Abstract
The association between dialysis facility size and mortality for patients undergoing hemodialysis remains largely unclear, and whether the relationship differs by race and ethnicity or among high-risk subgroups is not known. Using data from the USRDS, we analyzed mortality rates in 385,074 incident patients ages≥18 years who received in-center hemodialysis at 4633 dialysis facilities between 2003 and 2009. Facilities were categorized by the number of hemodialysis stations (1–5, 6–10, 11–15, 16–20, 21–25, 26–30, 31–35, 36–45, 46–60, and ≥61 stations). We found significantly higher mortality associated with facilities comprising ≤15 stations, and within this group, mortality increased as the number of stations decreased. The association with increased mortality was weaker for facilities with 16–30 stations, but >30 stations offered no additional survival benefit. The association between increased mortality and facilities with ≤15 stations was stronger for racial minorities and patients with diabetes or cardiovascular diseases. After adjustments, blacks had a 78% greater 1-year mortality risk in facilities with one to five stations, whereas whites had only a 26% greater risk. Notably, other patient-related events remained comparable across the categories assessed. In summary, these data suggest that hemodialysis care at small facilities associates with a significant increase in mortality that is only partially explained by measured patient case mix, other well defined facility characteristics, and geographic region. Future studies should investigate differences in processes of care and practices among hemodialysis facilities of different sizes.
The relationship between hospital volume or size and mortality has been extensively documented. Hospitals with a larger volume of patients have been associated with lower mortality rates on numerous surgical procedures (e.g., cancer surgery) and medical conditions (e.g., acute myocardial infarction).1,2 These relationships are likely because of the increased availability of specialized staff, equipment, and infrastructure dedicated to a given condition or procedure and better economies of scale to implement measures for quality improvement in larger hospitals. Moreover, it has been found that the magnitude of the volume–outcome association is stronger for black patients and several high-risk procedures and conditions, including pancreatic cancer, esophageal cancer, and AIDS.1
Across the United States, approximately 400,000 patients with ESRD undergo maintenance hemodialysis at dialysis facilities each year.3 However, only 51% of dialysis patients are still alive 3 years after the start of ESRD therapy.3 Thus, there is a pressing need to identify best practices that are associated with improved outcomes. Prior studies addressing the relationship of facility characteristics with outcome and processes of care have largely focused on profit status and chain affiliation,4–14 whereas the role of facility size remains largely unclear. We conducted a national study to examine the association between facility size and mortality among maintenance hemodialysis patients. Our objectives were to examine whether the size–outcome relationship exists among hemodialysis patients and if so, whether the relationship differs by race/ethnicity and high-risk subgroups after accounting for variation in patient, facility, and geographic characteristics.
Results
Patient and Facility Characteristics
Of 4633 facilities, 45 (0.97%) facilities had 1–5 stations, 491 (10.6%) facilities had 6–10 stations, 1267 (27.3%) facilities had 11–15 stations, 2562 (55.3%) facilities had 16–30 stations, and 268 (5.8%) facilities had more than 31 stations. The average number of stations was 18 (median=17; range=1–80). Compared with large facilities (≥31 stations), small facilities (≤15 stations) were more likely to be rural, have higher nurse-to-patient ratios, and locate in zip codes with higher socioeconomic status (SES) and the Midwest (Table 1). Additional examination of facilities with 1–5, 6–10, and 11–15 stations (Supplemental Table 1) revealed that 51% of facilities with 1–5 stations and 27% of facilities with 6–10 stations were not affiliated with national dialysis chains compared with only 15%–18% of larger facilities. Less than 4% (69 of 1803) of facilities with ≤15 stations were located in isolated rural areas.
Table 1.
Facility characteristics by size (n=4633)
| Facility Characteristics | Number of Stations in the Facility | ||
|---|---|---|---|
| 1–15 | 16–30 | ≥31 | |
| Number of facilities, n | 1803 (38.9%) | 2562 (55.3%) | 268 (5.8%) |
| For profit (%) | 89.1 | 91.3 | 85.4 |
| Chain ownership (%) | |||
| DCI | 4.3 | 4.4 | 5.6 |
| DaVita | 31.5 | 30.7 | 25.4 |
| Fresenius | 34.3 | 36.3 | 41.0 |
| Other chains | 11.3 | 13.3 | 9.7 |
| No chain affiliation | 18.7 | 15.3 | 18.3 |
| Median full-time RNs + LPNs per 100 patients | 7.4 | 6.2 | 5.5 |
| Facility zip code SES, mean ± SD | |||
| Median annual household income ($1000) | 41.1±14.9 | 40.4±14.5 | 35.6±13.0 |
| Percentage of persons in poverty | 12.5±8.0 | 14.5±9.2 | 18.2±9.4 |
| Percentage of black population | 12.8±19.2 | 19.9±23.8 | 30.4±27.7 |
| Percentage of persons with high school education | 79.7±10.3 | 78.0±11.9 | 74.8±12.4 |
| Urban (%) | 59.7 | 84.9 | 89.6 |
| Census region (%) | |||
| Northeast | 10.9 | 14.4 | 10.8 |
| Midwest | 29.3 | 16.5 | 19.4 |
| South | 44.8 | 50.6 | 50.7 |
| West | 15.0 | 18.5 | 19.0 |
RN, Registered Nurse; LPN, Licensed Practical Nurse; DCI, Dialysis Clinic, Inc.
