Abstract
Under physiologic conditions, significant amounts of plasma protein pass the renal filter and are reabsorbed by proximal tubular cells, but it is not clear whether the endocytosed protein, particularly albumin, is degraded in lysosomes or returned to the circulatory system intact. To resolve this question, a transgenic mouse with podocyte-specific expression of doxycycline-inducible tagged murine albumin was developed. To assess potential glomerular backfiltration, two types of albumin with different charges were expressed. On administration of doxycycline, podocytes expressed either of the two types of transgenic albumin, which were secreted into the primary filtrate and reabsorbed by proximal tubular cells, resulting in serum accumulation. Renal transplantation experiments confirmed that extrarenal transcription of transgenic albumin was unlikely to account for these results. Genetic deletion of the neonatal Fc receptor (FcRn), which rescues albumin and IgG from lysosomal degradation, abolished transcytosis of both types of transgenic albumin and IgG in proximal tubular cells. In summary, we provide evidence of a transcytosis within the kidney tubular system that protects albumin and IgG from lysosomal degradation, allowing these proteins to be recycled intact.
The main function of the kidney is to filter plasma, while at the same time, retain the majority of plasma proteins. However, a certain fraction of plasma proteins inevitably passes the glomerular filtration barrier. The most abundant and most studied plasma protein, albumin, is produced at a rate of about 15 g per day in humans.1 In the renal glomerulus, the albumin sieving coefficient (i.e., the transport rate of albumin across the glomerular filter in relation to water) has been estimated to be below 0.001.2–4 In more recent studies using intravital microscopy, the albumin sieving coefficient has been estimated to be significantly higher, although this result is still controversial.5–7 Thus, even when assuming a tight renal filtration barrier, significant amounts of albumin pass the glomerular filter—roughly in the range of 1 g per day in healthy humans, whereas less than about 0.05 g per day albumin can be detected in the final urine.
The filtered albumin is largely taken up by proximal renal tubular cells in an active process.8,9 Endocytotic uptake of filtered proteins is mediated by the multiligand receptors cubilin and megalin, which bind to albumin with high affinity through coated pits.10,11 The same is true for most other plasma proteins, specifically Igs. Endocytosis of albumin is specific in the sense that endocytosis of labeled albumin can be prevented by an excess of unlabeled albumin but not transferrin or lactalbumin.12 After endocytosis, the cubilin/megalin receptor complex dissociates from albumin at a pH < 6.5 in early endosomes during acidification,13 and it is recycled back to the apical plasma membrane through dense apical tubules.8,10 Thus, the cubilin/megalin receptor complex acts like a shuttle for apical endocytosis of albumin into the proximal tubular cells. However, although this pathway is well established, there is controversy whether filtered and reabsorbed albumin is exclusively subjected to lysosomal degradation into amino acids or whether at least a fraction is returned to the blood intact.5,14–19
In this study, we have addressed this controversy and traced the fate of filtered albumin within the kidney under physiologic conditions. For this purpose, we used a set of novel transgenic tools that allowed us to specifically spike the primary filtrate behind the filter with different versions of labeled albumin.
Results
To trace the fate of filtered albumin, an experimental approach was chosen where the primary filtrate was spiked with transgenic albumin. This approach has become feasible, because podocytes can be specifically targeted to express virtually any transgene using the 2.5-kb NPHS2 promoter (podocin promoter).20 Podocytes are submerged within the primary filtrate behind the filter, and thus, transgenic albumin is secreted from podocytes directly into the primary urine (Figure 1A, black arrows).
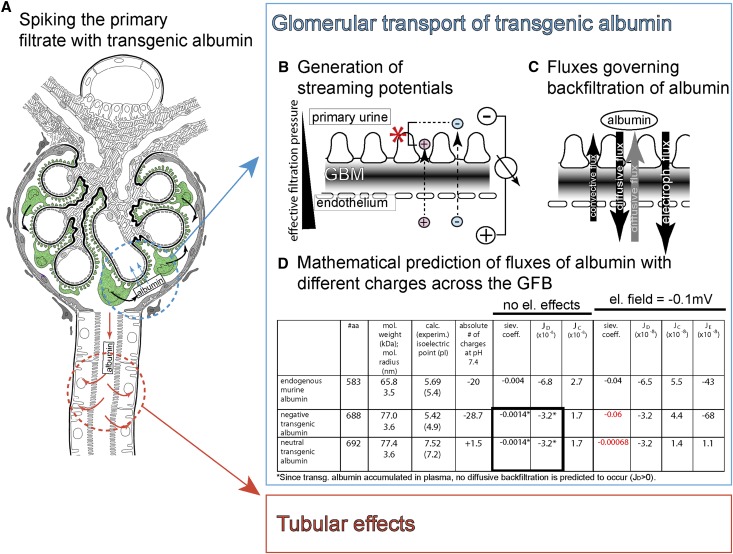
Figure 1.
Concept of spiking the primary filtrate with transgenic albumin and estimation of glomerular backfiltration. (A) The primary filtrate can be spiked with transgenic albumin by driving expression specifically within podocytes (green; black arrows, secretion of transgenic albumin into the primary filtrate). (B) Concept of streaming potentials. Within the glomerulus, an electrical field (streaming potential) is generated across the glomerular filtration barrier by filtration. Small plasma ions (Na+, Cl−, HCO3 −, or Ca++, etc.) interact with the charged filter during the filtration process and as a consequence, pass the filter with different speeds, generating a potential difference.23,24 (C) Three fluxes influence glomerular backfiltration of transgenic albumin: convection, diffusion (gray arrow; can also be oriented to the urine if transgenic albumin accumulated within the plasma), and electrophoresis (driven by the electrical field). (D) Mathematical estimations of the sieving coefficient of transgenic albumin from the primary filtrate into the capillary lumen with and without considering an electrical field across the glomerular filtration barrier (mathematical equations derived from ref. 24). dPI; GBM.
Mathematical Modeling of Glomerular Backfiltration of Albumin
Theoretically, transgenic albumin can be reabsorbed from the primary filtrate by two mechanisms: (1) within the glomerulus by backfiltration (Figure 1, blue circle) or (2) by tubular cells (Figure 1, red circle). How can glomerular backfiltration of transgenic albumin be estimated? To solve this problem, we first used a mathematical model.21,22 In addition, we have used an expansion of this model, which also considers electrical effects.23–25 This electrokinetic model proposes that an electrical field is generated across the glomerular filter by forced filtration of the small ions (e.g., sodium and chloride), which pass the filter at different speeds because of physical interactions with the charged filter surface (Figure 1B).25 Recently, this streaming potential has been experimentally verified by direct measurements.24
The following fluxes (J) governing backfiltration of transgenic albumin from the primary filtrate into the plasma were considered: diffusion (JD; driven by the difference in concentration of transgenic albumin between the primary filtrate and plasma), convection (JC; driven by the outward drag of the filtrate), and electrophoresis (JE; driven by the streaming potential) (Figure 1, C and D). Convection is the only flux, which will always be directed against backfiltration of transgenic albumin; therefore, it always bears a positive sign (Figure 1D). When disregarding electrokinetic effects (JE = 0), albumin is backfiltered exclusively by diffusion. When assuming that no transgenic albumin is present within the plasma, the mathematical model predicts that only about 1/1.000 of the synthesized transgenic albumin is backfiltered by diffusion (sieving coefficient = −0.0014) (Figure 1D). However, as shown below, we found that transgenic albumin accumulates within the plasma. Therefore, with increasing concentrations of transgenic albumin within the serum, diffusive backfiltration of transgenic albumin across the glomerular filtration barrier is predicted to decrease even more (Figure 1D, asterisk).
When considering an electrical field in the range of −0.1 mV, significant glomerular backfiltration is predicted to occur (about 6% of the total transgenic albumin within the primary filtrate) (Figure 1D, red). In the case of a modified albumin without significant charges (i.e., neutral albumin [Albneutral]), the sieving coefficient is predicted to drop by two orders of magnitude. In addition, the renal clearance of Albneutral (i.e., the sieving from plasma into the primary filtrate) is predicted to be higher than the renal clearance of Albnegative. Thus, electrical effects within the glomerulus can be detected by differences in the plasma concentration of negatively charged and neutralized albumin (i.e., the plasma concentration of Albnegative >> Albneutral if electrical effects are dominant). The mathematical model, therefore, predicts that glomerular backfiltration can be ruled out as a dominant effect for resorption of transgenic albumin from the primary filtrate as long as plasma concentrations are Albneutral ≥ Albnegative.
