.97) | 0.0016 |
| BMI category | < 0.0001 | |
| <20 kg/m2 | 0.99 (0.92 to 1.07) | 0.76 |
| 20–24.9 kg/m2 | 1 (reference) | |
| 25–29.9 kg/m2 | 0.96 (0.91 to 1.01) | 0.027 |
| ≥30 kg/m2 | 0.82 (0.77 to 0.86) | <0.0001 |
| Smoker | 1.04 (0.94 to 1.15) | 0.31 |
| Substance abuser | 0.87 (0.72 to 1.04) | 0.05 |
| Unemployed | 1.66 (1. 42 to 1.95) | <0.0001 |
| Inability to ambulate | 0.92 (0.82 to 1.03) | 0.06 |
| Inability to transfer | 1.05 (0.89 to 1.25) | 0.45 |
| In-center hemodialysisa | 1.09 (1.01 to 1.19) | 0.0060 |
| Comorbid conditions | ||
| AF | 1.40 (1.33 to 1.48) | <0.0001 |
| Hypertension | 1.25 (1.16 to 1.35) | <0.0001 |
| Diabetes mellitus | 1.13 (1.06 to 1.21) | <0.0001 |
| Congestive heart failure | 0.95 (0.88 to 1.01) | 0.035 |
| Coronary artery disease | 0.96 (0.92 to 1.01) | 0.064 |
| Peripheral vascular disease | 1.03 (0.97 to 1.09) | 0.28 |
| Prior cerebrovascular accident | 2.47 (2.34 to 2.61) | <0.0001 |
| Liu comorbidity score | ||
| 0–3 | 1 (reference) | |
| 4–6 | 1.00 (0.94 to 1.07) | 0.92 |
| ≥7 | 0.96 (0.86 to 1.07) | 0.34 |
ARR, adjusted rate ratio; CI, confidence interval.
In-center hemodialysis is contrasted to self-care dialysis, which consists of peritoneal dialysis and home hemodialysis.
Geographic Factors Associated with Stroke
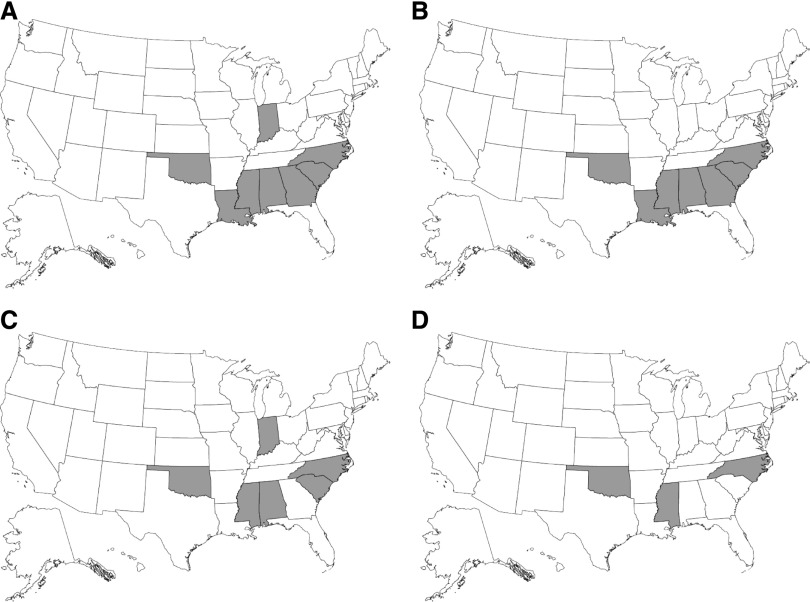
Figure 2 and Table 3 demonstrate geographic variation in ischemic stroke rates under various modeling strategies. Figure 2A shows observed-to-expected (O/E) ratios after adjustment only for age; Figure 2B, after adjustment for age and sex; Figure 2C, after adjustment for age, sex, and race; and Figure 2D, after full adjustment for factors listed Table 2. Figure 2A demonstrates that stroke rates are highest predominantly in the southern and southeastern United States, although the rate is also high in Indiana; eight states had O/E ratios significantly above unity, seven of which were in the South. After additional adjustment for sex (Figure 2B), all seven of the states with the rates significantly >1.0 were in the South, and after further adjustment for race (Figure 2C), five of six states were in the South. After full adjustment (Figure 2D), three states remained, all of which are in the South: The O/E ratio for North Carolina was 1.15 (99% confidence interal [CI], 1.04 to 1.27); for Oklahoma, 1.16 (99% CI, 1.01 to 1.34); and for Mississippi, 1.18 (99% CI, 1.03 to 1.34). Table 3 demonstrates the same phenomenon, facilitating comparison between states as the various modeling strategies were undertaken; it shows that the effect of state is modified by more thorough statistical adjustment. Of note, employment rate in the eight states with O/E ratios >1 was significantly, but modestly, lower than in the remaining states (4.2% versus 5.0%; P<0.0001).
