Abstract
Model systems demonstrate that progression to ESRD is driven by progressive podocyte depletion (the podocyte depletion hypothesis) and can be noninvasively monitored through measurement of urine pellet podocyte mRNAs. To test these concepts in humans, we analyzed urine pellet mRNAs from 358 adult and pediatric kidney clinic patients and 291 controls (n=1143 samples). Compared with controls, urine podocyte mRNAs increased 79-fold (P<0.001) in patients with biopsy-proven glomerular disease and a 50% decrease in kidney function or progression to ESRD. An independent cohort of patients with Alport syndrome had a 23-fold increase in urinary podocyte mRNAs (P<0.001 compared with controls). Urinary podocyte mRNAs increased during active disease but returned to baseline on disease remission. Furthermore, urine podocyte mRNAs increased in all categories of glomerular disease evaluated, but levels ranged from high to normal, consistent with individual patient variability in the risk for progression. In contrast, urine podocyte mRNAs did not increase in polycystic kidney disease. The association between proteinuria and podocyturia varied markedly by glomerular disease type: a high correlation in minimal-change disease and a low correlation in membranous nephropathy. These data support the podocyte depletion hypothesis as the mechanism driving progression in all human glomerular diseases, suggest that urine pellet podocyte mRNAs could be useful for monitoring risk for progression and response to treatment, and provide novel insights into glomerular disease pathophysiology.
Human glomerular diseases are heterogeneous but can collectively be viewed as a spectrum of podocytopathies.1 Podocyte depletion per se causes glomerulosclerosis in model systems2–5 and is associated with progressive glomerulosclerosis in humans.6–12 Podocyte damage causes damage to other podocytes13 and activation of the renin-angiotensin system (RAS), which, in turn, drives angiotensin II–dependent further podocyte detachment from destabilized glomeruli.14 In model systems glomerulosclerosis is initiated when >30% of podocytes have been lost from glomeruli.14 Quantitative podocyte depletion from glomeruli results in mesangial expansion (10%–20% depletion), adhesions to the Bowman capsule (30% depletion), FSGS (30%–50% depletion), and then global sclerosis (70%–90% depletion) associated with interstitial fibrosis.4,14 Model systems demonstrate that throughout the progression process podocytes continue to detach and appear in the urine, where they can be monitored noninvasively through quantitation of urine pellet mRNAs.14–17 Collectively, these findings describe key elements of the podocyte depletion hypothesis for progression of glomerular diseases, whereby progressive depletion of podocytes leads through a series of stages to glomerulosclerosis and ultimately to ESRD.
Although increased urine podocyte excretion has also been documented in some inflammatory and noninflammatory glomerular diseases in humans,12,18–35 the role of podocyte depletion in progression remains unproven. We therefore used urine pellet mRNAs to test the hypothesis that progression to ESRD in human glomerular diseases would also be associated with increased urine podocyte mRNAs, regardless of the underlying cause of glomerular injury. Because proteinuria is a well established marker of kidney disease progression36,37 but varies in extent and relationship to progression in different diseases, we also examined the relationship between proteinuria and rate of podocyte detachment. Establishing that human glomerular diseases follow the rules defined in model systems of progression would provide a foundation for understanding the progression mechanism in human glomerular diseases and the potential for urine podocyte mRNAs to be clinically useful.
Results
Characteristics of Patient and Control Groups
Table 1 shows demographics and clinical characteristics of patients with heterogeneous diseases encountered in pediatric and adult nephrology clinics. This includes newly diagnosed glomerular diseases as well as prevalent patients receiving treatments for their glomerular disease, or who were considered to be clinically stable and in remission that had occurred spontaneously or after treatment. Table 1 also shows that many patients were taking antihypertensive medications (including RAS inhibitors) and immunosuppressants.
Table 1.
Characteristics of the control and patient groups