Of 385,074 patients, 23.6% of patients were treated at facilities with 1–15 stations, 64.5% of patients were treated at facilities with 16–30 stations, and 11.9% of patients were treated at facilities with ≥31 stations (Table 2 and Supplemental Table 2); 30.1% of whites, 16.7% of blacks, and 15.4% of Hispanics received care at smaller facilities (≤15 stations). Compared with whites, black and Hispanic patients tended to be younger and of lower SES (employment, insurance, and income), and they were more likely to be women, have higher prevalence of hypertension and diabetes, and have lower prevalence of other comorbid conditions. Characteristics of patients of the same race/ethnicity seemed to be similar in these three broad facility size categories.
Table 2.
Patient characteristics by facility size and race/ethnicity
| Characteristics | White (n=202,977) | Black (n=125,877) | Hispanic (n=56,220) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1–15 Stations | 16–30 Stations | ≥31 Stations | 1–15 Stations | 16–30 Stations | ≥31 Stations | 1–15 Stations | 16–30 Stations | ≥31 Stations | |
| Number of patients, n | 61,101 (30.1%) | 126,039 (62.1%) | 15,837 (7.8%) | 21,068 (16.7%) | 82,809 (65.8%) | 22,000 (17.5%) | 8648 (15.4%) | 39,488 (70.2%) | 8084 (14.4%) |
| Men (%) | 57.1 | 57.7 | 57.8 | 49.7 | 49.8 | 50.7 | 56.6 | 55.8 | 55.1 |
| Age, mean ± SD (yr) | 66.7±14.0 | 66.8±14.2 | 65.6±14.6 | 59.3±14.8 | 58.2±14.9 | 57.9±14.8 | 59.2±15.2 | 59.0±15.2 | 58.2±14.8 |
| BMI, mean ± SD (kg/m2) | 29.0±7.7 | 28.7±7.6 | 28.6±7.7 | 29.4±8.2 | 29.4±8.1 | 29.5±8.2 | 28.4±7.1 | 28.2±7.0 | 28.3±7.1 |
| eGFR, mean ± SD (ml/min per 1.73 m2) | 11.1±4.9 | 10.9±4.8 | 10.3±4.6 | 10.6±5.0 | 10.2±4.8 | 9.9±4.6 | 10.0±4.6 | 9.8±4.6 | 9.5±4.5 |
| SES | |||||||||
| Employed (%) | 88.9 | 88.4 | 85.7 | 77.5 | 75.1 | 72.1 | 75.2 | 74.8 | 70.4 |
| Private insurance (%) | 57.8 | 58.9 | 57.1 | 37.7 | 37.0 | 34.9 | 32.5 | 30.1 | 27.6 |
| No insurance (%) | 6.7 | 7.9 | 8.9 | 11.2 | 13.3 | 14.8 | 14.3 | 16.3 | 19.1 |
| Race/ethnicity-specific zip code income ($1000) | 43.9±15.3 | 46.5±15.4 | 45.1±14.1 | 30.6±15.2 | 30.6±13.8 | 28.8±11.8 | 35.2±13.5 | 34.0±12.0 | 33.3±10.9 |
| Current smoker (%) | 7.1 | 6.6 | 7.6 | 6.6 | 6.8 | 8.7 | 3.0 | 2.5 | 2.9 |
| Cause of ESRD (%) | |||||||||
| Diabetes | 44.3 | 43.8 | 43.7 | 46.0 | 45.0 | 43.4 | 62.4 | 63.1 | 64.6 |
| Hypertension | 28.3 | 28.3 | 27.1 | 36.9 | 36.9 | 39.2 | 19.1 | 19.7 | 20.2 |
| GN | 6.6 | 7.1 | 7.7 | 5.0 | 5.6 | 5.6 | 6.4 | 5.7 | 5.1 |
| Other | 16.5 | 16.6 | 17.4 | 9.4 | 9.9 | 9.5 | 8.1 | 8.2 | 7.0 |
| Unknown | 4.3 | 4.1 | 4.1 | 2.7 | 2.6 | 2.3 | 4.1 | 3.3 | 3.2 |
| Comorbidity | |||||||||
| Hypertension (%) | 82.3 | 82.6 | 83.8 | 88.0 | 88.7 | 89.7 | 84.1 | 85.5 | 85.7 |
| Diabetes (%) | 52.4 | 51.3 | 52.7 | 54.5 | 53.5 | 54.2 | 64.8 | 65.8 | 66.5 |
| Cardiac failure (%) | 36.4 | 36.2 | 35.9 | 30.6 | 29.6 | 30.2 | 27.7 | 28.0 | 26.2 |
| Atherosclerotic heart disease (%) | 29.1 | 28.5 | 30.3 | 15.5 | 14.1 | 14.0 | 16.8 | 16.8 | 15.5 |
| Cerebrovascular disease (%) | 10.1 | 9.9 | 10.7 | 10.5 | 9.4 | 10.1 | 6.8 | 7.0 | 6.4 |
| Peripheral vascular disease (%) | 17.1 | 17.1 | 17.3 | 10.6 | 9.5 | 9.3 | 11.1 | 11.7 | 9.7 |
| Chronic obstructive pulmonary disease (%) | 12.7 | 11.9 | 12.0 | 5.7 | 5.4 | 5.7 | 4.2 | 3.2 | 2.8 |
| Pre-ESRD care | |||||||||
| Use of ESA before ESRD (%) | 30.0 | 32.7 | 33.8 | 23.8 | 25.1 | 26.4 | 22.9 | 24.1 | 22.5 |
| Receipt of pre-ESRD nephrologist care (%)a | 61.3 | 62.7 | 64.2 | 52.9 | 53.6 | 54.7 | 48.6 | 49.1 | 46.7 |
| AVF for first dialysis (%)a | 15.8 | 16.9 | 17.8 | 12.0 | 12.9 | 13.4 | 12.7 | 12.2 | 11.2 |
BMI, body mass index; eGFR, estimated GFR; ESA, erythropoiesis-stimulating agent; AVF, arteriovenous fistula.