Figure 2.
Strokes are generally more common in the southern United States. States with O/E adjusted odds ratios significantly >1 for new ischemic stroke, after successive adjustments. (A) Adjusted for age. (B) Adjusted for age and sex. (C) Adjusted for age, sex, and race. (D) Full multivariable adjustment.
Table 3.
States with an O/E adjusted rate ratios, for ischemic stroke remaining significantly >1.0, after successive adjustments
| State | Unadjusted | Age | Age, Sex | Age, Sex, Race | Fulla |
|---|---|---|---|---|---|
| MS | 1.27 | 1.36 | 1.32 | 1.19 | 1.18 |
| NC | 1.28 | 1.35 | 1.32 | 1.22 | 1.15 |
| OK | 1.18 | 1.22 | 1.20 | 1.21 | 1.16 |
| AL | 1.21 | 1.27 | 1.25 | 1.16 | – |
| SC | 1.24 | 1.31 | 1.27 | 1.15 | – |
| IN | 1.16 | 1.15 | – | 1.14 | – |
| GA | 1.14 | 1.20 | 1.17 | – | – |
| LA | 1.15 | 1.20 | 1.18 | – | – |
Significance was maintained for each state above using 99% confidence intervals. Note that Indiana is considered as being outside the “stroke belt” in some studies, but has been included in it by others. MS, Mississippi; NC North Carolina; OK, Oklahoma; AL, Alabama; SC, South Carolina; IN, Indiana; SC, South Carolina; IN, Indiana; GA, Georgia; LA, Louisiana.
Adjusted for age, sex, and race, as well as for the comorbidity and other factors shown in Table 2.
In the unadjusted model, only New Mexico had an O/E ratio <1 (0.74 [99% CI, 0.58 to 0.95]) while in the fully adjusted model, only New York had an O/E ratio <1 (0.90 [99% CI, 0.82 to 0.98]), demonstrating that, overall, variation >1.0 was far more common than variation <1.0.
Sensitivity Analyses
To assess the rigor of our analysis, we performed multiple sensitivity analyses. First, we performed identical modeling save elimination of the adapted Liu comorbidity index; final results were identical, with North Carolina, Mississippi, and Oklahoma again being the only states with O/E ratios for ischemic stroke significantly >1.0. Next, we used a more sensitive definition for ischemic strokes in which an additional 20% of strokes were included. Five (Alabama, Mississippi, North Carolina, Oklahoma, South Carolina) of the seven (New Jersey, Indiana) states with O/E ratios significantly >1.0 were in the South; the same general trend of progressive attenuation with greater adjustment was observed, starting from 14 (predominantly southern) states with O/E ratios significantly >1.0 when adjustment for age alone was undertaken. Finally, we modeled ischemic strokes (using the specific definition for ischemic strokes and including the adapted Liu index) in the dually eligible (Medicare and Medicaid) population; no states had O/E ratios significantly >1.0, probably due part to the more limited power of a sample size that was only 28.6% as large, but there was a clear trend toward higher point estimates, on average, for states in the stroke belt compared with those outside it. For example, Mississippi, North Carolina, and Virginia had the highest point estimates in both the fully adjusted model and the model adjusted for age alone.