| Category | Patients/Controls (n=649) | Samples (n=1143) | Age ± SD (Range) (yr) | Men (%) | eGFR ± SD (Range) | Median UPCR (IQR) | ACEI/ARB (%) | Immunosuppression, n (%) |
|---|---|---|---|---|---|---|---|---|
| Normal controls | 291 | 291 | 30.0±17.8 (3–89) | 123 (42) | NA | 0.05 (0.03–0.06) | 0 (0.0) | 0 (0.0) |
| Nephrotic syndrome complex | 91 | 288 | 16.9±15.2 (2–71) | 54 (58) | 111.2±43.2 (11–150) | 1.6 (0.1–6.1) | 47 (50.5) | 36 (38.7) |
| Pathologic diagnosis of MCD | 19 | 78 | 14.6±14.9 (3–54) | 9 (47) | 117±42 (40–150) | 0.9 (0.1–15.7) | 9 (47.4) | 14 (73.7) |
| Pathologic diagnosis of FSGS | 35 | 113 | 22.9±16.5 (3–71) | 21 (60) | 88±46 (11–150) | 2.4 (1.0–7.4) | 21 (60.0) | 6 (17.1) |
| Clinical diagnosis of SSNS | 16 | 34 | 10.8±6.3 (2–23) | 11 (69) | 145±11 (121–150) | 0.2 (0.1–4.5) | 2 (12.5) | 9 (56.3) |
| Clinical diagnosis of SDNS | 28 | 104 | 11.2±10.6 (2–51) | 13 (46) | 127±31 (40–150) | 2 (0.1–7.4) | 13 (46.4) | 18 (64.3) |
| Clinical diagnosis of SRNS | 26 | 94 | 16.7±13.1 (3–54) | 16 (62) | 103±46 (42–150) | 1.7 (0.1–6.9) | 18 (69.2) | 7 (26.9) |
| Other noninflammatory GNs | 114 | 226 | 52.8±44.0 (6–86) | 61 (57) | 44±35.7 (7–150) | 1.2 (0.4–3.0) | 84 (77.8) | 4 (3.7) |
| Diabetes-associated kidney disease | 62 | 123 | 56.9±20.2 (13–85) | 39 (63) | 32±18 (11–91) | 1.2 (0.3–3.1) | 52 (83.9) | 1 (1.6) |
| Hypertension-associated KD | 27 | 35 | 58.6±20.4 (14–86) | 16 (60) | 41±27 (7–136) | 0.4 (0.1–1.4) | 17 (63.0) | 0 (0.0) |
| Idiopathic membranous GN | 15 | 48 | 48.1±22.3 (12–79) | 8 (53) | 76±48 (20–150) | 2.2 (0.9–4.4) | 10 (66.7) | 4 (26.7) |
| Alport syndrome complex | 10 | 20 | 12.5±5.1 (6–23) | 4 (40) | 112±59 (16–150) | 1.9 (0.6–3.6) | 8 (80.0) | 0 (0.0) |
| Inflammatory GNs | 113 | 270 | 30.3±21.9 (5–82) | 47 (42) | 84.8±45.4 (8–150) | 0.7 (0.2–2.1) | 64 (57.7) | 37 (33.3) |
| Acute postinfectious GNs | 12 | 27 | 9.5±3.6 (6–19) | 8 (67) | 104±42 (28–150) | 1 (0.1–2.1) | 3 (25.0) | 0 (0.0) |
| HSP | 19 | 55 | 11.6±4.4 (5–19) | 12 (63) | 139±16 (100–150) | 0.3 (0.1–1.1) | 8 (42.1) | 5 (26.3) |
| IgA nephropathy | 20 | 48 | 34.9±22.4 (6–75) | 10 (50) | 71±47 (8–150) | 1.1 (0.3–2.0) | 14 (70.0) | 1 (5.0) |
| SLE nephropathies | 29 | 43 | 39.3±18.2 (14–82) | 4 (14) | 75±35 (27–150) | 0.8 (0.2–2.3) | 17 (58.6) | 17 (58.6) |
| MPGN (I, II, and III) | 14 | 44 | 25.1±18.1 (8–60) | 7 (50) | 100±48 (29–150) | 1.1 (0.3–2.5) | 11 (78.6) | 2 (14.3) |
| Pauci-immune GN | 19 | 53 | 45.2±24.6 (11–82) | 8 (42) | 56±37 (10–130) | 0.6 (0.3–2.0) | 12 (63.2) | 12 (63.2) |
| All progressors | 16 | 86 | 20.8±19.5 (2–76) | 9 (56) | 82±49 (11–150) | 4.9 (1.6–8.8) | 12 (75) | 8 (50.0) |
| eGFR progressors | 8 | 53 | 11.6±5.2 3–16 | 4 (50) | 102±41 (54–150) | 8.3 (4.9–22.9) | 4 (50.0) | 4 (50.0) |
| NS/FSGS progressors | 9 | 56 | 14.7±4.7 (4–21) | 4 (44) | 74±49 (11–144) | 6.7 (5.7–8.5) | 5 (55.6) | 6 (66.7) |
| Other GN progressors | 5 | 19 | 36.6±27.4 (15–73) | 3 (60) | 60±32 (18 92) | 2.6 (1.4–4.4) | 4 (80.0) | 4 (80.0) |
| Nonglomerular disease | ||||||||
| ADPKD | 10 | 15 | 45.6±8.8 (28–60) | 5 (50) | 56±34 (11–95) | 0.2 (0.1–0.8) | 8 (80.0) | 0 (0.0) |
| Uncategorized patients | 30 | 53 | 45.1±27.7 (5–86) | 12 (40) | 63±44 (5–150) | 0.5 (0.2–1.0) | 11 (36.7) | 5 (16.7) |
All progressors includes all patients with biopsy-proven GNs who halved their eGFR and/or progressed to ESRD requiring RRTs. eGFR progressors had biopsy-proven GNs who halved their eGFR during the observation period. NS/FSGS progressors are patients with nephrotic syndrome and FSGS who progressed to ESRD. Other GN progressors had non-FSGS biopsy-proven GNs who progressed to ESRD. Note the overlap of pathologic and clinical diagnoses in the nephrotic syndrome complex, where, for example, a patient with SRNS also has a biopsy diagnosis of FSGS. The progressor groups also contain individual patients from more than one group, as outlined in the Concise Methods. UPCR, urinary protein-to-creatinine ratio; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; NA, not applicable.
Table 2 shows demographic characteristics of 291 controls compared with the patient group demonstrating adequate numbers of patients within each subgroup. No significant differences in urine podocin corrected for urine creatinine (UPodCR) between age, sex, or race were observed within the control group (data not shown). However, urine nephrin mRNA decreased with age (P<0.01), consistent with rat glomerular aging.38,39 Therefore, all data shown are for podocin mRNA only.
Table 2.
