Abstract
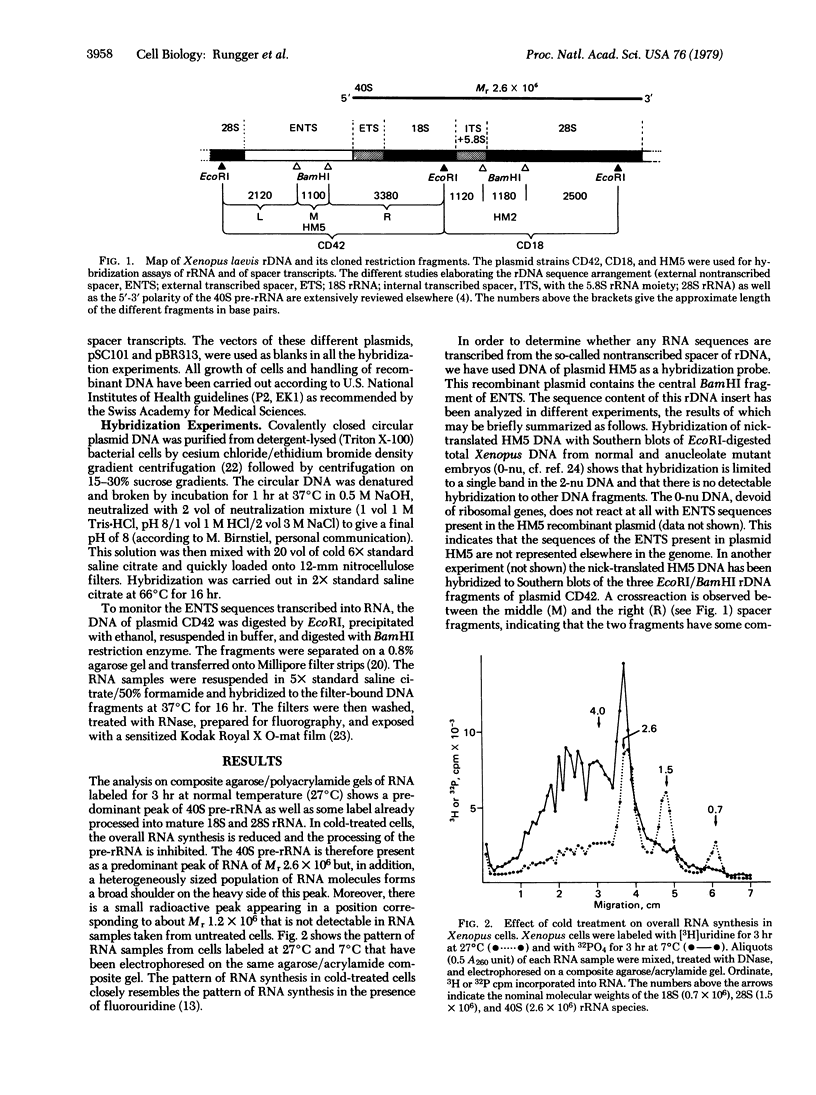
Untreated Xenopus cells synthesize RNA is increased and some sequences complementary to those of the nontranscribed spacer are found in the heavy shoulder of the 40S precursor rRNA peak. In such events transcription initiation seems to take place within the spacer because its middle and right BamHI endonuclease fragments are preferentially transcribed, whereas only few RNA sequences complementary to the left spacer fragment are found. It is concluded that at least some spacer regions contain promoters for transcription and can be transcribed either into a special class of "spacer transcripts" or into molecules covalently linked to rRNA precursor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A., Rogers E., Birnstiel M. Is gene amplification RNA-directed? Nat New Biol. 1973 Apr 25;242(121):226–230. doi: 10.1038/newbio242226a0. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Chipchase M., Speirs J. The ribosomal RNA cistrons. Prog Nucleic Acid Res Mol Biol. 1971;11:351–389. doi: 10.1016/s0079-6603(08)60332-3. [DOI] [PubMed] [Google Scholar]
- Boseley P., Moss T., Mächler M., Portmann R., Birnstiel M. Sequence organization of the spacer DNA in a ribosomal gene unit of Xenopus laevis. Cell. 1979 May;17(1):19–31. doi: 10.1016/0092-8674(79)90291-5. [DOI] [PubMed] [Google Scholar]
- Caston J. D., Jones P. H. Synthesis and processing of high molecular weight RNA by nuclei isolated from embryos of Rana pipiens. J Mol Biol. 1972 Aug 14;69(1):19–38. doi: 10.1016/0022-2836(72)90021-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSDALE T. R., FISCHBERG M., SMITH S. A mutation that reduces nucleolar number in Xenopus laevis. Exp Cell Res. 1958 Jun;14(3):642–643. doi: 10.1016/0014-4827(58)90175-7. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Spring H., Trendelenburg M. F., Krohne G. Morphology of transcriptional units of rDNA. Evidence for transcription in apparent spacer intercepts and cleavages in the elongating nascent RNA. Exp Cell Res. 1976 Jul;100(2):233–244. doi: 10.1016/0014-4827(76)90143-9. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A., Nikolaev N. Maturation of ribosomal ribonucleic acids and the biogenesis of ribosomes. Prog Biophys Mol Biol. 1976;31(2):95–144. doi: 10.1016/0079-6107(78)90006-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder R. H., Sollner-Webb B., Wahn H. L. Sites of transcription initiation in vivo on Xenopus laevis ribosomal DNA. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5402–5406. doi: 10.1073/pnas.74.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger D., Crippa M. In vivo transcription of the spacer sequences of rDNA in Xenopus. Exp Cell Res. 1977 Jun;107(1):227–237. doi: 10.1016/0014-4827(77)90404-9. [DOI] [PubMed] [Google Scholar]
- Rungger D., Crippa M. The primary ribosomal DNA transcript in eukaryotes. Prog Biophys Mol Biol. 1977;31(3):247–269. doi: 10.1016/0079-6107(78)90010-x. [DOI] [PubMed] [Google Scholar]
- Rungger D., Crippa M., Trendelenburg M. F., Scheer U., Franke W. W. Visualization of rDNA spacer transcription in Xenopus oocytes treated with fluorouridine. Exp Cell Res. 1978 Oct 15;116(2):481–486. doi: 10.1016/0014-4827(78)90476-7. [DOI] [PubMed] [Google Scholar]
- Scheer U., Trendelenburg M. F., Franke W. W. Transcription of ribosomal RNA cistrons. Correlation of morphological and biochemical data. Exp Cell Res. 1973 Jul;80(1):175–190. doi: 10.1016/0014-4827(73)90289-9. [DOI] [PubMed] [Google Scholar]
- Scheer U., Trendelenburg M. F., Krohne G., Franke W. W. Lengths and patterns of transcriptional units in the amplified nucleoli of oocytes of Xenopus laevis. Chromosoma. 1977 Mar 16;60(2):147–167. doi: 10.1007/BF00288462. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Loening U. E. 5'-Ends of ribosomal and ribosomal precursor RNAs form Xenopus laevis. Eur J Biochem. 1974 Mar 15;43(1):59–67. doi: 10.1111/j.1432-1033.1974.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Amos H. RNA metabolism in HeLa cells at reduced temperature. I. Modified processing of 45S RNA. J Cell Biol. 1971 Sep;50(3):818–829. doi: 10.1083/jcb.50.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Reeder R. H. A comparison of the structural organization of amplified ribosomal DNA from Xenopus mulleri and Xenopus laevis. J Mol Biol. 1975 May 15;94(2):151–161. doi: 10.1016/0022-2836(75)90074-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. S., Pitot H. C. Inhibition of ribosomal ribonucleic acid maturation in Novikoff hepatoma cells by 5-fluorouracil and 5-fluorouridine. J Biol Chem. 1973 Jan 10;248(1):63–68. [PubMed] [Google Scholar]
- Wilson M. C., Melli M., Birnstiel M. L. Reiteration frequency of histone coding sequences in man. Biochem Biophys Res Commun. 1974 Nov 27;61(2):404–409. doi: 10.1016/0006-291x(74)90971-1. [DOI] [PubMed] [Google Scholar]