For patients who completed the new CMS Medical Evidence form since 2005.
Unadjusted Mortality Rates by Facility Size Category
Overall, 19.8% of patients died within 1 year of follow-up (after the first 90 days of ESRD onset), and 43.0% of patients died within 5 years (after the first 90 days of ESRD onset; median follow-up=19.6 months), corresponding to 23.9 and 21.6 deaths per 100 patient-years, respectively. By race/ethnicity, deaths per 100 patient-years over 1-year follow-up times were 30.7 in whites, 17.8 in blacks, and 15.4 in Hispanics. The rates over 5-year follow-up times were 28.7 in whites, 16.0 in blacks, and 14.4 in Hispanics. Other events during 1-year follow-up times, including transplant or transfer out of the facility, were not substantially different across 10 facility size categories (Supplemental Table 3).
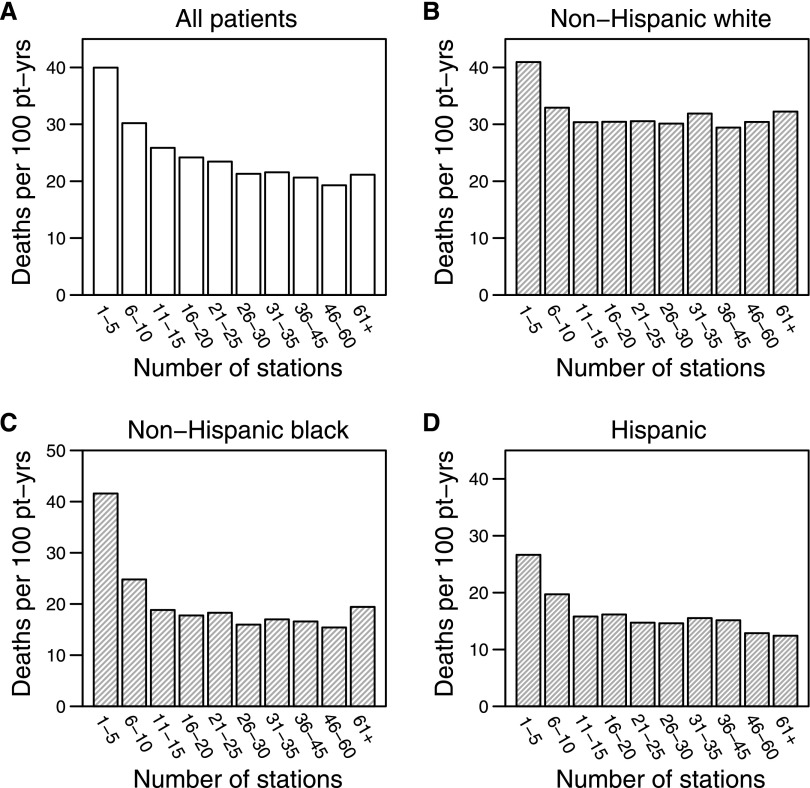
For 1-year mortality in all patients, facilities with 15 or fewer stations had significantly higher mortality rates, and this result was exacerbated as the number of stations decreased (Figure 1A). The higher mortality rates were also seen among facilities in the categories of 16–20 and 21–25 stations compared with those facilities with 26–30 stations. In the five smallest facility size categories (1–5, 6–10, 11–15, 16–20, and 21–25 stations), the crude 1-year mortality rates were 87%, 41%, 21%, 13%, and 10% higher, respectively, than the crude 1-year mortality rates in the reference category of 26–30 stations (all P<0.001) (Table 3). The strength of the relationship for 5-year mortality decreased but remained strongly significant (data not shown). There did not seem to be additional survival benefit as the number of stations increased beyond 30 stations.
Figure 1.
Unadjusted 1-year mortality rates after the first 90 days of ESRD onset by facility size category. Pt-yrs, patient-years.
Table 3.