Demographic characteristics of clinic and control populations
| Characteristics | Patients (n=358), n (%) | Controls (n=291) , n (%) |
|---|---|---|
| Age group | ||
| 0–10 yr | 62 (17.3) | 57 (19.6) |
| 11–18 yr | 98 (27.4) | 34 (11.7) |
| 19–39 yr | 51 (14.2) | 98 (33.7) |
| 40–59 yr | 57 (15.9) | 85 (29.2) |
| ≥ 60 yr | 90 (25.1) | 17 (5.8) |
| Men | 185 (51.7) | 125 (43.0) |
| Race | ||
| Asian | 13 (3.6) | 28 (9.6) |
| African-American | 54 (15.1) | 27 (9.3) |
| White | 268 (74.9) | 213 (73.2) |
| Other | 23 (3.4) | 23 (7.9) |
Correlations between Urine mRNA Markers
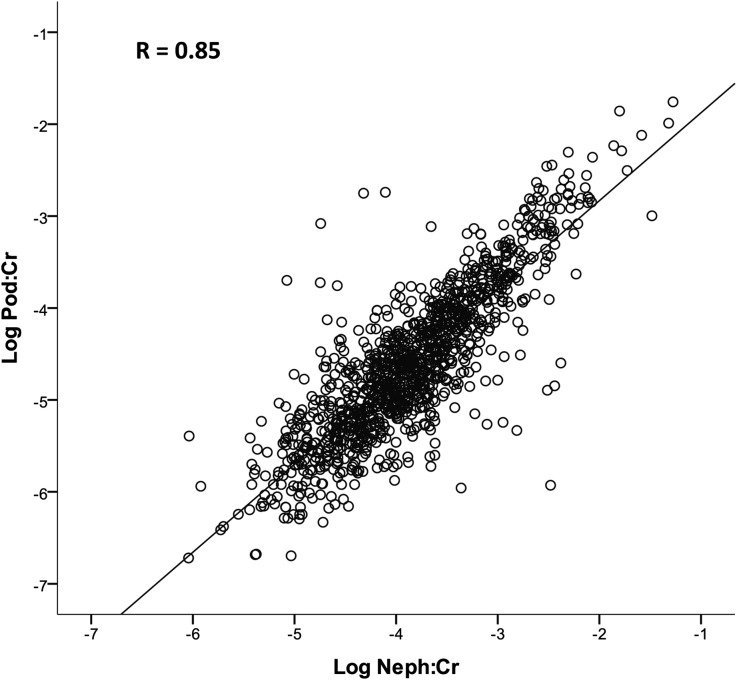
All data are expressed as moles of podocin cDNA per g creatinine (UPodCR) (see Concise Methods). Figure 1 shows correlations between two podocyte-specific mRNAs (podocin and nephrin) for all samples (n=1143). Podocin and nephrin mRNAs correlated closely with each other (r=0.85; P<0.001), as would be anticipated for products from the same cell.
Figure 1.
Two podocyte-specific mRNAs (podocin and nephrin) in urine samples correlate closely over 6 logs.
Contrasting Patient Populations with High and Low UPodCR
As a first step, we ordered the 358 clinic patients according to their averaged UPodCR values from all available urine samples from each patient. Table 3 compares the top and bottom 50 patients from this list. The 50 patients with highest UPodCR had active glomerular and kidney injury associated with both inflammatory and noninflammatory glomerulonephropathies, and 9 (18%) had progressed to ESRD requiring renal replacement therapies (RRTs) during the observation period. In contrast, those with a low UPodCR had stable disease or disease being maintained in an inactive state by treatments. None of the low-UPodCR cohort had progressed to ESRD requiring RRT during the observation period. Both groups had widely varying estimated GFR (eGFR) values, demonstrating that renal function did not strongly affect UPodCR. The high-UPodCR group tended to have higher urine protein-to-creatinine ratios (UProtCRs), although some patients with high UPodCR values had low UProtCR values and vice versa.
Table 3.
Characteristics of high and low UPodCR groups
| Characteristic | Highest UPodCR (n=50) | Lowest UPodCR (n=50) | P Value |
|---|---|---|---|
| Mean age ± SD (yr) | 19±18 | 32±22 | <0.01 |
| Age range (yr) | 2–80 | 7–78 | |
| Men (%) | 52 | 52 | NS |
| African American race (%) | 14 | 12 | NS |
| Mean urine UProtCR | 5.2±8.2 | 0.7±1.1 | <0.01 |
| UprotCR range | 0.4–25 | 0.04–5.2 | |
| Mean eGFR (ml/min per 1.73 m2) | 91±68 | 86±51 | NS |
| eGFR range at entry (ml/min per 1.73 m2) | 13–150 | 12–150 | |
| Renal biopsy diagnosis, n (%) | 37 (74) | 34 (68) | NS |
| Progressed to ESRD requiring RRT, n (%) | 9 (18) | 0 (0) | <0.01 |
| Angiotensin II blockade, n (%) | 38 (76) | 34 (68) | NS |
| Immunosuppression, n (%) | 29 (58) | 17 (34) | NS |
| NS and/or FSGS with UprotCR >5, n (%) | 9 (18) | 0 (0) | <0.01 |
| NS and/or FSGS with UprotCR <0.18, n (%) | 0 (0) | 11 (22) | <0.01 |
| Acute GN, n (%) | 20 (40) | 0 (0) | <0.01 |
| GNs in remission (PCR < 0.18) with or without treatment | 0 (0) | 13 (26) | <0.01 |
Clinic patients ordered according to whether their average measured UPodCR was high (in the top 50) or low (in the bottom 50). Total clinic n=358. Acute GN refers to any acute GN due to postinfectious GN, HSP, SLE, vasculitis, or similar conditions. RAS blockade refers to treatment with an angiotensin-converting enzyme Inhibitor or an angiotensin II receptor blocker. Immunosuppression includes a wide range of immunosuppressive agents (see Concise Methods).
Acute Glomerular Injury versus Remission from Acute GN
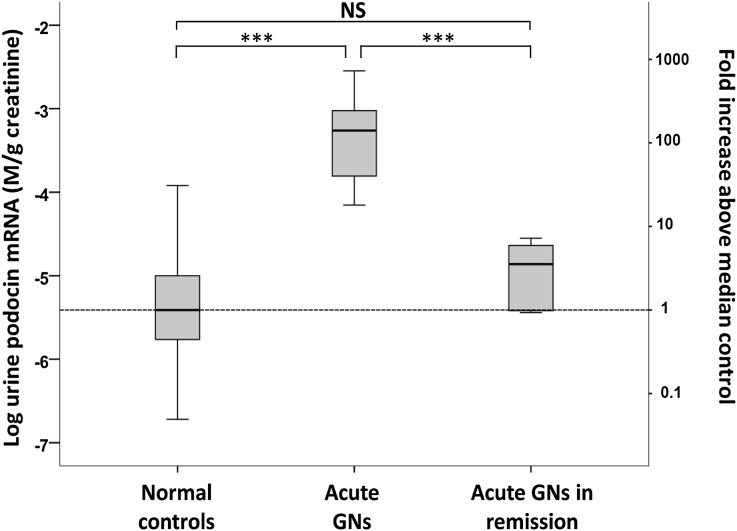
To define the urine mRNA marker profiles for active versus inactive disease, we analyzed urinary mRNAs in patients with acute inflammatory glomerulonephritides that usually remit with or without treatment. For this purpose we used acute postinfectious GN and Henoch-Schönlein purpura (HSP) nephritis at time of referral to the clinic with active disease (n=18) compared with samples obtained during remission 6–24 months later (n=13) and when the UProtCR had returned to within the normal range (≤0.18). Figure 2 shows that the rate of podocyte loss as reflected by the UPodCR median value was on average elevated 106-fold above the normal in active glomerular disease and had returned to the normal range in remission. These data demonstrate that UPodCR reflects active ongoing glomerular injury occurring at the time of urine collection. When the disease was no longer active, markers had returned toward normal levels.