Generation of Charge-Modified, Tagged Murine Albumin
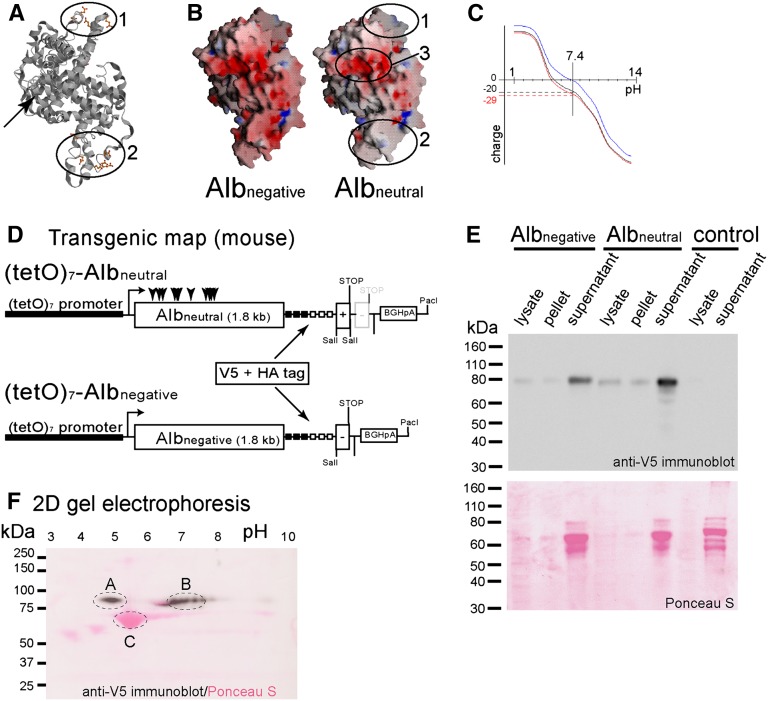
Given the considerations outlined above, two murine albumin molecules with different charges were generated. For neutralized albumin (Albneutral), the isoelectric point (pI) was shifted to a near-neutral pH by mutating 11 acidic amino acids within two patches on the surface of the molecule (Figure 2, A and B and Supplemental Figure 1). Mutating negatively charged amino acid residues within a third patch on the surface of the molecule (Figures 2B and 3) was not possible, because it resulted in precipitation of the molecule (data not shown). For sensitive and specific detection, 3xHA and 3xV5 tags accounting for the additional molecular weight were added to the C terminus of the transgenic albumins (Figure 2D and Supplemental Figure 1). Albnegative was predicted to consist of 690 amino acids, with a calculated molecular mass of 77 kDa and a pI of 5.42. Albneutral was predicted to consist of 692 amino acids, with a similar molecular mass of 77 kDa and a pI near neutral (pH 7.52). The tags added an additional 11.2 and 11.9 kDa, respectively.
Figure 2.
Characterization of tagged murine albumins with different charge. (A) Structure of wild-type mouse albumin. In two patches (1 and 2), negatively charged amino acids exposed to the surface of the molecule were mutated. The C terminus projects to the rear (arrow). (B) Electrostatic surface potential of negative (Albnegative) and neutralized albumin (Albneutral). The negative surface charge (red) was removed from patches 1 and 2. Mutation of a third negatively charged patch (3) caused precipitation of the molecule (not shown). (C) Predicted titration curve for wild-type albumin (black), Albnegative (red), and Albneutral (blue). At a physiologic pH of 7.4, Albnegative (red) is predicted to bear 29 negative charges. Albneutral is predicted to be close to neutral. (D) Transgenic map of the constructs used for pronuclear injection. (E) African green monkey kidney fibroblast (COS-7) cells were transiently transfected with both transgenic albumins. Controls were transfected with pcDNA3.1 only. After ultracentrifugation of the supernatants, corresponding amounts of the pellet and cellular lysates were subjected to SDS-PAGE and immunoblotting using V5 antiserum. Both negative and neutral transgenic albumin were detected predominantly within the supernatant as 80 kDa bands, indicating that both proteins were soluble and efficiently secreted. The prominent bands of 66 kDa on Ponceau S staining represent fetal calf albumin present in the culture media. (F) Two-dimensional gel electrophoresis (overlay of Ponceau S stain and immunoblot). (A) Albnegative and (B) Albneutral were mixed, subjected in equal amounts to two-dimensional gel electrophoresis (first dimension, isoelectric focusing [pH range 3–10]; second dimension, SDS-PAGE), and immunoblotted using an anti-V5 antiserum. (C) Unmodified BSA is visualized on the Ponceau S stain. Compared with BSA, (A) Albnegative has a higher molecular weight, and the isoelectric point is shifted to a lower pH as predicted. The isoelectric point of (B) Albneutral is shifted close to neutral.
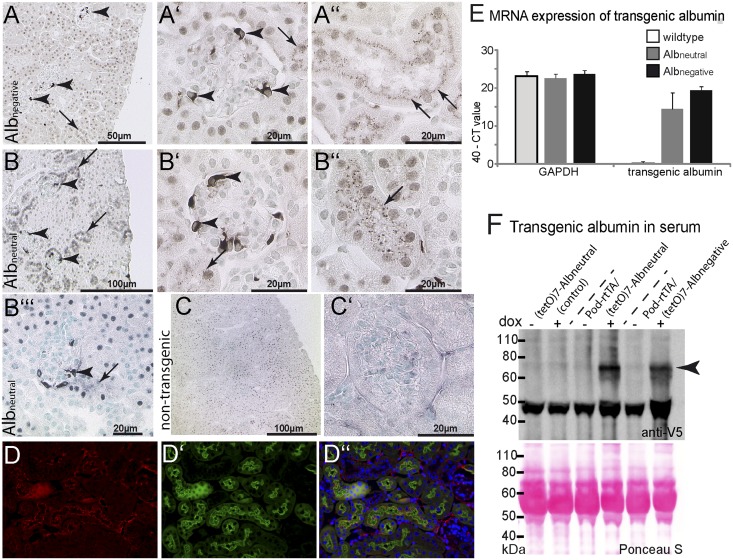
Figure 3.
Tracing transgenic albumin within the kidney. (A–C) Transgene expression was observed in a mosaic fashion in podocytes of both Pod-rtTA/Albnegative and/Albneutral mice (arrowheads in A–B″′). In both lines, transgenic albumin was also detected in apical granules within proximal tubular cells (arrows). (C and C′) No specific staining was observed in nontransgenic controls after induction dox. (A–C′) Immunohistochemical anti-V5 staining on paraffin sections. (D) When costaining for LTA FITC, transgenic albumin was detected in a granular pattern along the apical aspect within proximal tubular cells. (E) Expression of transgenic albumin mRNA was determined relative to endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in whole-kidney lysates by Taqman analysis using a transgen-specific probe. Data of six mice per group (wild type, Albneutral, and Albnegative) are shown as 40− Ct value, whereas a Ct value of 40 indicates that no PCR product was amplified. The difference in mRNA expression between Albneutral and Albnegative was not significant. (F) Detection of both transgenic albumins in the serum of transgenic POD-rtTA/Albneutral or Albnegative mice. Immunoblot analysis of sera of transgenic animals compared with their nontransgenic littermates before and on administration of dox. On administration of dox, V5-tagged albumin could be detected exclusively in the serum of double transgenic Albneutral or Albnegative mice.
To verify our predictions, both recombinant albumins were transiently overexpressed in HEK293 cells. Supernatants and cellular lysates were subjected to ultracentrifugation and subsequent SDS-PAGE and anti-V5 immunoblotting. As shown in Figure 2E, both Albnegative and Albneutral were efficiently secreted and remained soluble within the supernatant. Note that Albnegative and Albneutral showed slightly distinct electrophoretic mobility as a consequence of the charge modifications (Figures 2E and 4, D and E). To verify the charge modification further, supernatants containing both Albnegative and Albneutral were subjected to two-dimensional gel electrophoresis. In an overlay of an anti-V5 immunoblot and a Ponceau S staining, both recombinant Albnegative and Albneutral localized to the predicted pI of approximately 4.9 and 6.8–7.6 (Figure 2F, marks A and B, respectively). For comparison, native BSA (Figure 2F, mark C) is visualized on the same blot by Ponceau S staining (red).
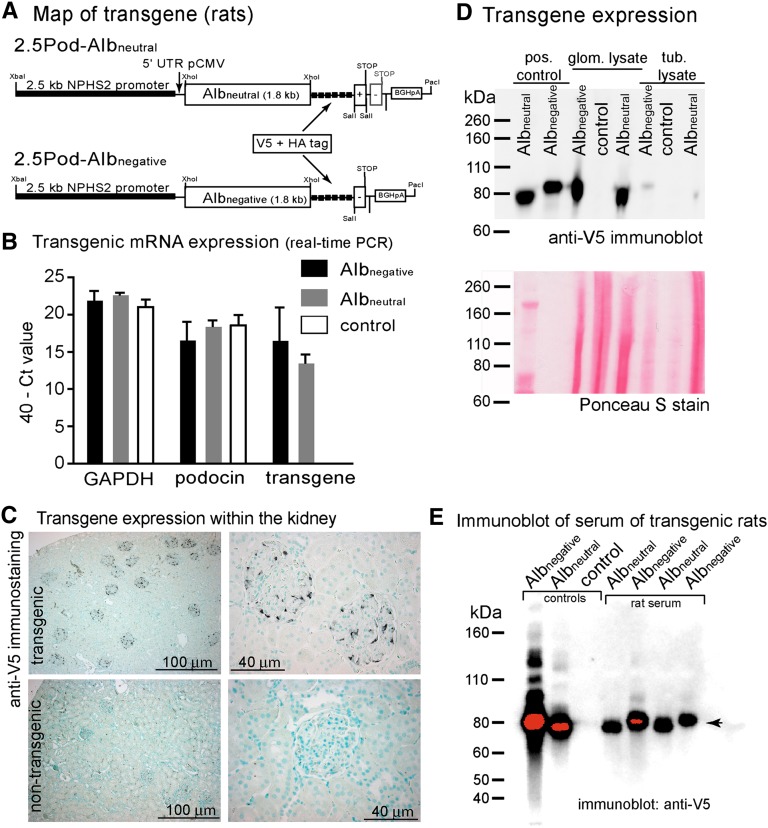
Figure 4.