Unadjusted and adjusted HRs of 1-year mortality for all patients
| Number of Stations | Proportion of Death (Deaths/Total) | Death Rate (n per 100 patient-yr) | Unadjusted Results | Adjusted Results: Model 1a | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P (versus Reference) | HR (95% CI) | P (versus Reference) | |||
| 1–5 | 30.4 (279/918) | 40.0 | 1.87 (1.45 to 2.40) | <0.001 | 1.26 (1.03 to 1.54) | 0.02 |
| 6–10 | 24.1 (4647/19,259) | 30.2 | 1.41 (1.32 to 1.51) | <0.001 | 1.13 (1.07 to 1.19) | <0.001 |
| 11–15 | 21.2 (14,963/70,640) | 25.9 | 1.21 (1.16 to 1.26) | <0.001 | 1.04 (1.00 to 1.08) | 0.03 |
| 16–20 | 20.0 (23,585/118,042) | 24.2 | 1.13 (1.09 to 1.18) | <0.001 | 1.04 (1.00 to 1.07) | 0.05 |
| 21–25 | 19.5 (17,344/89,149) | 23.4 | 1.10 (1.05 to 1.15) | <0.001 | 1.05 (1.01 to 1.09) | 0.01 |
| 26–30 | 17.9 (7385/41,145) | 21.3 | 1 (reference) | 1 (reference) | ||
| 31–35 | 18.2 (3602/19,826) | 21.6 | 1.01 (0.95 to 1.08) | 0.72 | 1.08 (1.03 to 1.14) | 0.002 |
| 36–45 | 17.4 (3125/17,911) | 20.6 | 0.97 (0.90 to 1.04) | 0.41 | 1.03 (0.97 to 1.09) | 0.34 |
| 46–60 | 16.5 (1118/6772) | 19.3 | 0.91 (0.81 to 1.01) | 0.09 | 0.98 (0.89 to 1.09) | 0.72 |
| ≥61 | 17.8 (251/1412) | 21.1 | 0.99 (0.86 to 1.14) | 0.92 | 1.16 (0.99 to 1.36) | 0.08 |
| Number of Stations | Adjusted Results: Model 2b | Adjusted Results: Model 3c | Adjusted Results: Model 4d | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P (versus Reference) | HR (95% CI) | P (versus Reference) | HR (95% CI) | P (versus Reference) | |
| 1–5 | 1.27 (1.03 to 1.56) | 0.02 | 1.31 (1.08 to 1.60) | 0.007 | 1.33 (1.09 to 1.63) | 0.005 |
| 6–10 | 1.12 (1.06 to 1.18) | <0.001 | 1.16 (1.09 to 1.22) | <0.001 | 1.17 (1.11 to 1.23) | <0.001 |
| 11–15 | 1.04 (1.00 to 1.08) | 0.03 | 1.05 (1.01 to 1.09) | 0.008 | 1.06 (1.02 to 1.10) | 0.002 |
| 16–20 | 1.04 (1.00 to 1.07) | 0.03 | 1.03 (1.00 to 1.07) | 0.05 | 1.04 (1.01 to 1.08) | 0.02 |
| 21–25 | 1.05 (1.01 to 1.09) | 0.006 | 1.05 (1.01 to 1.09) | 0.01 | 1.05 (1.01 to 1.09) | 0.006 |
| 26–30 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 31–35 | 1.08 (1.02 to 1.13) | 0.003 | 1.08 (1.03 to 1.13) | 0.003 | 1.07 (1.02 to 1.13) | 0.007 |
| 36–45 | 1.02 (0.97 to 1.08) | 0.39 | 1.02 (0.97 to 1.08) | 0.41 | 1.02 (0.97 to 1.08) | 0.42 |
| 46–60 | 0.98 (0.89 to 1.09) | 0.75 | 0.98 (0.89 to 1.08) | 0.67 | 0.97 (0.88 to 1.07) | 0.50 |
| ≥61 | 1.13 (0.98 to 1.32) | 0.10 | 1.14 (0.97 to 1.35) | 0.11 | 1.11 (0.92 to 1.34) | 0.29 |
Adjusted for patient demographic and clinical factors, including race/ethnicity, age, sex, body mass index, estimated GFR, cause of ESRD (diabetes, hypertension, GN, other, and unknown), presence of comorbid conditions (hypertension, diabetes, cardiac failure, atherosclerotic heart disease, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and cancer), lifestyle behaviors (smoking, alcohol, and drug dependence), immobility, and pre-ESRD use of erythropoiesis-stimulating agents (model 1).
Additionally adjusted for patient zip code race/ethnicity-specific income, health insurance, and employment (model 2).
Additionally adjusted for facility structural and geographic region, including for-profit status, chain ownership (Dialysis Clinic, Inc., DaVita, Fresenius, other chains, and no chain affiliation), urban/rural location, and US Census regions (Northeast, South, Midwest, and West; model 3).
Additionally adjusted for facility zip code SES, including median household income, percentage of persons who completed high school, percentage of persons in poverty, and percentage of black population (model 4).
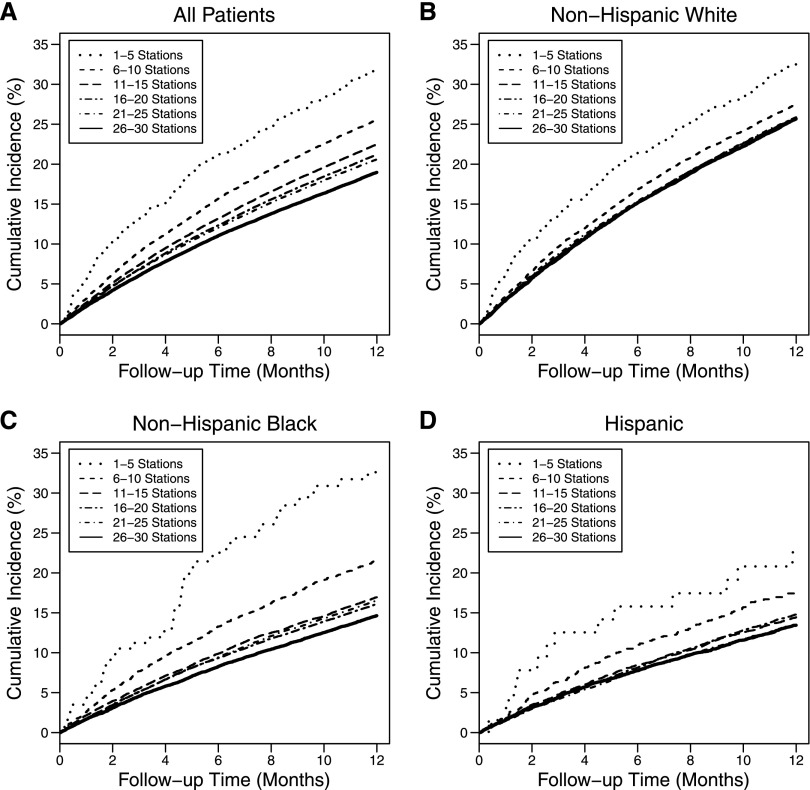
The increasing unadjusted mortality and decreasing facility size below 15 stations was strongest for blacks followed by Hispanics and whites (Figure 1 and Table 4). Figure 2 shows cumulative mortality over 1 year of follow-up, indicating that the higher mortality in smaller facilities, relative to the medium-sized facilities (26–30 stations), persisted throughout the entire 1-year follow-up time. Also, notably, the higher mortality in ≤10 stations was more significant in racial minorities, especially blacks, than whites.