Discussion
In this study, we sought to determine whether there was geographic variability in stroke rates by state and to what degree differences in patient characteristics could account for such variability. After adjustment for age alone (as the single most important factor associated with stroke), there was a distinct clustering of ischemic strokes in southern states, suggesting the presence of a “stroke belt” in long-term dialysis patients (of the states with increased risk, only Indiana was not in the South). Viewed another way, compared with the five states with the lowest stroke rates (none of which were in the South), the five states with the highest rates (all of which were in the South) had a 28% increase in strokes. However, as successive layers of adjustment were introduced (namely sex; race; and a comprehensive set of variables encompassing demographic, functional status, and comorbidity factors), the effect of geography was sequentially diminished. After full adjustment, the three states with significantly increased stroke rates (North Carolina, Mississippi, and Oklahoma) were all in the southern United States. Thus, while patient characteristics largely explain the increased risk of stroke in dialysis patients, a significantly increased risk is associated with residing in the stroke belt. Our main findings were robust to numerous sensitivity analyses.
The phenomenon of a stroke belt was first described as far back as 19654 and has been a continuing focus of public health research in the United States in the decades hence.2,3,7–10 These studies primarily examined stroke mortality, rather than incidence, and have varied considerably in their definitions of the stroke belt: For example, some studies have included Virginia and even Indiana,10 while others8,11 have not. We posited that because stroke mortality is probably related to stroke rates, there might well be increased stroke rates in dialysis patients residing in the southern United States. However, because there was variability in both analytical approaches and findings in the literature, we had no a priori hypothesis as to precisely which states might have higher-than-expected rates. Further, we did not necessarily expect that patterns in the general population would be explicitly replicated in the dialysis population. This study was merely designed to determine whether an ischemic stroke signal in dialysis patients would emerge from the southern United States.
Of note, evidence for a clustering of other cardiovascular disorders, such as congestive heart failure,12 has also been described in the southern and southeastern United States. Indeed, these issues are of sufficient public health interest such that the Centers for Disease Control and Prevention maintains an interactive website that can calculate risk of cardiovascular events, adjusted for age, sex, and race, at the state and even county level (http://apps.nccd.cdc.gov/DHDSPAtlas). However, much of the pioneering work on the stroke belt was undertaken without full consideration of how patient-level characteristics might be responsible for geographic variation in stroke. We sought not only to determine the degree of geographic variability present in stroke rates in dialysis patients but also to discern to what degree patient factors might be responsible for this phenomenon.
The present report appears to be the first such focused study on geographic variability in stroke rates in the dialysis population. That stroke would be more common in dialysis patients residing in the United States was not a foregone conclusion because the epidemiology of cardiovascular disease in dialysis patient differs substantially from that of the general population, a phenomenon controversially known as “reverse epidemiology.”13,14 In addition, patients undergoing long-term dialysis have close contact with the health care system, which includes routine measurement of BP and provides ample opportunity to assess for the presence of stroke-related symptoms. Nevertheless, our findings were generally concordant with previous work showing that regional differences in patient characteristics are responsible for the stroke belt in the general population.10 As was done by those investigators, we lected not to model merely the characteristics directly related to stroke but also a broad range of potentially contributing factors.10 This was done to minimize confounding by dialysis patients’ clinical characteristics that might differ across states. It is important to note that although the signal of a stroke belt diminishes with successive layers of statistical adjustment, patient characteristics are likely to be inherent to various geographic regions and may therefore be only weakly modifiable, if at all. In this sense, less adjusted models may be more insightful in helping to formulate public health strategies designed to address risk factors that are found more commonly in the southern United States.
Relatively few studies have explicitly examined factors associated with ischemic strokes. As with our study, most investigators15–17 (but not all18) show age to be a significant risk factor associated with stroke, a finding that would be expected from the general population. The role of sex has been less fully explored; one study showed no association between sex and stroke,15 whereas another suggested a trend in which female sex was associated with stroke.16 The role of race is yet more difficult to characterize: Seliger et al.15 showed that African American race was associated with new strokes (ischemic and hemorrhagic combined) only in the setting of previously established cardiovascular disease; in individuals without preexisting disease, African America race was associated with lower stroke rates. Sozio et al.16 also showed that African American race was inversely associated with stroke risk. These findings, combined with our own, suggest that the role of race in stroke risk may be nuanced and dependent on a milieu of other medical risk factors. Additionally, the role of body mass index does not seem to have been previously explored. While an inverse relationship exists between body mass index and mortality in the dialysis population,13 it was unclear to us whether this would be the case for ischemic stroke. That we found this to be the case suggests that the phenomenon of reverse epidemiology might affect stroke risk as well.