Characterization of transgenic rats. (A) Map of the transgenic constructs. The 2.5-kb NPHS2 promoter was used to drive expression of either neutralized or negative murine albumin (identical to the transgene used in mice). The 5′ untranslated region (UTR) of the cytomegaly virus promoter and the polyadenylation signal of bovine growth hormone was used (BGHpA) to enhance expression. (B) mRNA expression in sieved glomeruli relative to the endogenous genes GAPDH and podocin as qualitatively determined by RT-PCR. Data are given as 40− Ct value. Ct value indicates the number of cycles until the fluorescence signal reached the threshold. A Ct value of 40 cycles indicates that the fluorescence signal did not reach the threshold until cycle 40, and therefore, no PCR product was amplified. (C) The transgene expression within the kidney was verified by anti-V5 immunohistological staining (methyl green counterstain). In representative images of Albneutral transgenic animals, a specific staining was observed exclusively within the glomeruli (identical images were obtained for Albnegative animals). No reactivity was observed in nontransgenic controls. (D) SDS-PAGE and anti-V5 immunoblotting of glomerular and tubular lysates. Recombinant albumin within the supernatant of transiently transfected African green monkey kidney fibroblast (COS-7) cells served as positive control. Within glomerular lysates, a distinct band of the predicted size was observed in transgenic animals for either construct. No band was observed in nontransgenic controls. In tubular lysates, a weak signal of intact transgenic albumin could be observed. Nonspecific labeling of heavy and light chain of murine Ig is observed in the serum at 55 and 28 kDa. Loading was verified by a Ponceau S stain. (E) Immunoblot of serum from transgenic Albneutral and Albnegative rats (two samples each). Controls from supernatants of transfected HEK293 cells were loaded on the left. Intact transgenic albumin is detected in similar amounts in transgenic rats (arrowhead).
Inducible Podocyte-Specific Expression of Differently Charged Albumin in Transgenic Mice
Transgenic (tetO)7-Albneutral and (tetO)7-Albnegative mice were generated by pronuclear injection (transgenic map) (Figure 2D); 9 transgenic Albneutral founders of 43 littermates (20%) and 12 transgenic Albnegative founders of 29 littermates (41%) were identified by PCR analysis of genomic DNA from tail biopsies. To drive doxycycline-inducible expression specifically in podocytes, Albneutral and Albnegative founders were crossbred with Pod-rtTA mice26 (Supplemental Figure 2A). In double transgenic F1 offspring, transgene expression was evaluated by immunohistology and blotting after 7 days of induction with doxycycline, and the highest expressing founder line was chosen for all additional experiments—termed (tetO)7-Albnegative and (tetO)7-Albneutral (Supplemental Figure 2).
Transgenic Albumins Are Taken Up by Proximal Tubular Cells and Accumulate Intact in Plasma
On induction with doxycycline (dox), transgenic albumin was expressed specifically in podocytes in a mosaic fashion (Figure 3, A–C′). Regardless of the molecular charge, both transgenic albumins were detectable within vesicles of proximal tubular cells in decreasing intensities proportional to the distance from the glomerulus (Figure 3, A″, B″, and B″′, arrows). No uptake was observed within glomerular parietal epithelial cells. Albumin-filled vesicles were localized close to the apical brushborder, which is shown in a costaining for Lotus tetragonolobus agglutinin (Figure 3, D–D″). mRNA expression of both types of transgenic albumin was similar in total kidney lysates (Figure 3E).
To test whether transgenic albumin passes the proximal tubular cells intact, plasma from both double transgenic mice and single transgenic controls was subjected to SDS-PAGE and subsequent immunoblotting after induction with dox for 10 days (Figure 3F). Transgenic Albnegative as well as Albneutral were detected in similar amounts within the plasma (n=10). These results suggest that the transgenic albumin reached the plasma from the primary filtrate by transcytosis.
To investigate potential glomerular backfiltration as a significant contributor for accumulation of transgenic albumin within the serum, the concentrations of transgenic albumin within the primary filtrate were compared with the serum. For this purpose, double transgenic Pod-rtTA/(tetO)7-Albneutral and Pod-rtTA/(tetO)7-Albnegative mice were induced with dox for 5 days. Next, the kidneys were removed from anesthetized animals and immediately shock-frozen in liquid nitrogen within less than 3 seconds. Transgenic albumin was stained using a polyclonal rabbit anti-HA antibody. Luminal staining of Bowman’s space was generally weaker compared with the endokapillary compartment of the respective glomerulus (Supplemental Figure 3, arrowheads), suggesting that concentrations of transgenic albumin were higher in the serum. Under such conditions, glomerular backfiltration of transgenic albumin by diffusion cannot occur.
Generation of Transgenic Rats to Exclude Extrarenal Expression by Reciprocal Transplantation Studies
Before postulating tubular transcytosis of transgenic albumin, potential aberrant transcriptional activity of the transgene had to be ruled out in extrarenal tissues. No ectopic expression of the 2.5-kb human NPHS2 (podocin) promoter (2.5-kb podocin promoter) has been reported so far, and within the kidney, the podocin promoter is specific for podocytes. However, if the promoter was transcriptionally active in only a small extrarenal cell population, transgenic albumin could be secreted intact from this tissue and accumulate in plasma.
To exclude extrarenal activity of the 2.5-kb NPHS2 promoter, transgenic rats were generated for renal transplant experiments. For these experiments, transgene expression was driven directly and constitutively by the 2.5-kb podocin promoter (Figure 4A). Pronuclear injection of 2.5Pod-Albnegative and 2.5Pod-Albneutral yielded 6 transgenic animals of 29 injected animals and 5 transgenic animals of 19 injected animals, respectively. Transgene mRNA expression was confirmed in rats transgenic for both constructs compared with nontransgenic rats (Figure 4B). Immunohistochemistry for the V5 tag yielded a staining pattern specific for podocytes for both constructs (Figure 4C). Protein expression was further validated by immunoblotting for the V5 tag in glomerular lysates (Figure 4D). Tubular lysates were weakly positive. Within the plasma of transgenic animals, intact transgenic albumin was detectable independent of molecular charge, which is in line with our results in transgenic mice (Figure 4E, arrowhead). Again, both transgenic albumins could be detected in similar amounts within the plasma.
Exclusion of Extrarenal Transgene Expression: Transplanting Transgenic Kidneys into Nontransgenic Recipient Rats
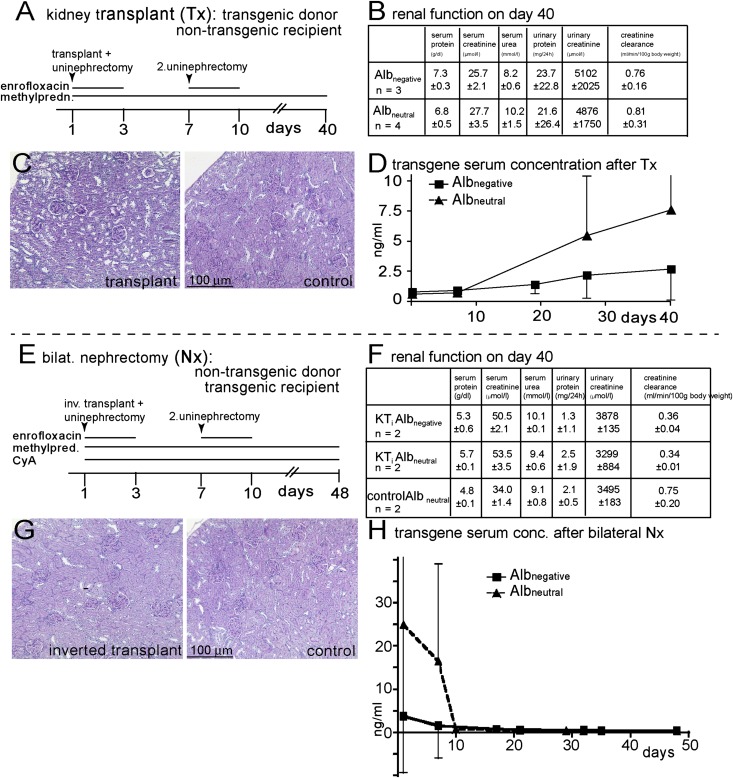
Seven kidney transplants using both transgenic 2.5Pod-Albnegative and 2.5Pod-Albneutral donor kidneys from different founder lines were performed. Immunodeficient nude rats were used as recipients. In a two-step procedure, both endogenous kidneys were removed to verify preserved graft function (Figure 5, A and B). Corticosteroids were administered to rule out formation of antibodies against transgenic albumin, even in nude immunodeficient rats. No significant histologic alterations were observed after 40 days within the graft compared with controls (Figure 5C). Serum concentrations of both transgenic albumins increased progressively after transplantation, which was determined by a sandwich ELISA from plasma (Figure 5D). Transgenic albumin in plasma could only be derived from the transplanted kidney, ruling out an extrarenal source.
Figure 5.
Reciprocal kidney transplantation experiments in transgenic rats. (A) Experimental design where the kidneys of seven transgenic rats (from different found lines) were transplanted into nontransgenic recipients. (B) Renal function test before euthanization showed no abnormalities. (C) Renal histology of the transplanted kidneys was normal in all seven rats. (D) Concentrations of transgenic albumin in the serum as determined by ELISA showed a progressive increase over time. (E) In the reverse experiment, nontransgenic allogenic kidneys were transplanted into transgenic recipients of different founder lines, which were subsequently nephrectomized. (F and G) Renal function tests and histology again showed no abnormalities. (H) After the second nephrectomy, transgenic albumin concentrations fell rapidly below detection levels, indicating that the transgenic albumin was not derived from an extrarenal source.
In the reciprocal experiment, both kidneys were removed from transgenic rats (Figure 5, E–H). To allow survival for an observation period of 50 days, transgenic rats received nontransgenic allogenic kidneys (requiring immunosuppression with cyclosporine and corticosteroids). As expected, removal of the transgenic kidneys resulted in reduction of both transgenic albumins below detection levels—again confirming that the kidneys are the major source of transgenic albumin in the plasma.