Table 4.
Unadjusted and adjusted HRs of 1-year mortality for each race/ethnicity group
| Number of Stations | White | Black | Hispanic | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P (versus Reference) | HR (95% CI) | P (versus Reference) | HR (95% CI) | P (versus Reference) | |
| Unadjusted results | ||||||
| 1–5 | 1.36 (1.02 to 1.81) | 0.04 | 2.58 (1.96 to 3.39) | <0.001 | 1.82 (1.13 to 2.94) | 0.02 |
| 6–10 | 1.09 (1.03 to 1.16) | 0.005 | 1.55 (1.36 to 1.76) | <0.001 | 1.35 (1.09 to 1.66) | 0.006 |
| 11–15 | 1.01 (0.97 to 1.05) | 0.70 | 1.18 (1.09 to 1.27) | <0.001 | 1.08 (0.95 to 1.23) | 0.24 |
| 16–20 | 1.01 (0.97 to 1.05) | 0.62 | 1.11 (1.05 to 1.18) | <0.001 | 1.10 (1.00 to 1.22) | 0.05 |
| 21–25 | 1.01 (0.97 to 1.06) | 0.53 | 1.14 (1.08 to 1.22) | <0.001 | 1.01 (0.91 to 1.11) | 0.90 |
| 26–30 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 31–35 | 1.06 (0.99 to 1.13) | 0.09 | 1.06 (0.99 to 1.15) | 0.11 | 1.06 (0.89 to 1.27) | 0.51 |
| 36–45 | 0.98 (0.90 to 1.06) | 0.58 | 1.04 (0.96 to 1.13) | 0.37 | 1.04 (0.90 to 1.20) | 0.64 |
| 46–60 | 1.01 (0.90 to 1.14) | 0.88 | 0.97 (0.86 to 1.09) | 0.57 | 0.88 (0.64 to 1.22) | 0.44 |
| ≥61 | 1.07 (0.96 to 1.18) | 0.21 | 1.22 (1.03 to 1.44) | 0.02 | 0.85 (0.69 to 1.05) | 0.12 |
| Adjusted resultsa | ||||||
| 1–5 | 1.26 (1.00 to 1.57) | 0.05 | 1.78 (1.35 to 2.33) | <0.001 | 1.14 (0.67 to 1.95) | 0.62 |
| 6–10 | 1.10 (1.03 to 1.17) | 0.003 | 1.38 (1.26 to 1.51) | <0.001 | 1.28 (1.07 to 1.53) | 0.008 |
| 11–15 | 1.03 (0.98 to 1.07) | 0.21 | 1.13 (1.05 to 1.21) | <0.001 | 1.07 (0.96 to 1.20) | 0.23 |
| 16–20 | 1.01 (0.97 to 1.05) | 0.61 | 1.09 (1.03 to 1.15) | 0.003 | 1.06 (0.97 to 1.15) | 0.19 |
| 21–25 | 1.02 (0.98 to 1.07) | 0.28 | 1.12 (1.05 to 1.18) | <0.001 | 0.99 (0.90 to 1.08) | 0.80 |
| 26–30 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 31–35 | 1.09 (1.02 to 1.16) | 0.01 | 1.07 (0.99 to 1.15) | 0.08 | 1.12 (0.96 to 1.30) | 0.15 |
| 36–45 | 0.99 (0.92 to 1.06) | 0.67 | 1.07 (0.99 to 1.16) | 0.09 | 1.08 (0.96 to 1.21) | 0.20 |
| 46–60 | 1.01 (0.89 to 1.14) | 0.88 | 0.97 (0.86 to 1.10) | 0.68 | 0.93 (0.75 to 1.16) | 0.53 |
| ≥61 | 1.18 (0.93 to 1.49) | 0.16 | 1.17 (0.99 to 1.38) | 0.07 | 0.96 (0.68 to 1.35) | 0.80 |
From the fully adjusted model (model 4) for each racial/ethnic group (additionally adjusted for facility zip code SES, including median household income, percentage of persons who completed high school, percentage of persons in poverty, and percentage of black population).
Figure 2.
Cumulative incidence of mortality over 1 year of follow-up after the first 90 days of ESRD onset by facilities with 1–5, 6–10, 11–15, 16–20, 21–25, and 26–30 stations.
Adjusted Results
The strong association of facility size and mortality below 15 stations was attenuated but still significant after adjustment for demographic factors, including race and ethnicity, and clinical factors as well as after sequential adjustments for patient SES, facility characteristics and geographic region, and facility zip code SES (Table 3). In the fully adjusted model for 1-year mortality (model 4), patients in facilities with 1–5, 6–10, and 11–15 stations had a 33% (hazard ratio [HR], 1.33; 95% confidence interval [95% CI], 1.09 to 1.63), 17% (HR, 1.17; 95% CI, 1.11 to 1.23), and 6% (HR, 1.06; 95% CI, 1.02 to 1.10) higher risk of death, respectively, than those patients treated in facilities with 26–30 stations. For facilities with ≥31 stations, only those facilities with 31–35 stations had significantly higher adjusted 1-year mortality (HR, 1.07; 95% CI, 1.02 to 1.13) than those facilities with 26–30 stations.