In terms of comorbid conditions, diabetes15–18 and a history of a cerebrovascular accident16,18 seem to be reliably associated with stroke risk, concordant with our findings. However, the potential role of other traditional risk factors is somewhat unclear. Hypertension, for example, has been associated with stroke in some15 but not all18 studies, and even the findings on the role of AF are discordant,17,19–21 although the most comprehensive study to date reported that, in the setting of AF, dialysis patients had a 1.8-fold increased risk of stroke compared with non–dialysis-dependent individuals.22 Such discrepancies in the literature suggested that even basic relationships between common comorbid conditions and stroke risk are not fully understood in the dialysis population. We suggest that our study, the largest of its kind to date, may help clarify these relationships.
Our findings should be interpreted in the context of important limitations. First, our outcomes were based on claims, rather than clinical data such as degree of BP control. Although our claims-based approach is imperfect, it seems unlikely that this would introduce bias in a way that varies by geography. Second, consistent with many approaches,23 we primarily employed stroke codes that used only the first three digits. This could result in inclusion of events that were not infarcts. However, >98% of the codes used in our primary definition did not have the fifth position “0.” It therefore likely that the overwhelming majority of events captured were ischemic strokes, especially when our analytic safeguards based on length of stay and the presence of a carotid endarterectomy are considered. Furthermore, our sensitivity analysis, which included code 433, was deliberately designed to be more liberal; the same general pattern of increased strokes in the south was found. We did not examine hemorrhagic strokes, which are also common in the dialysis population; whether a stroke belt exists for hemorrhagic strokes should also be a subject of future study.
Additionally, our primary analysis used only individuals who were Medicare-eligible from the start of dialysis, which means they were likely to be older than individuals who acquired Medicare at a later date; our results may not be generalizable to the general dialysis population. Another limitation is that our claims-based algorithm was likely capable of detecting only individuals with permanent, as opposed to paroxysmal, AF. Because paroxysmal AF, which constitutes a stroke risk similar to that provided by permanent AF, is much harder to ascertain from claims data, our analysis is likely to underestimate the stroke risk conferred by all types of AF. AF was treated with special scrutiny, as it was treated as a time-dependent covariate; in reality, patients accumulate comorbid events continuously. However, such an analysis would be extraordinarily complex and would require robust code-based algorithms for every comorbidity in order to reliably identify them. Finally, we did not censor at change of dialysis modality, but misclassification due to this is likely to be small, since 95% of long-term dialysis patients use in-center HD.
One major potential factor that our analysis was unable to account for is regional differences in treatment, such as hypertension treatment, anemia management, and, in the case of persons with permanent AF, treatment with warfarin. Further studies should investigate whether regional differences in treatment approaches, which seem likely to exist, are potentially responsible for our findings.
These limitations are probably counterbalanced by the large sample size, the richness of the data used (i.e., the Centers for Medicare & Medicaid [CMS] 2728 form combined with claims data), and our use of multiple sensitivity analyses, which generally supported the findings of the primary analysis. We suggest that the analytic cohort we constructed is likely to be broadly similar to more contemporary dialysis patients. For example, data from the U.S. Renal Data System1 suggest that racial composition of dialysis patients is generally stable. Hypertension as a cause of ESRD has become slightly more common, and GN slightly less common. Other subtle changes are that the mean age of dialysis initiation has increased slightly, as has the mean estimated GFR at initiation. Given general stability in the United States dialysis population, our findings are likely to persist into the near future at least. However, caution should also be used in attempting to extrapolate out findings to non–United States populations because stroke rates appear to vary substantially by country.24
In conclusion, we found significant geographic variability in stroke rates in long-term dialysis patients. The highest rates were generally in southern states. Adjustment for patient-level characteristics accounted for the majority of this geographic variability, suggesting that the burden of stroke risk factors is higher in the southern states, but strokes rates remained significantly higher than average in a few southern states. These findings suggest that regionally targeted stroke-related health care efforts, such as screening new dialysis patients for symptoms of previous strokes, alerting providers to a have a high index of suspicion for investigating possible cerebrovascular disease, or educating dialysis patients about the importance of seeking emergency care for stroke symptoms, might be beneficial and should be a focus of future study.