Transcytosis within the Proximal Tubules Depends on the Neonatal Fc Receptor
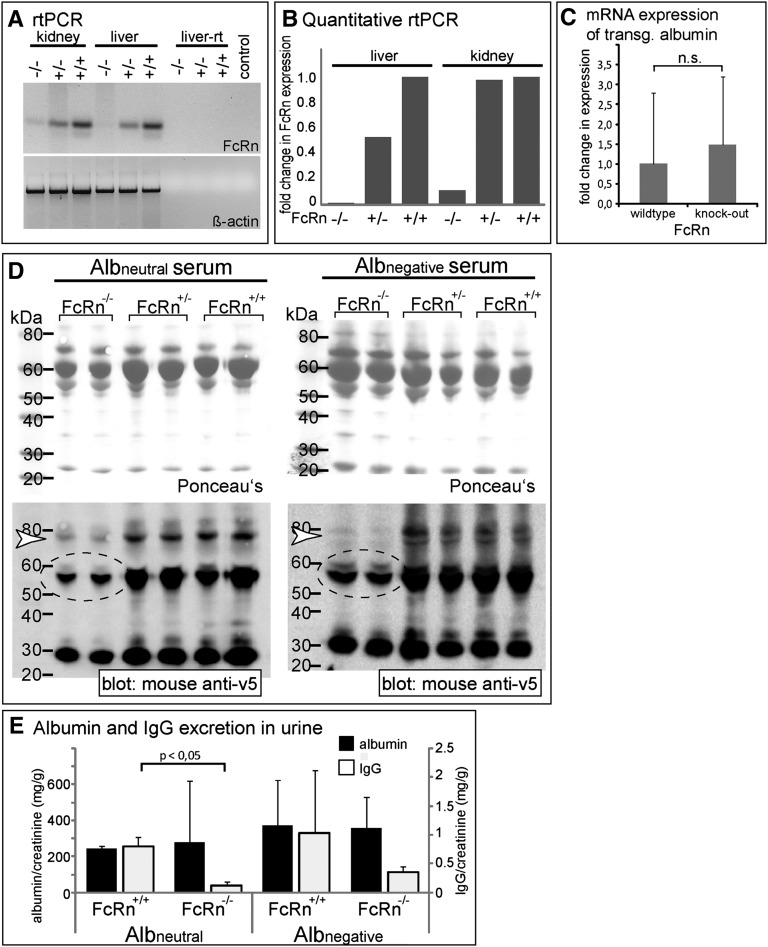
Albumin is taken up by proximal tubular cells through the cubilin/megalin complex.26 Subsequently, resorbed albumin is transferred into early endosomes. We tested whether the neonatal Fc receptor (FcRn) is involved in sorting resorbed albumin from early endosomes during acidification to prevent its subsequent lysosomal degradation. For this purpose, FcRn-deficient mice (Fcgrttm1dcr)27 were crossbred to our double transgenic Pod-rtTA/Albnegative and Pod-rtTA/Albneutral mice (Figure 6). FcRn transcripts were nearly absent in the homozygous knockout animals (Figure 6, A and B); however, a slight residual activity was noted in the kidney. Conversely, it was verified that inactivation of FcRn did not alter expression levels of transgenic albumin (Figure 6C). Endogenous Ig levels were reduced in the homozygous FcRn knockout animals as the consequence of the lower half-life of Ig in these animals (Figure 6D, dotted circles). FcRn-deficient mice did not excrete increased amounts of protein, consistent with the notions that protein uptake into the tubular cells is mediated by other multiligand receptors (e.g., cubilin/megalin) and that binding of albumin to FcRn occurs after endocytosis in the early endosomes during acidification. However, plasma levels of both transgenic albumins were significantly reduced exclusively in homozygous FcRn knockout mice (Figure 6D, white arrowheads). As shown in Figure 6E, FcRn deficiency did not result in proteinuria because of a defect in reabsorption of albumin or IgG. In contrast, lower amounts of IgG were excreted within the urine of FcRn-deficient mice—most likely because of the decreased IgG levels within the serum.
Figure 6.
FcRn is essential for transcytosis. (A) mRNA expression is significantly decreased in FcRn-deficient mice (Fcgrttm1dcr) as estimated by RT-PCR from total RNA. β-Actin controls show cDNA loading and quality. (B) Quantification was performed by real-time RT-PCR. The fold change in gene expression relative to a mean of wild-type liver/kidney was calculated using the ΔΔCt method. (C) No influence of FcRn on the expression of transgenic albumin mRNA. mRNA expression of transgenic albumin was performed by real-time RT-PCR in FcRn knockout mice. The fold change in gene expression relative to the mean of wild-type kidney was calculated using the ΔΔCt method (n=6 per group). No significant difference was detected between FcRn knockout and wild-type animals. (D) Immunoblot analysis of sera of Pod-rtTA/Albneutral/FcRn−/− and Pod-rtTA/Albnegative/FcRn−/− mice. Lanes were loaded with 5 µl serum and immunoblotted. In the serum of FcRn-deficient mice, significantly less transgenic albumin was detected (white arrowheads). Likewise, Ig heavy and light chains were decreased in the serum of FcRn-deficient mice (dotted circles), confirming functional inactivation of FcRn in our mouse model.28,40 (E) Urinary excretion of endogenous albumin and IgG in FcRn knockout versus wild-type mice was estimated by ELISA (normalized to creatinine concentrations). No significant differences were observed, although a decreased urinary excretion of IgG was noted in FcRn knockout mice, which is most likely as a consequence of the decreased IgG levels in the serum (n=2 per group).
Tracing IgG and Albumin across Proximal Tubular Cells in FcRn-Deficient Mice
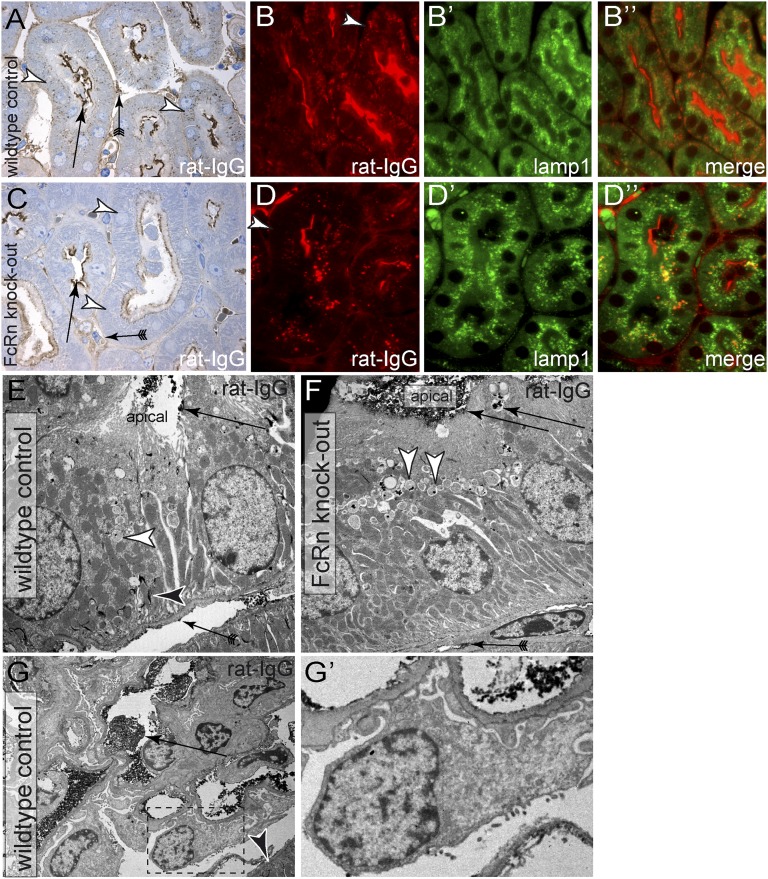
Other than albumin, the FcRn also binds IgG within the early endosomes and rescues both molecules from lysosomal degradation.28,29 To confirm a role of FcRn in a transcytotic mechanism of proximal tubular cells, rat IgG was traced in FcRn-deficient mice (Figure 7). For this purpose, rat antiamino-peptidase A IgG (anti-APA) was injected intravenously into FcRn-deficient mice and wild-type controls. In our mixed genetic background, anti-APA induced mild proteinuria for approximately 24 hours, allowing increased quantities of IgG to pass into the primary filtrate.30 When visualizing rat IgG with a specific antibody, anti-APA was still detected within tubulointerstitial capillaries and along the brush border of proximal tubule cells (Figure 7, A–D″, arrows). In wild-type controls, anti-APA rat IgG was detected on the apical as well as along the basolateral aspect of proximal tubular cells (Figure 7, A–B″, arrowheads). In FcRn-deficient mice, anti-APA remained confined along the apical aspect of proximal tubular cells. This distribution was observed in all proximal tubular cells (Figure 7, C–D″, arrowheads). Immunoelectron microscopy confirmed these findings (Figure 7, E and F). In wild-type animals with a preserved transcytosis mechanism, the intact rat Ig epitope was detected in vesicles along the apical aspect of proximal tubular cells and within the extracellular compartment of the basolateral plasmalemmal invaginations (Figure 7E, arrowheads). Of note, rat IgG was still present within the tubulointerstitial capillaries in wild-type and knockout animals. Because backdiffusion of rat IgG from the capillaries into the basolateral labyrinth of proximal tubular cells should occur independent of FcRn expression, backdiffusion cannot account for our findings. Within the glomerulus, no significant uptake of rat IgG was detected in podocytes or parietal epithelial cells (Figure 7, G and G′). These findings support our above-described finding that rat Ig is subject to transcytosis across the proximal tubular cells, similar to albumin.
Figure 7.