Figure 2.
UPodCR reflects active glomerular diseases. At presentation of acute GN, defined as postinfectious GN or HSP, the UPodCR was on average 106-fold higher than in controls (P<0.001). In remission, 6–12 months after presentation and after return of the urine UProtCR to within the normal range, the UPodCR had returned to normal levels. These data show that the UPodCR reflects active disease at the time the urine sample is collected. The box represents median and interquartile ranges, and error bars present 1.5-fold x the interquartile range below 25th and above 75th percentile. Means were compared using ANOVA with post hoc Bonferroni correction for multiple comparisons. ***P<0.001. NS, not significant.
Progressors Have Increased Levels of Urine Podocyte mRNA
To test the podocyte depletion hypothesis for progression, we defined three groups of progressors with biopsy-proven glomerular disease (see also Concise Methods and Table 1). These groups were (1) eGFR progressors, whose eGFR more than halved during the observation period (n=8); (2) nephrotic syndrome/FSGS progressors (n=9), with nephrotic syndrome and biopsy-proven FSGS-type glomerulosclerosis who reached ESRD during the observation period; and (3) other GN progressors (n=5), who reached ESRD with a biopsy diagnosis of non–FSGS-type glomerular diseases (membranoproliferative GN [MPGN] ×2, IgA nephropathy, anti–glomerular basement membrane antibody (anti-GBM) disease, and Alport complex). Some patients who halved their eGFR also progressed to ESRD, so they appear in two of the three progressor groups.
As shown in Figure 3 the eGFR progressor, other GN progressor, and FSGS progressor groups had average UPodCR values 100-fold, 31-fold, and 92-fold above control values, respectively (P<0.001 for all groups versus controls). There was no statistical difference between the progressor groups. They were therefore combined to form a single all-progressor group (n=16) that had UpodCR values an average of 79-fold higher than those of controls (P<0.001). All clinic samples (excluding those from progressors) (n=342) had on average an 8-fold higher UPodCR level than control samples (P<0.001 versus control), but a statistically lower average UPodCR than did each of the progressor groups (P<0.001). However, the range for the clinic group was wide.
Figure 3.
All progressor groups have persistently high UPodCR levels. The normal range derived from single samples of urine from individuals without kidney disease at left was compared with the average UPodCR values for each patient, demonstrating that the urine samples from kidney clinic patients in general had on average an 8-fold higher level of UPodCR than controls (P<0.001). The eGFR progressor group (n=8), GN progressor group (n=5) and FSGS progressor group (n=9) had 100-, 31-, and 92-fold higher average levels of UpodCR, respectively, compared with the normal control median (P<0.001). Each progressor group also had higher levels than the average clinic UPodCR value for nonprogressor patients (P<0.001). The range for the clinic patients was large, including individuals with normal levels and very high levels. The three progressor groups were not statistically different and so were combined as a single all-progressor group with an average that was 79-fold higher than normal (P<0.001). The box represents median and interquartile ranges, and error bars present 1.5-fold x the interquartile range below 25th and above 75th percentile. Means were compared using ANOVA with post hoc Bonferroni correction for multiple comparisons. ***P<0.001.
Clinic Patients with All Types of Glomerular Disease Have Increased Levels of Urine Podocytes mRNAs
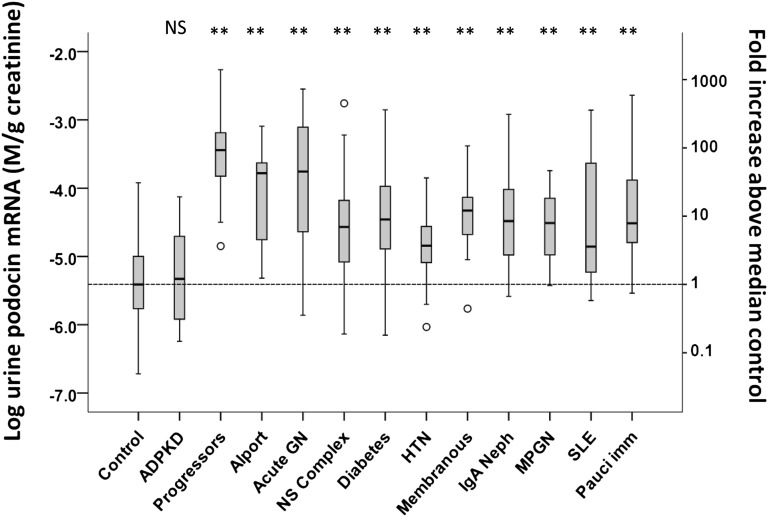
Patients were grouped according to underlying disease diagnosis (Table 1). The average level of UPodCR from all samples available for each patient (n=1–23) was used for analysis to assess steady state UPodCR to the extent possible. Figure 4 shows levels of UPodCR in the defined all-progressor group at 79-fold above normal. However, all forms of glomerular disease evaluated had significantly increased levels of UPodCR compared with the normal control group (P<0.001). In each disease category, there were individual patients with very high levels of UPodCR in the range of the all-progressor group. In contrast, patients with PKD had normal levels of UPodCR. These data are compatible with the conclusion that within the clinic population for each glomerular disease group there are patients with high levels of UPodCR who would be predicted to be at higher risk for progression, and some patients with lower levels of UPodCR who would be predicted to be at low risk for progression.