Concise Methods
Study Design and Data Sources for Analysis
We performed a retrospective cohort analysis of incident, Medicare-eligible long-term dialysis patients. We also performed a secondary analysis in a subcohort of dually eligible (Medicare and Medicaid) long-term dialysis patients; these patients are a particularly vulnerable group who have similar socioeconomic status, which enables us to minimize the potential confounding from this factor. Medicare is a federally funded program for which nearly all adults with ESRD are entitled, regardless of age; although not all individuals receiving long-term dialysis are Medicare enrollees, most are. Medicaid, a public insurer funded jointly by federal and state governments, provides broad medical care benefits including prescription drugs for low-income or medically needy patients. The data sources and strategy used for linking Medicare and Medicaid patients have been previously described25,26 and are provided in more detail in Supplemental Appendix 1.
Study Cohort and Rationale for Analytic Approach
The cohorts consisted of individuals >18 years of age who initiated long-term dialysis on or after January 1, 2000; survived at least 90 days after dialysis initiation; and were continuously enrolled in Medicare (primary analysis) or both Medicare and Medicaid (secondary analysis) from dialysis initiation. Last date of enrollment was October 2, 2005 (to permit a minimum of 90 days of possible follow-up). Briefly, to ensure that all Medicare claims were observable, we studied only individuals who were Medicare-eligible from the time of dialysis initiation and for whom Medicare was the primary payer. Individuals were censored at the time that they lost Medicare eligibility, if this occurred. For the dually eligible cohort (Medicare plus Medicaid) substudy analysis, individuals were censored when they lost Medicare or Medicaid coverage. Patients enrolled in any form of managed care plan (e.g., those in Arizona or Tennessee) or in the Department of Veterans Affairs health system were also excluded because claims data were not available. Of note, persons undergoing long-term dialysis were generally not enrolled in Medicare managed care plans prior to 2006. Additional criteria for censoring were receipt of a kidney transplant or death on or before December 31 2005.
Covariates and Descriptive Variables
Demographic and clinical variables were drawn from the CMS 2728 dialysis intake form, a mandatory requirement of Medicare-subsidized dialysis that describes patients’ clinical history. A variety of covariates were considered as potential risk factors for ischemic stroke (Supplemental Appendix 1). Because previous stroke is a strong predictor of future stroke, we examined the cohort for stroke claims in the first 90-day run-in period before the start of the observation window; an individual was considered to have a preexisting stroke if either a history of a cerebrovascular accident (either ischemic or hemorrhagic) was declared on the CMS 2728 form or if a stroke claim appeared during the 90-day run-in period; strokes occurring after this period (i.e., during the observation window) were therefore considered “incident” strokes. Additionally, we supplemented the medical information from the CMS 2728 form with a modified form of the Liu comorbidity index27 (a summary measure of comorbidity burden), as described in Supplemental Appendix 1. Because permanent AF is a unique risk factor for stroke that is poorly captured in the CMS 2728 form, requires complex algorithms to capture from claims data, and develops over time in a substantial number of dialysis patients, we treated AF as a time-dependent covariate, an approach that permitted us to determine the onset of the disorder relative to new ischemic strokes captured in the observation window; details in this are described in Supplemental Appendix 1.
For the geographic analysis, the southern states were considered to be those from three of the nine official U.S. Census Bureau geographic divisions: Oklahoma, Texas, Arkansas, and Louisiana from the “west south central” region; Mississippi, Tennessee, Alabama, and Kentucky, from the “east south central” region; and the eight southeastern states of the “south Atlantic” region.
Stroke Outcomes
Our primary outcome was ischemic stroke rate (taking into account that some patients had multiple strokes). We used recent information on the sensitivity and specificity of stroke-related International Classification of Diseases, Ninth Revision (ICD-9), claims to identify ischemic strokes from Medicare data.23 A specific approach, in which only the codes with higher specificities for ischemic strokes were used, was the primary approach. A sensitivity analysis was performed in which we used a broader range of stroke codes.