Altered distribution of Ig in tubular cells of FcRn-deficient mice. To test whether tubular handling of Igs is FcRn-dependent, rat anti-APA was injected intravenously into (A–B″) wild-type control littermates or (C–D″) FcRn-deficient mice. After 16 hours, animals were euthanized, and their kidneys were immunostained using rabbit anti-rat IgG-horseradish peroxidase. (A and C) On semithin kidney sections (Toluidin blue counterstain), the rat IgG (anti-APA) was detected within tubulointerstitial capillaries and along the brushborder of proximal tubule cells (arrows) from where it was endocytosed. (A–B″) In wild-type controls, (A, arrowheads) anti-APA distributed in vesicles throughout the entire proximal tubular cells. (B, arrowhead) Basolateral staining was also apparent in immunofluorescent staining. (C) In FcRn-deficient mice, anti-APA remained confined to the apical aspect of proximal tubular cells. (D–D″) This finding was observed in all proximal tubular cells (arrowhead) and confirmed by immunofluorescent staining. (D) Basolateral staining of IgG did not colocalize with lamp1 (marker for late endosomes and lysosomes). (E and F) These findings were confirmed by anti-rat Ig immunoelectron microscopy. Rat Ig was detected within the tubular lumen and bound to the apical brushborder (black precipitates; arrow). (E) In wild-type mice, rat Ig was detected as black precipitates within vesicles throughout the cell (white arrowhead). Along the basolateral aspect of proximal tubular cells, rat IgG accumulated extracellularly within the plasmalemmal infoldings (black arrowhead), consistent with our immunofluorescent staining. (F) In FcRn-deficient mice, rat Ig was detected exclusively within vesicles along the apical aspect of the cells (arrowheads). No rat Ig antigen accumulated along the extracellular basolateral aspect. (E and F) Within the tubulointerstitial capillaries, rat Ig was detected (arrows with tails). Because no rat Ig was detected within the basolateral plasma membrane invaginations (basolatheral labyrinth) of proximal tubular cells in FcRn-deficient mice, it must have reached this location by transcytosis and subsequent exocytosis across the cells. (G and G′) Within the glomeruli of the same mice, podocyte effacement as a consequence of anti-APA was observed. Rat IgG was visualized by immunoelectron microscopy within the glomerular capillary lumen (arrow) and again in the basolateral labyrinth of proximal tubular cells (arrowhead). No significant uptake of rat IgG/anti-APA could be detected in podocytes in either (G and G′) FcRn wild-type or knockout animals (not shown).
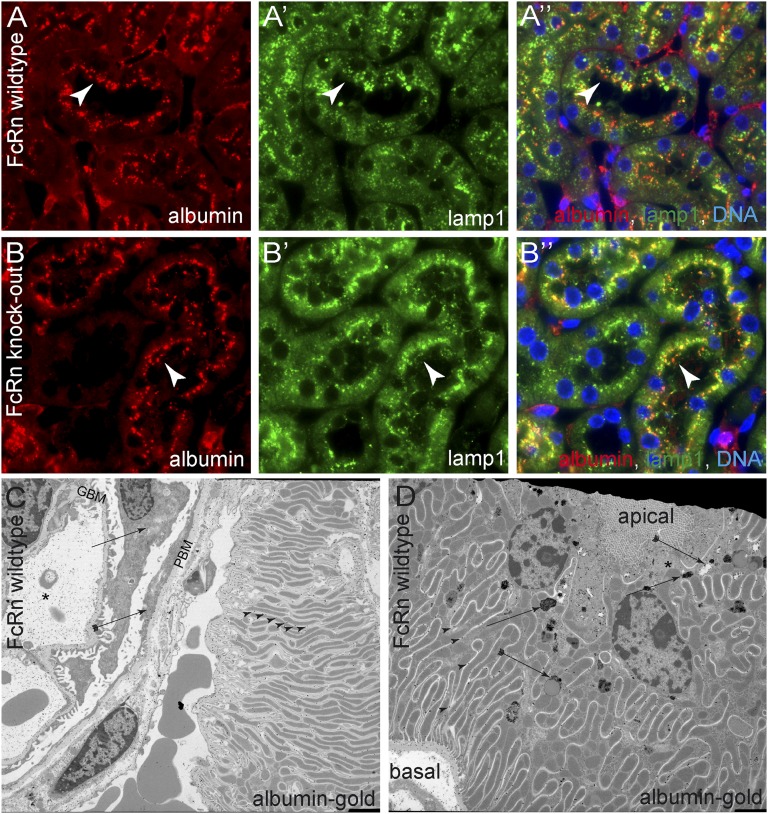
Colocalization studies with reabsorbed endogenous albumin and the endosomal/lysosomal marker lamp1 (www.uniprot.org/uniprot/P11279) did not show a different distribution in FcRn wild-type or knockout animals (Figure 8, A and B″). Nevertheless, when analyzing the distribution of gold-labeled albumin by electron microscopy, gold particles were enriched within the apical resorption vesicles, confirming our results. In addition, gold particles were found throughout the extracellular compartment of the plasmalemmal invaginations (labyrinth) (Figure 8, C and D), suggesting that albumin is efficiently transcytosed to the basolateral aspect of the cells. In contrast to the rat IgG immune complexes, albumin is potentially cleared away rapidly into the circulation, and therefore, no increased basolateral staining can be observed by immunofluorescent staining.
Figure 8.
Subcellular distribution of filtered albumin in proximal tubular cells. (A) In FcRn wild-type mice, endogenous albumin was costained with lamp1 (a marker for late endosomes and lysosomes) in proximal tubular cells. Albumin was localized in a punctate pattern (presumptive resorption vacuoles) along the apical aspect of proximal tubular cells (arrowheads). Partial colocalization with lamp1 was noted. (B) In FcRn knockout mice, the distribution of endogenous albumin within resorption vesicles and lamp1-positive vesicles was similar to wild-type mice (arrowheads). (C) To trace filtered albumin on ultrastructural levels, mice were injected intravenously with gold-labeled BSA (albumin gold) and analyzed 15 minutes later. Glomerular capillaries (asterisk) and the GBM contained significant amounts of albumin gold, whereas no significant uptake was observed in podocytes (arrow). Small quantities of albumin gold were present in parietal epithelial cells (arrow with tail). High amounts of albumin gold were found within the extracellular plasmalemmal infoldings of the basolateral labyrinth of proximal tubular cells (arrowheads). (D) Apico/basal cross-section across a proximal tubular cell of a mouse injected with albumin gold. As observed by immunofluorescent staining, resorption vesicles on the apical aspect of the cell contain gold particles in different concentrations (arrows). The distribution of gold particles seemed polarized in some vesicles (consistent with a potential sorting process; arrows with tails). Throughout the entire basolateral labyrinth, gold particles were present extracellularly. In contrast to the tracing experiments with rat IgG (see below), albumin gold no longer accumulated within the basolateral labyrinth, explaining why no basolateral staining was obvious by immunofluorescence (A and B). Note that the basolateral plasmalemmal invaginations (labyrinth) reached up to the apical aspect of the cell where the resorption vesicles were localized (asterisk). GBM, glomerular basement membrane; PBM, parietal basement membrane.
Discussion
The major finding of the present study is that filtered and reabsorbed albumin is transcytosed in an intact fashion, at least in part, back into the blood. By directly spiking the primary urine with tagged albumin, the subsequent fate of filtered albumin was traced in vivo over extended periods of time under physiologic conditions. The discovered mechanism is relevant to prevent physiologic losses of albumin, which would amount to approximately 1 g albumin per day in humans. Although the exact capacity of tubular transcytosis is still unknown, the fact that even low-grade proteinuria (e.g., orthostatic proteinuria of approximately 1 g per day) exceeds the resorptive capacity of the tubular system argues in favor of a low-capacity transcytosis mechanism.31
Significant extrarenal transcriptional activity of the podocyte-specific promoter fragment was ruled out by renal transplantation experiments in different transgenic rat founder lines. Although low-grade expression in other tissues cannot be ruled out entirely, our experiments show that the bulk of the transgenic albumin in the blood was derived from the podocytes within the transgenic kidneys.
In the literature, it is well established that virtually all proteins, which are endocytosed through coated pits, are subsequently degraded into amino acids within lysosomes.11 However, the experimental evidence indicating that albumin should also be degraded in lysosomes is mainly derived from studies performed in vitro.32 There are studies, however, that have already found evidence that albumin is handled differently, and these studies are in good agreement with our results. In the amphibian Necturus, iodinated albumin was injected into the lumen of proximal tubuli, and subsequently, a small fraction of intact labeled albumin could be recovered from the blood.33 In a second study, isolated rabbit proximal tubules were perfused with 0.03 mg/ml albumin. Most of the albumin was degraded and released into the peritubular solution. However, a small fraction remained macromolecular and presumably intact.34 The Comper group traced tritium-labeled albumin in the renal venous blood in isolated rat kidneys.35 They found a transient increase in radioactivity as early as less than 2 minutes after application of the labeled albumin, which was not apparent for other labeled proteins.35 More recently, it could be shown that albumin degradation products typically found in the urine36 are not formed within proximal tubular cells of the kidney but rather, are formed in other tissues.37 By two-photon microscopy, fluorescently labeled albumin was observed within endocytotic vesicles of proximal tubules in vivo.38 Some of the vesicles fused with the basolateral membrane during the observation period, indicating a transcytosis mechanism.
Another major finding of this study relates to charge-modified (i.e., neutralized) albumin. Our analyses in vitro and in vivo showed that the charge modification did not impair solubility or biologic behavior of the molecule. Specifically, neutralized albumin was also efficiently taken up by proximal tubular cells and subsequently, found in the blood. This finding indicates that neutralized albumin still bound to the cubilin/megalin and FcRn complex. Thus, the dox-inducible transgenic mouse model should be useful to spike virtually any body compartment with transgenic albumin of different charges to investigate permeability and/or charge effects in these tissues.