By racial/ethnic groups, after the adjustments, the higher mortality risks at the small facilities persisted but were attenuated and more robust among black patients (Table 4). For 1-year mortality, blacks treated in facilities with 1–5 stations had an adjusted HR of 1.78 (95% CI, 1.35 to 2.33) compared with their counterparts in facilities with 26–30 stations, whereas whites had an HR of 1.26 (95% CI, 1.00 to 1.57). There was a trend to higher 1-year mortality rates in the largest size category (≥61 stations) for blacks (HR, 1.17; 95% CI, 0.99 to 1.38) and whites (HR, 1.18; 95% CI, 0.93 to 1.49) but not Hispanics. Additional adjustment for pre-ESRD nephrologist care and vascular access type in the first outpatient dialysis for patients who completed the 2005 form did not alter the main findings. The sensitivity analysis that censored the time when the patients were first transferred out yielded similar results (Supplemental Table 4).
In the fully adjusted model for 1-year mortality, among patients with the same comorbid conditions with respect to either presence or absence of diabetes or cardiovascular diseases, blacks (HR ranging from 1.28 to 1.48; P<0.001) and whites (HR ranging from 1.09 to 1.12; P<0.02) always had a greater risk of death at smaller facilities (≤10 stations) than their counterparts in facilities with 26–30 stations. Among Hispanics, there was an increased mortality rate at smaller facilities only in the presence of diabetes (HR, 1.35; P=0.005) or cardiovascular diseases (HR, 1.39; P=0.003), but there were no significant differences in their absence.
Discussion
Capacity, as a measure for facility size, is an important component of structural characteristics that affects the processes of care and outcomes.15–17 Prior studies have mostly focused on other structural characteristics, such as profit status or chain ownership,4–14 whereas the relationship between facility size and outcome has received limited attention. The studies that discussed this relationship used patient volume as a measure for facility size. Frankenfield et al.18 found little influence of the number of patients treated in the facility on intermediate outcomes, such as dialysis dose and anemia management, but did not assess mortality. Eisenstein et al.19 found a relationship between fewer facility patients and increasing mortality. The data between 1996 and 1999 used in the study by Eisenstein et al.19 might not reflect many of the recent changes in dialysis care and payment reform. For example, the number of dialysis facilities has almost doubled over the last 15 years (from 3095 facilities in 1996 to 5869 facilities in 2010).3 The present study, however, is the first to use the number of stations in the facility as a measure of facility size, which reflects the capacity of the facility structure. The present study is also the first to examine whether the relationship between facility size and patient mortality is modified by race/ethnicity. Our findings were derived after extensive adjustment for potential confounding by differences in patients, facility characteristics, and geographic region.
We found that, when the number of stations was 15 or smaller, there was a significant increase in mortality as the number of stations decreased (Figure 1). Furthermore, blacks and Hispanics had a greater increase in mortality than whites at small facilities, although they overall had lower absolute mortality rates than whites. These findings, although attenuated after multiple adjustments, remained significant in most instances. It should be noted that, during this study period, the differences in other types of events across the 10 facility size categories were comparable (Supplemental Table 3), suggesting that these mortality differences could not be attributed to reasons related to transplantation or transfer out of the facility. In hemodialysis, where there is practically universal coverage under the Medicare program, these large differences in mortality rates among facility sizes were not only unexpected but in fact, quite alarming.
Similar to numerous prior studies for surgical conditions that identified some thresholds,20 there seemed to be a threshold size of approximately 15 stations, where the association between facility size and mortality seemed to weaken from 16 to 26–30 stations. Above 30 stations, there did not seem to be a relationship of increasing size and reducing mortality. No prior study has documented an association between increasing volume and worse outcome.1 However, we found a trend to higher mortality rate in facilities with ≥61 stations for blacks and whites, albeit not statistically different after covariate adjustments (Table 4).
Most of the 1803 facilities with 1–15 stations were located in zip code areas with higher SES, a characteristic that has commonly been associated with better health outcomes. These facilities also tended to have higher nurse-to-patient ratios, which may have to do with the regulation that mandates the presence of a registered nurse on site. In addition, we found that the mortality rates were higher in Southern and Western US facilities, zip codes with a higher proportion of blacks, and urban facilities. However, after adjusting for patient case mix as well as facility and geographic factors, the higher mortality risk at the small facilities compared with the medium-sized category persisted and remained strongly significant, indicating that facility size is independently associated with outcome. These results show that facility size is a strong indicator for facility quality care, which should deserve great attention.
The reasons for the higher mortality risk in smaller dialysis facilities with ≤15 stations are unclear. Our results were markedly attenuated after the multiple adjustments as shown in Tables 3 and 4, reflecting that this finding was partially attributable to patient case mix, other facility characteristics, and geographic region. Importantly, however, the increased mortality risk below 15 stations persisted after the adjustments, suggesting the existence of contributors that might be unmeasured or unexamined. One possibility is the potential financial constraints faced by the small facilities because of the lack of economy of scale, thus limiting the opportunities for advancing expertise, developing rigorous clinical care protocols, and implementing measures for quality improvement. Consequently, small facilities may lack experience to care for diabetic and cardiovascular patients and racial/ethnic minorities. Although hospital-based dialysis units were excluded from the present analysis, there might still be some residual confounding, such as severity of key clinical and disease conditions, leading to higher mortality risk in small facilities. Here, we have identified an important gap in health outcomes, which should be amenable to improvement, but it will require the collection of more information about structure, processes of care, and practice differences among facilities with different sizes.