For the primary approach for identifying ischemic strokes, we used the strategy of Go et al.28 The appearance of codes 434 or 436 in the primary position of an inpatient claim was first assessed; if these were not present, the appearance of code 362.3 was sufficient to make the diagnosis of an ischemic stroke. If the code 434 or 436 was present and accompanied by a fatal hospitalization, an ischemic stroke was attributed. If 434 or 436 was present and the hospitalization was nonfatal, the length of the hospitalization was considered. If the hospitalization duration was ≥48 hours, an ischemic stroke was assessed; if the hospitalization was <48 hours, the presence of a carotid endarterectomy (ICD-9 code 381.2) was determined. If a carotid endarterectomy was not present an ischemic stroke was assessed, while the presence of a carotid endarterectomy meant that the stroke was not assessed. The sensitive approach differed only by treating code 433 analogously to 434 and 436.
Statistical Analyses
We generated descriptive statistics (means and SDs for continuous variables and frequencies for categorical variables) to illustrate how individuals who experienced ischemic strokes differed from those who did not. Bivariate analyses comparing each of the explanatory variables by use versus nonuse were performed by using the Pearson chi-squared test or t test, as appropriate. To identify independent factors associated with ischemic stroke, we generated a Poisson generalized linear mixed model29 with stroke rate (number of strokes per unit of exposure time) being regressed simultaneously on all a priori selected explanatory variables as fixed effects and with state modeled as a random effect. Although cause of ESRD was not included among these a priori selected variables, we included, in modified form, the Liu comorbidity index,27 which incorporates cause of ESRD. The time-dependent nature of permanent AF as an explanatory variable was handled by creating two separate rows of data for patients who experienced AF, with the first row contributing person-time to the no-AF group and the second row contributing person-time to the AF group. The Poisson model fit was assessed by ensuring that the ratio of the generalized chi-squared test statistic to its degrees of freedom was close to 1, indicating that there were no major concerns with overdispersion in the model (i.e., the observed variability in stroke rates was close to the variability expected under the Poisson model).
After developing the Poisson generalized linear mixed model, we examined the variability in stroke rates by state of residence and compared states. For each state, we determined whether the observed number of incident strokes, called the observed (O) value, was above or below the expected (E) value given the total exposure time for persons belonging to that state. The random effect estimates for each state calculated by our model facilitated the O/E rate ratio comparisons. Specifically, we obtained the estimates of the random effects for each state because these variables modify each state’s log-rates of ischemic stroke from the overall cross-state (fixed) model effects. Exponentiation of these estimates generated state-specific observed versus expected (O/E) adjusted rate ratios adjusted for the effect of other covariates. Using the estimated SEMs of the predictions, we estimated confidence intervals for these state-specific O/E rate ratios. Because of the large sample size of our cohort, statistical significance was inferred only for P<0.01. All statistical analyses were done with SAS software, version 9.2 (SAS Institute, Inc., Cary, NC ). We then incorporated an approach used by others to illuminate patient characteristics associated with states’ stroke rates:3 sequentially adjusted for age; then age and sex; then for age, sex, and race; and, finally, for all factors based on our generalized linear mixed model, which accounted for individual-level characteristics.
Sensitivity Analyses
To examine the robustness of our results, we performed several sensitivity analyses. First, as stated above, we repeated the analysis with a more sensitive method for identifying ischemic stroke. Second, we performed the analysis both with and without the modified Liu comorbidity index as a summary measure of overall illness burden. Third, we performed an analysis in the cohort of dually eligible (Medicare & Medicaid) dialysis patients, which creates a more homogenous cohort based upon socioeconomic status.
Compliance and Protection of Human Research Participants
The research protocol was approved by the institutional review board at the University of Kansas Medical Center (KUMC). The work was undertaken in accordance with the principles of the Declarations of Helsinki. Data Use Agreements between KUMC and the U.S. Renal Data Service and CMS were in place.
Disclosures
None.
Acknowledgments
The authors thank Connie Wang, MD and Amanda Gellhaus for technical assistance with manuscript preparation.
Funding for this study was provided by National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) grants K23 DK085378 (J.B.W.) and R01 DK080111 (T.I.S.), by a National Kidney Foundation Young Investigator Award (J.B.W.), and by a Sandra A. Daugherty Foundation Grant (J.B.W.).