In the present study, the use of differently charged albumin was instrumental to detect a potentially significant glomerular backfiltration. Although we concede that a certain component of glomerular backfiltration can never be ruled out entirely, we took great care to be able to exclude a major contribution of backfiltration. When assuming that the standard models of glomerular filtration are true (including the pore theory, the slit diaphragm model, the endothelial cell model, the gel hypothesis, the gel permeation hypothesis, and the albumin retrieval hypothesis [all models are summarized in ref. 25]), glomerular backfiltration can only be driven by diffusion and/or convection. Because the transgenic albumin is secreted behind the filter, convection will always counteract backfiltration. When we estimated backdiffusion using the mathematical model developed by a group of experts in the field,21,22 the sieving coefficient (amount of transgenic albumin passing the filter) was predicted to be in the range of −0.0014 (the negative sign indicates the reverse flux of transgenic albumin across the glomerular filter).
However, it is possible that the sieving coefficient is actually even lower, because transgenic albumin accumulated within the serum over time, suggesting that the concentration of transgenic albumin in the serum became higher than within the primary filtrate. This result argues against the notion that significant glomerular backfiltration of transgenic albumin occurs if the standard models of glomerular filtration are true.
When assuming the novel electrokinetic model of glomerular filtration,25 charge effects also need to be considered. For this purpose, tracing experiments were performed in duplicate in vivo using the novel neutralized albumin. According to the electrokinetic model, negatively charged albumin is predicted to be backfiltered by diffusion and electrophoresis, whereas neutralized albumin will be backfiltered by diffusion alone. As shown in Figure 1D, the predicted difference in glomerular backfiltration of the differently charged albumins is about two orders of magnitude. However, in all of our experiments, we observed only minor differences between negatively charged and neutralized transgenic albumin. Therefore, when assuming the electrokinetic model of glomerular filtration, a significant influence of glomerular backfiltration is unlikely.
The third major finding of this study identified an essential role of the MHC-related Fc receptor for IgG (FcRn) for transcytosis of albumin and IgG. As shown by our experiments in FcRn-deficient mice, FcRn-dependent transcytosis likely also applies for IgG. This finding fits well with the published literature defining a similar role for the FcRn complex in other tissues. The FcRn complex mediates transcytosis of maternal IgG across the placenta and the fetal small intestine to passively immunize the fetus. In adults, it protects albumin and IgG from lysosomal degradation in virtually all cells (also outside the kidney) by binding both proteins with high affinity at low pH in the acidifying endosomes and thus, protecting both from a lysosomal pathway, returning them to the extracellular compartment.29,39 In FcRn knockout mice, the half-life of albumin and IgG have been shown to be reduced (28.7 versus 39.9 hours and 29.5 versus 66.1 hours, respectively), resulting in slightly reduced serum albumin levels in FcRn-deficient mice.40 Thus, the FcRn-mediated salvage pathway explains the unusually long half-life of these proteins. The renal contribution of albumin turnover has been investigated in the work by Sarav et al.,40 which showed that transplanting a wild-type kidney into FcRn-deficient mice (endogenous kidneys were removed) normalized serum albumin levels—consistent with our results and supporting that this mechanism is physiologically relevant. As nicely shown in the work by Weyer et al. 10, small but significant amounts of albumin are excreted in cubilin/megalin knockout mice in the urine, consistent with a low glomerular albumin sieving coefficient and consistent with a low but significant renal turnover of albumin by transcytosis. Sarav et al. 40 also noticed that the half-life of IgG does not depend on renal FcRn, which is also consistent with our results, because only very small amounts of IgG pass into the primary filtrate; therefore, transcytosis of IgG is not relevant in the absence of proteinuria. Consistently, we were unable to detect IgG within proximal tubular cells in nonproteinuric mouse kidneys (data not shown). FcRn is expressed in many tissues, including the vasculature, lung, skin, gut, spleen, and kidney. In the kidney, FcRn is expressed in podocytes and along the brush border of proximal tubular cells.41–43 In vitro, proximal tubular cells were shown to actively bind and transcytose human albumin and IgG in an FcRn-dependent manner.44 During the transition from early to late endosomes, the pH drops (acidification), and albumin as well as IgG are released from the cubilin/megalin complex (Figure 9). However, at pH<6.4, FcRn binds albumin and IgG with high affinity and prevents its sorting and degradation within a lysosomal compartment.28,29 The data of the present study show that FcRn is essential to protect the albumin and IgG, which has been reclaimed from the primary filtrate by the cubilin/megalin receptor complex, from lysosomal degradation. Instead, in the presence of FcRn, both proteins are shuttled to the basolateral surface of the proximal tubular cells and returned to the blood.
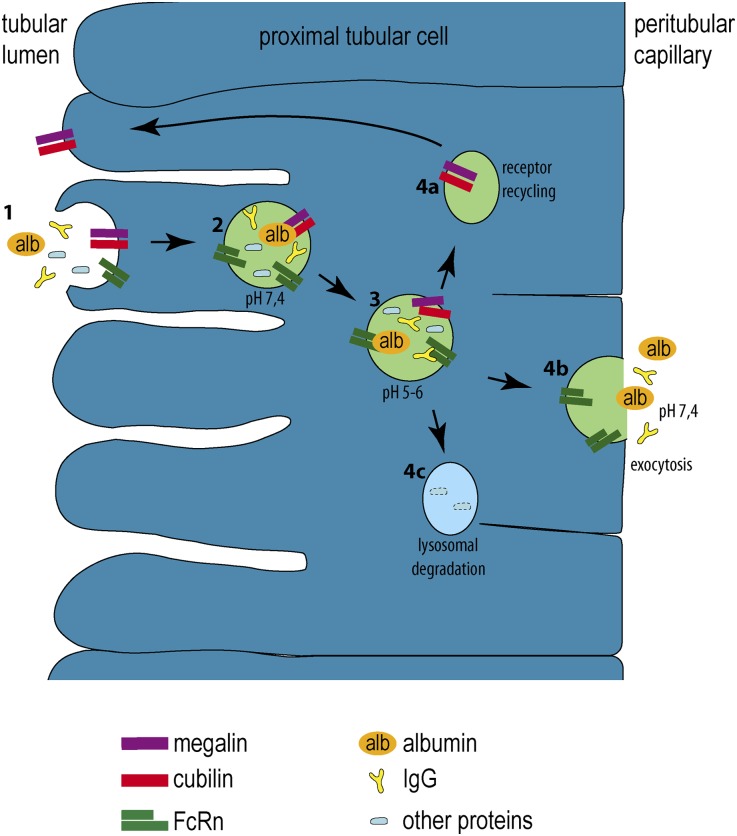
Figure 9.
Schematic for transcytosis in proximal tubular cells. (1) From the tubular lumen, albumin (alb, orange), IgG (yellow), and other proteins (blue) are bound to the brushborder of proximal tubular cells through the cubilin/megalin complex. (2 and 3) Acidification in early endosomes results in a release of the bound proteins from the cubilin/megalin complex. At a pH of 5–6, albumin and IgG bind to FcRn, whereas other proteins do not. (4a) The cubilin/megalin complex is recycled back to the apical brushborder. (4b) FcRn-bound proteins are sorted to the basolateral aspect of the cell from where they are released. (4c) The remaining proteins are destined for lysosomal degradation.
In summary, in the present study, the primary filtrate was specifically spiked with transgenic albumin with different net charge. By tracing the fate of transgenic albumin, we provide conclusive evidence for the existence of a transcytosis mechanism for filtered albumin and IgG across tubular cells in an FcRn-dependent fashion.
Concise Methods
Transgenic Mouse and Rat Models
All experiments were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein–Westfalen (50.203.2 AC 7; 87–51.04.2010.A155). Animals were housed under standard specific pathogen-free conditions (22°C, 50% humidity, 12-hour light cycle) with free access to tap water and standard animal chow. If not specified otherwise, an equal distribution of males and females and Pod-rtTA mice have been described previously.26 Albnegative/neutral cDNA (Geneart) was cloned into the pTRE2 plasmid (Clontech) or the p2.5hNPHS2 plasmid20 for transgenic mice or rats, respectively. Genotyping from tail biopsies was performed by PCR using the following primers: TetOn.fwd (5′AATCG AGATG CTGGA CAGGC ATCAT ACCCA3′); TetOn.rev (5′GGCAT AGAAT CGGTG GTAGGT GTCTCT CTT3′); Albnegative.fwd (5′CCATG CTTCT CTGCTC TGACAG TTGAT GA3′); Albnegative.rev (5′GGATT GGTAA TTGGT TTCCC GCTTC TTCTG3′); Albneutral.fwd (5′TGATC GCCTT CAGCC AGTAC CTGCA GAAGT3′); Albneutral.rev (5′GTTCT CCTTG AAGCTG GTGCA CATGGC3′); FcRnko.fwd (5′GGAAT TCCCA GTGAA GGGC3′); FcRnwt.fwd (5′GGGAT GCCAC TGCCCTG 3′); and FcRnwt/ko.rev (5′CGAGC CTGAGA TTGTCAA GTGTATT3′). Mice were kept on a 50% Sv129 mixed genetic background. To induce transgene expression, animals received dox hydrochloride (1 g/L, 5% sucrose; Fargon, Barsbüttel, Germany) through the drinking water ad libitum.
Mice were anesthetized using ketamin/xylazine, blood and urine were obtained through heart and bladder puncture, and the kidneys were perfused through the left heart ventricle with 3% paraformaldehyde in PBS and harvested. Pieces of the kidneys were embedded in Tissue Tec (Miles Inc,, Iowa City, IA) and snap-frozen in liquid nitrogen or fixed in 4% formalin and embedded in paraffin.