Figure 4.
All glomerular disease groups have increased UPodCR compared to control and ADPKD. The average UPodCR value for all clinic urine samples collected for the combined-progressor group was 79-fold higher than for healthy controls. In all other glomerular groups evaluated, the UPodCR was highly significantly increased above the normal median (P<0.001). This includes Alport syndrome complex (chronic hereditary nephritis), Acute GNs (postinfectious GN and HSP), the nephrotic syndrome complex (MCD, SSNS, SDNS, SRNS, and FSGS), diabetes-associated glomerular disease, hypertension-associated glomerular disease, idiopathic membranous nephropathy, IgA nephropathy, MPGN, SLE-related glomerular diseases, and pauci-immune vasculitides. The only renal disease in which the UPodCR was not elevated was PKD, a nonglomerular progressive disease. In each disease group there were individual patients with high UPodCR levels predicted to be at increased risk for progression and those with levels in the normal range who would be predicted to be at lower risk for progression. The box represents median and interquartile ranges, and error bars present 1.5-fold x the interquartile range below 25th and above 75th percentile. Means were compared using ANOVA with post hoc Bonferroni correction for multiple comparisons. **P<0.001. NS, not significant.
Nephrotic Syndrome Complex (MCD, Steroid-Sensitive Nephrotic Syndrome, Steroid-Dependent Nephrotic Syndrome, Steroid-Resistant Nephrotic Syndrome, and FSGS)
Figure 5 shows the breakdown of different groups within the nephrotic syndrome complex, including MCD, steroid-sensitive nephrotic syndrome (SSNS), steroid-dependent nephrotic syndrome (SDNS), steroid-resistant nephrotic syndrome (SRNS), and FSGS (nonprogressors who did not fulfill criteria for progression) and FSGS progressors. The FSGS-progressor group had 121-fold elevated median levels of UPodCR compared with the normal median (P<0.001). All subgroups, including FSGS (nonprogressor), MCD, SDNS, SRNS, and SSNS, at time of proteinuria (UprotCR ≥ 2) had significantly elevated UPodCR levels (P<0.001) at 10-, 13-, 14-, 16-, and 16-fold above the median control, respectively (P<0.001 for each subgroup versus control). However, all groups (except the SSNS group) had upper ranges that were >100-fold above the normal median, compatible with their known potential for progression. This suggests that individual patients within these groups are at potential risk for becoming podocyte depleted and developing FSGS unless their rate of podocyte detachment could be reduced by treatment. In all five subgroups, treatment-related reduction in proteinuria to within the normal range was also associated with reduction in the UPodCR values to 2–4-fold above the normal range (not significantly different from control).
Figure 5.
All nephrotic syndrome complex groups have high UPodCR in association with higher level proteinuria. The FSGS progressor group (n=9) had on average a 92-fold higher median level of UPodCR compared with the normal median (P<0.001). Patients with a biopsy diagnosis of MCD or FSGS (who did not meet criteria for progression) had on average a 10- and 13-fold higher median UpodCR, respectively (P<0.001 compared with normal control), although the range varied up to >100-fold above normal, well within the FSGS progressor group range. The clinical descriptors SRNS, SDNS, and SSNS had similar median UPodCR values that were 16-, 14- and 16-fold above the control median, respectively. There were no significant differences in UPodCR values between FSGS, MCD, SDNS, SRNS, or SSNS groups with or without proteinuria. The patients with nephrotic syndrome complex as a whole who had been brought into remission by various treatments, resulting in reduction of proteinuria into the normal range (UProtCR ≤0.18), had significantly reduced UPodCR values toward the normal range (not significantly different from control). The box represents median and interquartile ranges, and error bars present 1.5-fold x the interquartile range below 25th and above 75th percentile. Means were compared using k-way ANOVA with post hoc Bonferroni correction for multiple comparisons. **P<0.001. NS, not significant.