The data reported here have been supplied by the U.S. Renal Data System (DUA#2007-10 & 2009-19) and CMS (DUA#19707). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012111077/-/DCSupplemental.
References
- 1.United States Renal Data System: USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Lanska DJ: Geographic distribution of stroke mortality in the United States: 1939-1941 to 1979-1981. Neurology 43: 1839–1851, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Labarthe DR, Hu J, Yoon S, Howard VJ: Regional differences in African Americans’ high risk for stroke: The remarkable burden of stroke for Southern African Americans. Ann Epidemiol 17: 689–696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borhani NO: Changes and geographic distribution of mortality from cerebrovascular disease. Am J Public Health Nations Health 55: 673–681, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, Goldsmith DJ: Vascular calcification in chronic kidney disease. Clin Sci (Lond) 119: 111–121, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, Vittinghoff E, Lin F, Shlipak MG: The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med 141: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Lackland DT, Bachman DL, Carter TD, Barker DL, Timms S, Kohli H: The geographic variation in stroke incidence in two areas of the southeastern stroke belt: The Anderson and Pee Dee Stroke Study. Stroke 29: 2061–2068, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, Temple EM, Howard VJ: Estimated 10-year stroke risk by region and race in the United States: Geographic and racial differences in stroke risk. Ann Neurol 64: 507–513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glymour MM, Kosheleva A, Boden-Albala B: Birth and adult residence in the Stroke Belt independently predict stroke mortality. Neurology 73: 1858–1865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao Y, Greenlund KJ, Croft JB, Keenan NL, Giles WH: Factors explaining excess stroke prevalence in the US Stroke Belt. Stroke 40: 3336–3341, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Yang D, Howard G, Coffey CS, Roseman J: The confounding of race and geography: How much of the excess stroke mortality among African Americans is explained by geography? Neuroepidemiology 23: 118–122, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Mujib M, Zhang Y, Feller MA, Ahmed A: Evidence of a “heart failure belt” in the southeastern United States. Am J Cardiol 107: 935–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC: Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol 43: 1439–1444, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K: What is so bad about reverse epidemiology anyway? Semin Dial 20: 593–601, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Seliger SL, Gillen DL, Tirschwell D, Wasse H, Kestenbaum BR, Stehman-Breen CO: Risk factors for incident stroke among patients with end-stage renal disease. J Am Soc Nephrol 14: 2623–2631, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, Powe NR, Parekh RS: Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: The choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis 54: 468–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez-Perales C, Vázquez E, García-Cortés MJ, Borrego J, Polaina M, Gutiérrez CP, Lozano C, Liébana A: Ischaemic stroke in incident dialysis patients. Nephrol Dial Transplant 25: 3343–3348, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Power A, Chan K, Singh SK, Taube D, Duncan N: Appraising stroke risk in maintenance hemodialysis patients: A large single-center cohort study. Am J Kidney Dis 59: 249–257, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Wiesholzer M, Harm F, Tomasec G, Barbieri G, Putz D, Balcke P: Incidence of stroke among chronic hemodialysis patients with nonrheumatic atrial fibrillation. Am J Nephrol 21: 35–39, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A: Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis 46: 897–902, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, Kerr PG, Young EW, Robinson BM: Atrial fibrillation in hemodialysis patients: Clinical features and associations with anticoagulant therapy. Kidney Int 77: 1098–1106, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C: Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 367: 625–635, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH: A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf 21[Suppl 1]: 100–128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis M, Rigler SK, Mukhopadhyay P, Spertus JA, Zhou X, Hou Q, Shireman TI: Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 23: 112–118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, Spertus JA, Shireman TI: Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis 58: 73–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, Hou Q, Shireman TI: The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 81: 469–476, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE: Anticoagulation therapy for stroke prevention in atrial fibrillation: How well do randomized trials translate into clinical practice? JAMA 290: 2685–2692, 2003 [DOI] [PubMed] [Google Scholar]
- 29.McCulloch C, Searle S: Generalized, Linear, and Mixed Models, New York, John Wiley & Sons, Inc., 2001 [Google Scholar]