Immunostaining
Immunofluorescent and immunohistochemical staining were performed on 2-µm paraffin sections as described previously.45 The following antibodies were used: mouse monoclonal anti-V5 (1:500, R960–25; Invitrogen, Carlsbad), rabbit antisynaptopodin (1:100, sc50459; Santa Cruz Biotechnology, CA), goat antialbumin (1:200, A90–134A; Bethyl Laboratories, Montgomery, TX), and fluorescein labeled L. tetragonolobus lectin or L. tetragonolobus agglutinin (1:100, FL-1321; Vector Laboratories, Burlingame, CA). Isotype-matched irrelevant antibodies were used as controls. The secondary antibodies were as follows: donkey anti-rabbit, -mouse, or -rat, Dylight 488, Dylight 647, or Dylight 549 (1:200; Dianova, Hamburg, Germany), and Hoechst 33342 (Sigma-Aldrich, St. Louis, MO). Images were collected using a Leica DMRX microscope (Leice Microsystem GmbH, Wetzlar, Germany) combined with the Diskus program (Diskus Kameras, Koenigswinter, Germany) and Adobe Photoshop CS5.
cDNA Isolation and Real-Time PCR
Total RNA was isolated from tissues using the RNeasy Mini Kit (Quiagen, Hilden, Germany) according to the manufacturer’s protocol. Sample purity and RNA content were determined by ultraviolet spectrophotometry. Reaction mix for cDNA synthesis was 1 µg total RNA, 10 µl RNAse-free water, 1 µl random primer (250 ng/µl; Roche, Mannheim, Germany), 1.5 µl dNTP mix (10 mM each; Amersham, Piscataway, NJ), 0.7 µl RNAsin (Promega, Mannheim, Germany), 6 µl Moloney Murine Leukemia Virus (M-MLV) reverse transcription buffer, and 1 µl M-MLV reverse transrcriptase (Invitrogen, Carlsbad). The reaction mix was incubated for 10 minutes at 25°C and afterward, 1 hour at 42°C. For normal PCR cDNA analysis, the Albneutral and FcRn genotyping primers and the following β-actin primers were used: Actin.fwd (5′GTGGG CCGCT CTAGGC ACCAA3′) and Actin.rev (5′CTCTT TGATGT CACCGC ACGAT TTC3′). The ABI Prism 7300 sequence detector (Applied Biosystems, Foster City) was used for real-time PCR analysis. Here, the reaction mix contained 0.75 µl cDNA and the qPCR Core Kit for SYBR Green I (Eurogentec, Seraing, Belgium) according to manufacturer’s instructions, and it was incubated for 2 minutes at 50°C followed by 40 cycles for 15 seconds at 95°C and 1 minute at 58°C. Primer Express software (Applied Biosystems) was used for the design of the following Taqman primers and probes: GAPDH.fwd (5′GGCAA ATTCAA CGGCA CAGT3′); GAPDH.rev (5′AGATG GTGAT GGGCT TCCC3′); GAPDH probe (5′AAGGCCGAGAATGGGAAGCTTGTCATC3′); FcRn.fwd (5′CTGAG AACGG AAATC GTTGC TAA3′); FcRn.rev (5′TTAGC AGGAA CTCGC TCTCC TT3′); transgenic albumin: Albxx.fwd (5′GAAGC GGGAA ACCAA TTCC3′); Albxx.rev (5′GTTTG GGATT GGCTT TCCG3′); Albxxprobe (5′CTCTC CTGGG TCTGG ATTCC ACCCC A3′); mpodocin.fwd (5′GAGGC ACAAA GACAG GCCAA3′); mpodocin.rev (5′ACTCA GAGGC AGCTT TTTCC C3′). In Figure 6, expression levels were determined using the comparative cycle threshold method (ΔΔCt method).
Immunoblot Analysis
The glomeruli and tubules were isolated as described46 and lysed in radioimmunoprecipitation assay buffer containing protease inhibitors (Sigma-Aldrich). Protein concentration was determined with a BC Assay for Protein quantification (Uptima, Montlucon Cedex, France). For SDS-PAGE, NuPAGE 4%–12% Bis Tris Zoom Gels (Invitrogen) were loaded with denaturated protein lysates buffered in Laemmli followed by a transfer onto nitrocellulose membranes (Protan; Whatman, Dassel, Germany). After blotting, the membranes were stained reversibly with Ponceau S (Sigma) to control transfer efficiency and compare protein loading, and they were blocked for 1 hour in RotiBlock (Carl Roth). The primary antibodies were as follows: mouse monoclonal anti-V5 or anti-V5–horseradish peroxidase antibody (Invitrogen) and rabbit polyclonal anti-FcRn (Santa Cruz Biotechnology, Santa Cruz, CA) followed by horseradish peroxidase-conjugated secondary antibodies (anti-rabbit, anti-mouse; Vector). Signals were visualized by ECL or Super Signal Femto (Pierce, Thermo Scientific) and detected by LAS-3000 (Fujifilm).
Two-Dimensional SDS-PAGE
Proteins were separated in the first dimension by isoelectric focusing using the Ettan IGPhor 3 IEF System and Immobiline DryStrips pH 3–10 (both GE Healthcare, Uppsala, Sweden) and the second dimension by SDS-PAGE in NuPAGE 4%–12% Bis-Tris ZOOM gels (Invitrogen). All steps were performed according to manufacturer’s protocol.
Rat Transplantation Studies
Rat heterotopic renal transplantation with end-to-end ureteral anastomosis, uninephrectomy of the ipsilateral recipient kidney at day 0, and uninephrectomy (through flank incision) of the contralateral recipient kidney after 7 days were performed as described.47 Animals received enrofloxacin (2.5 mg/kg body wt) by intraperitoneal injection prophylactically every 12 hours for 3 days after each surgery. Immunosuppression was with cyclosporine A (2 mg/kg body wt) by subcutaneous injection daily and/or methylprednisolone (2 mg/kg body wt) by subcutaneous injection daily (as specified above).
Cell Culture
Cells were cultured in an HERAcell incubator (Thermo Fisher Scientific) at 37°C in an atmosphere containing 5% CO2. HEK293 cells (gift from R. Hausmann, Institute of Pharmacology, RWTH University Hospital of Aachen, Aachen, Germany) were maintained in DMEM F12 + Glutamax (31331; Invitrogen) supplemented with 10% FCS (Biowest, Nuaillé, France) and 1% penicillin-streptomycin solution (15070–063; Invitrogen). Cells were passaged by incubation with 2 ml 0.25% trypsin with EDTA (25200–72; Invitrogen) for 2 minutes and diluted with 8 ml cell culture medium. For transient transfection, Albnegative or Albneutral was cloned into pcDNA3.1 using TransIT-LT1 (2300, ratio of 5:2; Mirus, Madison, WI) according to the manufacturer’s instructions.
Ultracentrifugation
Two milliliters supernatant of HEK293 cells transfected with either Albnegative or Albneutral or empty controls were ultracentrifuged at 72,000 × g for 1 hour in an Optima L 80 XP (Beckman Coulter, Krefeld, Germany) using a Ti 70 rotor. Ultracentrifugation supernatants were decanted, and each ultracentrifugation pellet was resuspended in 500 μl 1× RIPA buffer.
Anti-HA/V5 Sandwich ELISA
Dilution series for the standard was prepared with 1% BSA-PBS; 96-well plates (655074; Greiner Bio-One) were coated with 100 μl/well anti-HA antibody (H6908; Sigma) diluted 1:20,000 in coating buffer (0.1 M NaHCO3, pH 9.6) and incubated overnight at 4°C. After removing the coating solution, plates were blocked with 300 μl/well synthetic blocking buffer (37515; Thermo Fisher Scientific, Schwerte, Germany) for 10 minutes at room temperature on a wave platform shaker (model 3011; GFL). Blocking solution was decanted, and probes, standards, and blanks (100 μl each) were pipetted in duplicate and incubated for 1 hour at room temperature on a wave platform shaker. Plates were washed three times with 300 μl/well phosphate and tris buffered saline for 5 minutes each on a wave platform shaker. Afterward, plates were incubated with 100 μl/well anti-V5 horseradish peroxidase-conjugated antibody (R961–25; Invitrogen) diluted 1:50,000 in PBS for 30 minutes at room temperature on a wave platform shaker. Plates were thoroughly washed six times with 300 μl/well PBST on a vibrating microtiter plate shaker for 1 minute each. PBST was shaken out well, and 100 μl/well substrate (37074; Thermo Scientific) were applied. Within 30 minutes, plates were measured on a Tecan infinite M200 (Tecan, Crailsheim, Germany) using iControl 1.4 in chemoluminescence mode.
Endogenous Albumin and IgG ELISA
All mice were placed in metabolic cages for 24 hours for urine collection. Urinary creatinine was assessed using an autoanalyzer (vitros 250; Ortho Clinical Diagnostics, Rochester, NY). Urinary levels of endogenous albumin and IgG were estimated using the Mouse Albumin ELISA Quantitation Set and the Mouse IgG ELISA Quantitation Set (Bethyl Laboratories, Montgomery, TX).