Relationship between Proteinuria, Podocyte Detachment Rate, and Progression for All Samples
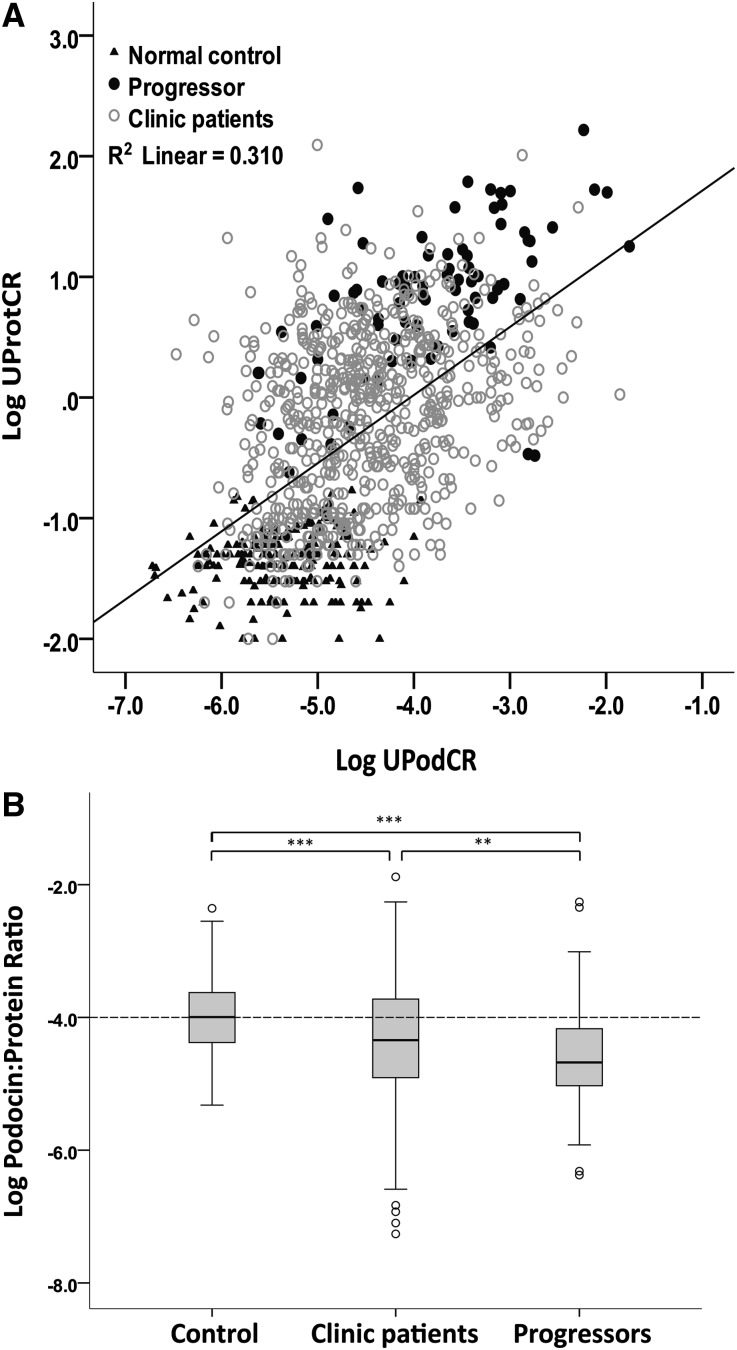
Figure 6A and Table 4 show individual data points for all clinic patients and controls. There was a significant correlation between proteinuria (UProtCR) and podocyte detachment rate (UPodCR) (R=0.57; P<0.001). In Figure 6A, progressors (filled circles) appear predominantly above the regression line, suggesting that not only do they have high UPodCR values but also they also have an unexpectedly high level of proteinuria for the amount of podocyte detachment. This is confirmed by expressing data as the UpodCR-to-UProtCR ratio (Figure 6B).
Figure 6.
Proteinuria correlates with the rate of podocyte detachment (UPodCR)and is relatively increased in progressors. (A) Control samples (filled triangles), progressors (filled circles), and other clinic samples (open circles) are shown. See Table 4 for statistical data. Individual data points are shown (n=1143). The overall correlation R is 0.56 (P<0.001). Note that the progressors tend to lie above the regression line. (B) The same data presented as the urine podocin-to-protein ratio, confirming that progressors have a significantly lower ratio than do controls or other clinic patients. Therefore, although the rate of podocyte detachment is higher for progressors, they have an even higher rate of proteinuria than would be predicted from the UPodCR. The box represents median and interquartile ranges, and error bars present 1.5-fold x the interquartile range below 25th and above 75th percentile. Means were compared using ANOVA with post hoc Bonferroni correction for multiple comparisons. **P=0.003, ***P<0.001.
Table 4.
Different relationships between UPodCR and UProtCR in different disease groups
| Category | Urine Samples (n) | Slope | R | P Value | Slope Comparisons | ||||
|---|---|---|---|---|---|---|---|---|---|
| P versus SSNS | P versus MCD | P versus FSGS | P versus FSGS Progressors | P versus All Progressors | |||||
| Clinic samples | 852 | 0.39 | 0.43 | <0.001 | <0.001 | <0.001 | NS | NS | NS |
| Control samples | 291 | 0.06 | 0.12 | NS | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| All samples | 1143 | 0.57 | 0.56 | <0.001 | 0.01 | <0.01 | NS | NS | <0.001 |
| All progressors | 86 | 0.40 | 0.57 | <0.001 | 0.001 | <0.001 | NS | NS | – |
| SSNS | 34 | 0.94 | 0.78 | <0.001 | – | NS | <0.01 | <0.01 | 0.001 |
| MCD | 78 | 0.89 | 0.70 | <0.001 | NS | – | <0.01 | <0.01 | <0.001 |
| SDNS | 104 | 0.60 | 0.56 | <0.001 | NS | NS | NS | NS | <0.05 |
| SRNS | 94 | 0.80 | 0.66 | <0.001 | NS | NS | 0.02 | <0.01 | <0.001 |
| FSGS | 113 | 0.51 | 0.62 | <0.001 | <0.01 | <0.01 | – | NS | NS |
| FSG progressors | 56 | 0.43 | 0.61 | <0.001 | <0.01 | <0.01 | NS | – | NS |
| Diabetes-associated kidney disease | 123 | 0.49 | 0.46 | <0.001 | <0.01 | <0.01 | NS | NS | NS |
| SLE-associated GNs | 43 | 0.44 | 0.66 | <0.001 | <0.01 | <0.01 | NS | NS | NS |
| Acute GNs | 82 | 0.32 | 0.57 | <0.001 | <0.001 | <0.001 | 0.02 | NS | NS |
| Hypertension-associated kidney disease | 35 | 0.41 | 0.33 | NS | NS | NS | NS | NS | NS |
| MPGNs | 44 | 0.20 | 0.23 | NS | 0.001 | <0.001 | 0.05 | NS | NS |
| IgA nephropathy | 48 | 0.19 | 0.13 | NS | <0.001 | <0.001 | 0.001 | 0.02 | 0.01 |
| Pauci-immune GN | 53 | 0.11 | 0.14 | NS | <0.001 | <0.001 | <0.001 | 0.02 | 0.02 |
| Alport complex | 20 | −0.05 | 0.07 | NS | <0.001 | <0.001 | 0.001 | <0.001 | <0.01 |
| Membranous GN | 48 | −0.10 | 0.12 | NS | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
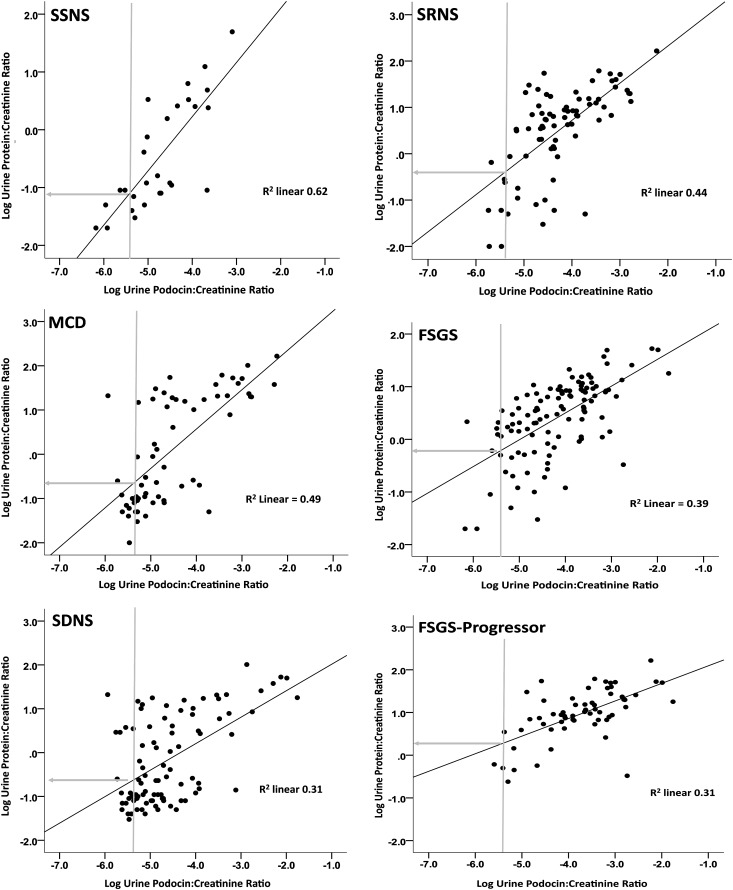
See also Figures 6–8. For all samples (clinic plus control), UPodCR correlated significantly but not strongly with UProtCR (R=0.57, R2=0.31), although the correlations for clinic samples alone were weaker (R=0.39, R2=0.15). The slope of UPodCR and UProtCR correlations varied markedly for different disease groups. The slope for MCD and SSNS showed an almost 1:1 relationship, thereby demonstrating a close association between podocyte detachment rate and proteinuria. In marked contrast, the UPodCR/UprotCR slope flattened significantly in FSGS and particularly in FSGS progressors, such that changes in the rate of podocyte detachment would have a much smaller effect on proteinuria (see also Figure 7). Other disease groups, including diabetes, SLE, and acute GNs, also showed a significant relationship between UPodCR and UProtCR. In contrast for MPGNs, IgA nephropathy, pauci-immune GNs, Alport complex, and Membranous GN, no significant correlation was observed between UPodCR and UProtCR. NS, not significant.