There are limitations to our current findings. First, our main analyses linked the baseline facility to outcome, regardless of whether patients were transferred to other facilities later. To assess the robustness of these main findings, we performed sensitivity analysis that censored for the time when the patients were first transferred and obtained similar results. Second, our multivariate models did not adjust for some other aspects of care, such as dialysis dose or dialyzer reuse, that might be associated with outcomes. There might have been residual confounding from other factors not captured, such as severity of specific illnesses (only a yes/no ascertainment of comorbid conditions in the US Renal Data System [USRDS]) or others. Third, because information on individual patient income was not available, we used zip code race/ethnicity-specific income that might have introduced bias. The validity of the lifestyle variables, such as smoking, alcohol, and drug dependence, is limited because of the data being self-reported without laboratory verification. Despite multiple adjustments, we cannot exclude the influence of potential changes in baseline comorbid conditions, vascular access, and other variables over time on facility differences in the mortality rates. Fourth, transferring between units might have limited the robustness of 5-year mortality as attributed to dialysis unit size.
In summary, we show the robust finding that maintenance hemodialysis at small facilities is associated with a significant increase in mortality, which is only partially explained by the measured patient case mix, other well defined facility characteristics, and geographic region. Black and Hispanic patients as well as patients with diabetes and cardiovascular diseases in small facilities were particularly susceptible to death compared with patients with similar demographic and clinical characteristics in larger facilities. Future work to identify the reasons for these differences in mortality rates among facilities of various sizes and especially, these racial/ethnic- and disease-specific subgroups may help inform focused strategies to improve clinical outcomes.
Concise Methods
Data Sources
We used the USRDS.21 The patient-level data, such as ESRD onset date, demographics, and clinical characteristics, were obtained from the Centers for Medicare and Medicaid Services (CMS) Medical Evidence Report (CMS-2728) in the USRDS. The facility-level data, including the number of hemodialysis stations, chain affiliation, and facility zip code, were from the annual CMS Facility Survey (CMS-2744) in the USRDS. In addition, we obtained zip code socioeconomic data from the 2000 US Census Zip Code Tabulation Areas Files.22
Study Population
We identified all adult patients (≥18 years) beginning the first maintenance dialysis between October 1, 2003 and December 31, 2009 who had not previously received kidney transplantation, resided in the 50 states or the District of Columbia, and were white, black, or Hispanic. We did not include Asians and Native Americans, because there were very few patients in those racial/ethnic groups in smaller facilities. Using the USRDS 90-day rule for stable modality, we only included those patients who survived the first 90 days since ESRD onset and received in-center hemodialysis at day 91 for at least 60 days. Of 481,561 eligible patients, we excluded 16,784 (3.5%) patients with missing CMS Facility Survey information or facility zip codes that could not be linked to a zip code in the 2000 US Census data. We excluded another 52,750 (10.9%) patients treated in hospital-based facilities to achieve a more homogeneous study population, because hospital-based patients have a much higher mortality risk than those patients in freestanding facilities.23 Of the remaining 412,027 patients, 6.5% of patients were excluded because of missing patient covariates. The final cohort included 385,074 patients (52.7% white, 32.7% black, and 14.6% Hispanic) at 4633 dialysis facilities. This project was approved by the Institutional Review Board at the University of Virginia.
Study Variables
The primary outcome of interest was 1-year survival from day 91 of the dialysis initiation, with censoring at transplant, dialysis modality switch, or administrative end of study (August 31, 2010). The primary predictor was facility size, defined as the number of hemodialysis stations in the facility where the patient received dialysis at day 91 from his/her dialysis initiation, regardless of whether the patient was subsequently transferred to other facilities during the study period. However, we performed sensitivity analysis that censored for the follow-up time when the patient was first transferred out. We also examined survival at 5 years from day 91 of the dialysis initiation.
Patient race and ethnicity (non-Hispanic African American/black, non-Hispanic Caucasian/white, and Hispanic) were classified in the CMS Medical Evidence form. The following covariates at the onset of ESRD were sequentially added to the statistical models: (1) demographic and clinical factors, including age, race/ethnicity (in the analysis for overall population), sex, body mass index, estimated GFR, cause of ESRD, comorbid conditions (hypertension, diabetes, all cardiac diseases, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and cancer), lifestyle behaviors (smoking, alcohol, and drug dependence), and immobility as well as pre-ESRD use of erythropoiesis-stimulating agents; (2) patient SES, including employment status at 6 months before ESRD onset, health insurance (private, Medicare, or Medicaid/none), and income (income was substituted by race/ethnicity-specific median household income within the residential zip code, because it was not available in the USRDS); (3) facility structural and geographic characteristics, including for-profit status, chain ownership, urban/rural location, and four US Census regions (urban/rural location was determined according to facility zip code using Rural–Urban Commuting Area Codes24,25); and (4) facility zip code SES, including median household income, percentage of persons who completed high school, percentage of persons in poverty, and percentage of black population. Additionally, we included two pre-ESRD care indicators (nephrologist care and vascular access type used for the first outpatient dialysis) in patients who have these data available since 2005.
Statistical Analyses
We first used nonparametric smoothing techniques implemented in Cox regression to model the relationship of facility size as a continuous predictor and mortality.26 The results revealed an exponential increase in mortality risk as facility size decreased. Therefore, we grouped facility size into 10 categories (1–5, 6–10, 11–15, 16–20, 21–25, 26–30, 31–35, 36–45, 46–60, and ≥61 stations). The association between facility size category and survival was examined in Cox regression, with the middle group (26–30 stations) as the reference. CIs and P values were computed using empirical (sandwich) standard errors that incorporated intrafacility correlations.27 We performed unadjusted analysis and analyses sequentially adjusted for the four sets of variables for overall population and each racial/ethnic group. To further investigate whether the relationships differed by comorbid conditions, we repeated similar analyses for several subgroups according to the presence/absence of diabetes or cardiovascular diseases. A number of sensitivity analyses were performed, including one analysis with survival time censored at first transfer and one analysis additionally adjusted for two pre-ESRD care indicators.