APA Injection and Immunoelectron Microscopy
Mice received an intravenous injection of 4 mg rat monoclonal antibody against APA (ASD-37/41).30 After 16 hours, animals were proteinuric and euthanized; 1-mm kidney slices were fixed in 10 mM periodate, 75 mM lysine, and 2% paraformaldehyde (pH 6.2) at 4°C for 3 hours. The slices were washed in PBS for 30 minutes, cryoprotected by immersion in 2.3 M sucrose solution for 1 hour, and afterward, snap-frozen in liquid nitrogen; 20-μm cryosections were incubated with a peroxidase-labeled rabbit anti-rat IgG (Dako Glostrup, Denmark) diluted in PBS containing 1% BSA followed by three washes with PBS. The sections were incubated in PBS, pH 7.4, containing diaminobenzidine medium for 10 minutes followed by diaminobenzidine with the addition of 0.003% H2O2 for 7 minutes. The sections were washed in water, postfixed in Palade buffer containing 1% OsO4 for 30 minutes at 4°C, dehydrated, and embedded in Epon812 (Merck, Darmstadt, Germany). Ultrathin sections were examined in a JEOL 1200 EX2 electron microscope (JEOL, Tokyo, Japan).
Protein modifications and plots were performed using the GRASP software.48 Albumin gold-injected mice were derived from and described in a previous study.49
Disclosures
None.
Acknowledgments
This work was supported by funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under Grant HEALTH-F5-2008-223007 STAR-TREK (to U.K.), a START grant by the Medical Faculty of the RWTH Aachen (to S.K.S.), TP17, TP14, TP22, and TP25 SFB/Transregio 57 of the German Research Foundation (DFG; to M.K., P.B., J.F., M.J.M.), DFG Grant BO 3755/1-1 (to P.B. and B.S.), Boost Fund OPBo45 of the Excellence Initiative by the German Federal and State Governments (DFG; to M.G. and M.J.M.), and the NephCure Foundation (F001; B.S. and M.J.M.).
U.K., P.B., J.F., and M.J.M. are members of the SFB/TRR57 Consortium Mechanisms of Organ Fibrosis (DFG).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010018/-/DCSupplemental.
References
- 1. Peters T, Jr: Serum albumin: Recent progress in the understanding of its structure and biosynthesis. Clin Chem 23: 5–12, 1977. [PubMed] [Google Scholar]
- 2. Haraldsson B, Nyström J, Deen WM: Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Tojo A, Endou H: Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol 263: F601–F606, 1992. [DOI] [PubMed] [Google Scholar]
- 4. Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW, Thakker RV, Unwin RJ, Wrong O: Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int 60: 1885–1892, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Christensen EI, Birn H, Rippe B, Maunsbach AB: Controversies in nephrology: Renal albumin handling, facts, and artifacts! Kidney Int 72: 1192–1194, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Jarad G, Miner JH: Update on the glomerular filtration barrier. Curr Opin Nephrol Hypertens 18: 226–232, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Birn H, Christensen EI: Renal albumin absorption in physiology and pathology. Kidney Int 69: 440–449, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Maunsbach AB: Albumin absorption by renal proximal tubule cells. Nature 212: 546–547, 1966. [DOI] [PubMed] [Google Scholar]
- 10. Weyer K, Storm T, Shan J, Vainio S, Kozyraki R, Verroust PJ, Christensen EI, Nielsen R: Mouse model of proximal tubule endocytic dysfunction. Nephrol Dial Transplant 26: 3446–3451, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Gekle M: Renal tubule albumin transport. Annu Rev Physiol 67: 573–594, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Gekle M, Mildenberger S, Freudinger R, Silbernagl S: Endosomal alkalinization reduces Jmax and Km of albumin receptor-mediated endocytosis in OK cells. Am J Physiol 268: F899–F906, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Gekle M, Mildenberger S, Freudinger R, Silbernagl S: Functional characterization of albumin binding to the apical membrane of OK cells. Am J Physiol 271: F286–F291, 1996. [DOI] [PubMed] [Google Scholar]
- 14. Norden AG, Unwin RJ: Is the albumin retrieval hypothesis a paradigm shift for nephrology? J Am Soc Nephrol 23: 569–571, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Pollock CA, Poronnik P: Albumin transport and processing by the proximal tubule: Physiology and pathophysiology. Curr Opin Nephrol Hypertens 16: 359–364, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Haraldsson B: Tubular reabsorption of albumin: It’s all about cubilin. J Am Soc Nephrol 21: 1810–1812, 2010. [DOI] [PubMed] [Google Scholar]
- 17. de Borst MH: On the origin of albuminuria. Kidney Int 72: 1409, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Russo LM, Bakris GL, Comper WD: Renal handling of albumin: A critical review of basic concepts and perspective. Am J Kidney Dis 39: 899–919, 2002. [DOI] [PubMed] [Google Scholar]
- 19. Christensen EI, Verroust PJ: Interstitial fibrosis: Tubular hypothesis versus glomerular hypothesis. Kidney Int 74: 1233–1236, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Dechadilok P, Deen WM: Hindrance factors for diffusion and convection in pores. Ind Eng Chem Res 45: 6953–6959, 2006. [Google Scholar]
- 22. Rippe B, Haraldsson B: Transport of macromolecules across microvascular walls: The two-pore theory. Physiol Rev 74: 163–219, 1994. [DOI] [PubMed] [Google Scholar]
- 23. Hausmann R, Grepl M, Knecht V, Moeller MJ: The glomerular filtration barrier function: New concepts. Curr Opin Nephrol Hypertens 21: 441–449, 2012. [DOI] [PubMed] [Google Scholar]
- 24. Hausmann R, Kuppe C, Egger H, Schweda F, Knecht V, Elger M, Menzel S, Somers D, Braun G, Fuss A, Uhlig S, Kriz W, Tanner G, Floege J, Moeller MJ: Electrical forces determine glomerular permeability. J Am Soc Nephrol 21: 2053–2058, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moeller MJ, Tenten V: Renal albumin filtration: Alternative models to the standard physical barriers. Nat Rev Nephrol 9: 266–277, 2013. [DOI] [PubMed] [Google Scholar]
- 26. Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB: Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998–2003, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL: The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol 170: 3528–3533, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL: The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 197: 315–322, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roopenian DC, Akilesh S: FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol 7: 715–725, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Dijkman HB, Gerlofs-Nijland ME, van der Laak JA, Wetzels JF, Groenen PJ, Assmann KJ: Podocyte changes after induction of acute albuminuria in mice by anti-aminopeptidase A mAb. Nephron, Exp Nephrol 94: e85–e93, 2003. [DOI] [PubMed] [Google Scholar]
- 31. Robinson RR, Lecocq FR, Phillippi PJ, Glenn WG: Fixed and reproducible orthostatic proteinuria. III. Effect of induced renal hemodynamic alterations upon urinary protein excretion. J Clin Invest 42: 100–110, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrell N, Ricci KB, Groszek J, Marmerstein JT, Fissell WH: Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnol Bioeng 109: 797–803, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scott WN, Maude DL, Shehadeh I, Solomon AK: Inulin and albumin absorption from the proximal tubule in necturus kidney. Science 146: 1588–1590, 1964. [DOI] [PubMed] [Google Scholar]
- 34. Park CH, Maack T: Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest 73: 767–777, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eppel GA, Osicka TM, Pratt LM, Jablonski P, Howden BO, Glasgow EF, Comper WD: The return of glomerular-filtered albumin to the rat renal vein. Kidney Int 55: 1861–1870, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Gudehithlu KP, Pegoraro AA, Dunea G, Arruda JA, Singh AK: Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int 65: 2113–2122, 2004. [DOI] [PubMed] [Google Scholar]
- 37. Weyer K, Nielsen R, Christensen EI, Birn H: Generation of urinary albumin fragments does not require proximal tubular uptake. J Am Soc Nephrol 23: 591–596, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA: Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23: 447–457, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderson CL, Chaudhury C, Kim J, Bronson CL, Wani MA, Mohanty S: Perspective— FcRn transports albumin: Relevance to immunology and medicine. Trends Immunol 27: 343–348, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, Quigg RJ: Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol 20: 1941–1952, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haymann JP, Levraud JP, Bouet S, Kappes V, Hagège J, Nguyen G, Xu Y, Rondeau E, Sraer JD: Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 11: 632–639, 2000. [DOI] [PubMed] [Google Scholar]
- 42. Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kacskovics I, Kis Z, Mayer B, West AP, Jr, Tiangco NE, Tilahun M, Cervenak L, Bjorkman PJ, Goldsby RA, Szenci O, Hammarström L: FcRn mediates elongated serum half-life of human IgG in cattle. Int Immunol 18: 525–536, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Kobayashi N, Suzuki Y, Tsuge T, Okumura K, Ra C, Tomino Y: FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol 282: F358–F365, 2002. [DOI] [PubMed] [Google Scholar]
- 45. Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kabgani N, Grigoleit T, Schulte K, Sechi A, Sauer-Lehnen S, Tag C, Boor P, Kuppe C, Warsow G, Schordan S, Mostertz J, Chilukoti RK, Homuth G, Endlich N, Tacke F, Weiskirchen R, Fuellen G, Endlich K, Floege J, Smeets B, Moeller MJ: Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS One 7: e34907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kunter U, Floege J, von Jürgensonn AS, Stojanovic T, Merkel S, Gröne HJ, Ferran C: Expression of A20 in the vessel wall of rat-kidney allografts correlates with protection from transplant arteriosclerosis. Transplantation 75: 3–9, 2003. [DOI] [PubMed] [Google Scholar]
- 48. Nicholls A, Sharp KA, Honig B: Protein folding and association: Insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11: 281–296, 1991. [DOI] [PubMed] [Google Scholar]
- 49. Sicking EM, Fuss A, Uhlig S, Jirak P, Dijkman H, Wetzels J, Engel DR, Urzynicok T, Heidenreich S, Kriz W, Kurts C, Ostendorf T, Floege J, Smeets B, Moeller MJ: Subtotal ablation of parietal epithelial cells induces crescent formation. J Am Soc Nephrol 23: 629–640, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]