UPodCR and UProtCR Relationships in Nephrotic Syndrome Complex
As shown in Figure 7 and Table 4 for both SSNS and MCD, the UPodCR and UProtCR slope was almost 1 (0.94 and 0.89, respectively). This suggests a direct causal relationship between the underlying pathologic process (effaced foot processes) and both proteinuria and podocyte detachment rate. It also implies that if the podocyte detachment rate were to be reduced by treatment to the normal median, then proteinuria would follow and vice versa, compatible with clinical expectation for lack of progression in these conditions if proteinuria can be reduced to within the normal range. In contrast in FSGS and particularly in the FSGS-progressor group, the slope relating UPodCR and UProtCR was significantly flatter (0.51 and 0.43, respectively; P<0.001 compared with SSNS and MCD). This progressive change in slope from SSNS through MCD, SDNS, SRNS, and FSGS to FSGS progressors is compatible with the concept that uncompensated podocyte depletion results in bare areas of GBM with enhanced protein leak. It also means that if the podocyte detachment rate were to be decreased to the normal median that proteinuria would persist at levels at least 10-fold above normal in FSGS and progressors. Proteinuria is therefore a less sensitive marker of rate of podocyte detachment for FSGS than for SSNS/MCD.
Figure 7.
The correlation slope for UPodCR versus UProtCR is flatter for FSGS and FSGS progressors than for SSNS and MCD, which suggests that change in proteinuria is a less sensitive marker of progression in FSGS. Nephrotic syndrome subgroups are shown as defined in Table 1 as SSNS, SDNS, SRNS, MCD, FSGS, and FSGS progressor patients (see Concise Methods). Individual data points are shown. The calculated slope relating UPodCR to UProtCR is shown for each data set (see also Table 4). Note that the slope in SSNS and MCD is significantly steeper than for other subgroups. In contrast, the slope is significantly flatter for FSGS and flattest for FSGS progressors. The vertical gray line in each panel shows the median normal control UPodCR value. The horizontal gray line shows the predicted average level of proteinuria (UPodCR) corresponding to median control value for UPodCR derived from the slope line, where the Log −1 and Log 0 correspond to UProtCR values of 0.1 and 1, respectively. This change in slope reflects a changed relationship between podocyte detachment rate and proteinuria, probably resulting from uncompensated podocyte detachment leaving a bare area of leaky glomerular basement membrane, as demonstrated in model systems.14,16 As a consequence of this altered slope, if a hypothetical treatment were to successfully reduce the rate of podocyte detachment to the control median value, significant proteinuria would persist in FSGS but not in SSNS/MCD. Proteinuria is therefore a very sensitive marker of the podocyte detachment rate in SSNS and MCD, but a less sensitive marker of the rate of podocyte detachment in FSGS and particularly in FSGS progressors.
UPodCR and UProtCR Relationships in Other Glomerular Diseases
Figure 8 and Table 4 show correlation analysis for other GN groups. In diabetes, systemic lupus erythematosus (SLE), acute GNs, hypertension-associated GN, and MPGNs the correlations between UPodCR and UProtCR were not significantly different from FSGS. In contrast, in membranous glomerulopathy (and also IgA nephropathy, Alport complex, and pauci-immune GN), UPodCR and UProtCR were not significantly correlated, suggesting that in these conditions proteinuria and podocyte detachment rate are not closely related. This implies that proteinuria will be a less reliable readout for the rate of podocyte detachment and therefore for progression in these conditions.