Disclosures
None.
Acknowledgments
The authors thank the staff at the US Renal Data System for their assistance in providing the US Renal Data System data.
This work is funded by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant 5R01DK084200-02. In addition, K.C.N. is supported in part by National Institutes of Health Grants U54MD007598, UL1TR000124, P30AG021684, and P20-MD000182.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013010033/-/DCSupplemental.
References
- 1.Halm EA, Lee C, Chassin MR: Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med 137: 511–520, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Fareed N: Size matters: A meta-analysis on the impact of hospital size on patient mortality. Int J Evid-Based Healthc 10: 103–111, 2012 [DOI] [PubMed] [Google Scholar]
- 3.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 4.McClellan WM, Soucie JM, Flanders WD: Mortality in end-stage renal disease is associated with facility-to-facility differences in adequacy of hemodialysis. J Am Soc Nephrol 9: 1940–1947, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Fink JC, Zhan M, Blahut SA, Soucie M, McClellan WM: Measuring the efficacy of a quality improvement program in dialysis adequacy with changes in center effects. J Am Soc Nephrol 13: 2338–2344, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Fink JC, Blahut SA, Briglia AE, Gardner JF, Light PD: Effect of center- versus patient-specific factors on variations in dialysis adequacy. J Am Soc Nephrol 12: 164–169, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Thamer M, Zhang Y, Kaufman J, Cotter D, Dong F, Hernán MA: Dialysis facility ownership and epoetin dosing in patients receiving hemodialysis. JAMA 297: 1667–1674, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Collins AJ, Ebben JP, Gilbertson DT: EPO adjustments in patients with elevated hemoglobin levels: Provider practice patterns compared with recommended practice guidelines. Am J Kidney Dis 49: 135–142, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Cotter DJ, Thamer M: The effect of dialysis chains on mortality among patients receiving hemodialysis. Health Serv Res 46: 747–767, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DK, Chertow GM, Zenios SA: Reexploring differences among for-profit and nonprofit dialysis providers. Health Serv Res 45: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehrotra R, Khawar O, Duong U, Fried L, Norris K, Nissenson A, Kalantar-Zadeh K: Ownership patterns of dialysis units and peritoneal dialysis in the United States: Utilization and outcomes. Am J Kidney Dis 54: 289–298, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Van Wyck D, Robertson J, Nissenson A, Provenzano R, Kogod D: Relationship among length of facility ownership, clinical performance, and mortality. Clin J Am Soc Nephrol 5: 248–251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutner N, Bowles T, Zhang R, Huang Y, Pastan S: Dialysis facility characteristics and variation in employment rates: A national study. Clin J Am Soc Nephrol 3: 111–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szczech LA, Klassen PS, Chua B, Hedayati SS, Flanigan M, McClellan WM, Reddan DN, Rettig RA, Frankenfield DL, Owen WF, Jr.: Associations between CMS’s Clinical Performance Measures project benchmarks, profit structure, and mortality in dialysis units. Kidney Int 69: 2094–2100, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Donabedian A: The quality of care. How can it be assessed? JAMA 260: 1743–1748, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Donaldson MS: ebrary Inc. Measuring the Quality of Health Care: A Statement by the National Roundtable on Health Care Quality, Division of Health Care Services, Institute of Medicine, 1999. Available at: http://site.ebrary.com/lib/yale/Doc?id=10057002 Accessed December 2, 2012 [PubMed]
- 17.Spertus JA, Radford MJ, Every NR, Ellerbeck EF, Peterson ED, Krumholz HM, Acute Myocardial Infarction Working Group of the American Heart Association. American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke : Challenges and opportunities in quantifying the quality of care for acute myocardial infarction: Summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation 107: 1681–1691, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Frankenfield DL, Sugarman JR, Presley RJ, Helgerson SD, Rocco MV: Impact of facility size and profit status on intermediate outcomes in chronic dialysis patients. Am J Kidney Dis 36: 318–326, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Eisenstein EL, Sun JL, Anstrom KJ, Stafford JA, Szczech LA, Muhlbaier LH, Mark DB: Re-evaluating the volume-outcome relationship in hemodialysis patients. Health Policy 88: 317–325, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ross JS, Normand SL, Wang Y, Ko DT, Chen J, Drye EE, Keenan PS, Lichtman JH, Bueno H, Schreiner GC, Krumholz HM: Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med 362: 1110–1118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 22.US Department of Commerce; US Census Bureau: ZIP Code Tabulation Areas (ZCTA), 2012. Available at: http://www.census.gov/ Accessed March 1, 2012
- 23.Plough AL, Salem SR, Shwartz M, Weller JM, Ferguson CW: Case mix in end-stage renal disease. Differences between patients in hospital-based and free-standing treatment facilities. N Engl J Med 310: 1432–1436, 1984 [DOI] [PubMed] [Google Scholar]
- 24.USDA Economic Research Service: Rural-Urban Commuting Area Codes, 2012. Available at: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx Accessed March 1, 2012
- 25.University of Washington: RUCA Rural Health Research Center, 2012. Available at: http://depts.washington.edu/uwruca/ruca-maps.php Accessed February 1, 2012
- 26.Therneau TM, Grambsch PM: Modeling Survival Data: Extending the Cox Model, New York, Springer, 2000 [Google Scholar]
- 27.Lin DY, Wei LJ: The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84: 1074–1078, 1989 [Google Scholar]