Figure 8.
Different glomerular diseases have different relationships between rate of podocyte detachment and proteinuiria, which suggests that proteinuria is a less sensitive marker of progression in some glomerular diseases (e.g., membranous nephropathy). Groups are shown as in Table 1. Acute GNs are combined postinfectious GN and HSP; SLE-associated glomerular diseases; diabetes-associated kidney disease; IgA nephropathy, pauci-immune GN, and membranous GN. Individual data points are shown. Table 4 provides details of slopes and slope comparisons. The vertical gray line denotes the normal median value for UPodCR. The horizontal gray line projects the UProtCR value corresponding to the median UPodCR value provided by the slope, where the Log −1 and Log 0 correspond to UProtCR values of 0.1 and 1, respectively. Note the significant differences between disease groups with respect to the slope relating UPodCR to UprotCR, with the relationship being significant in acute GNs, SLE, and diabetes, where the slope is not significantly different from FSGS, as shown in Figure 7 and Table 4. In contrast, UPodCR and UProtCR did not correlate for IgA nephropathy, pauci-immune GNs, or membranous nephropathy, and these conditions were significantly different from both the FSGS and the SSNS/MCD groups (Table 4). Note from the intercept of the gray line on the vertical axis that for membranous GN reducing the rate of podocyte detachment to the normal median level would be projected not to affect proteinuria significantly, as might be expected for membranous nephropathy. In contrast, reduction of the rate of podocyte detachment for diabetes, SLE, and acute GNs would be expected to reduce proteinuria.
Discussion
Every nephrologist knows that the pellet obtained from bench-top centrifugation of fresh urine contains important information that can guide clinical decision-making. In this report we demonstrate that urine pellet mRNA can also be used to estimate the rate of podocyte detachment from glomeruli in humans. An essential aspect of this work is that it is based on recent understanding of the biology of progression in glomerular diseases developed and validated in model systems that have unequivocally proven the direct causative relationship between podocyte depletion, glomerulosclerosis, and progression to ESRD, in part through use of this urine pellet mRNA technology.1–5,14–17 As outlined in the introduction, these concepts are collectively captured as the podocyte depletion hypothesis. No other mechanism of progression of glomerular diseases that is independent of podocyte injury and depletion has yet been proven to exist.5 This systematic approach, where each step of the way has been quantitatively proven in model systems, is necessary to serve as a foundation for translating the concept of podocyte depletion to the clinic for improved prevention of progression.
In this context we demonstrate that patients who actually progress, as determined by a >50% reduction in eGFR and/or reaching ESRD, have a markedly increased rate of urine podocyte detachment, whatever their underlying renal pathology (79-fold above control). An independent cohort of patients with Alport complex who progress to ESRD over 20–30 years40 also have an increased rate of podocyte detachment (23-fold above control). Prior reports using urine markers have focused primarily on disease “activity.”12, 18–35 As is emphasized by the acute GN studies presented in this report, there is an important difference between disease activity at a point in time and progression. Persistent high-level podocyte detachment over time is associated with progression.
We show that all glomerular diseases seen in adult and pediatric clinics can have an increased podocyte detachment rate. This result therefore establishes that accelerated podocyte loss rate detectable in urine is a general phenomenon found in all glomerular diseases, but not in nonglomerular diseases (e.g., autosomal dominant PKD). Whatever the underlying glomerular disease, patients with persistently high levels can be expected to be at increased risk for progression. In contrast, patients with levels in the normal range because of treatment or spontaneous remission can be expected to be at reduced risk for progression. This comprehensive analysis therefore opens up the potential for urine podocyte assays to be generally applicable to all glomerular diseases.
In designing this study we had anticipated that some forms of podocyte depletion would be caused by the death of podocytes in situ or to failure of the podocyte replacement mechanism. In both of these scenarios podocyte mRNAs in urine might be expected to be decreased in association with progression. But this is not what we observed. The likely explanation is that two major podocyte detachment amplification mechanisms are nonspecifically triggered by podocyte injury for any cause. These are (1) the “podocyte damage damages podocytes” mechanism described by Ichikawa and colleagues13 and (2) the “angiotensin 2 amplifies the rate of detachment of podocytes as a consequence of any glomerular injury” that we documented as the mechanism by which angiotensin 2 blockade protects kidneys from progression.14 Through both of these mechanisms, essentially any cause of podocyte injury can result in accelerated detachment of podocytes which become detectable in urine. Most patients with proteinuria in this study were treated with RAS inhibitors, so we cannot determine the extent to which this treatment reduced the rate of podocyte detachment as would have been expected from model systems14 and previously reported in diabetes-associated kidney disease.31
Contrary to what might have been expected and previously reported using podocyte counting methods, which are much less sensitive than the mRNA method,20 the rate of podocyte detachment was increased in MCD/SSNS in direct proportion to proteinuria. MCD/SSNS is not generally considered to be a progressive glomerular disease, although MCD progression to FSGS and steroid-responsiveness of some patients with pathologic diagnoses of FSGS are both well established clinical phenomena. This remarkable result supports the concept that the development of FSGS-type pathology is a function of the rate and duration of podocyte injury and detachment rather than a fundamental component of the underlying disease. According to this scenario, development of FSGS-type pathology is a two-phase process consisting of initiating events driving podocyte injury and dysfunction for any cause and secondary events associated with accelerated podocyte death and detachment that, if they exceed the capacity of podocytes to adapt by hypertrophy14,38,39 or replacement,41,42 will inevitably result in areas of absent podocytes (bare areas of glomerular basement membrane) that the glomerulus will defend against excessive protein loss by sclerosis, resulting in the typical FSGS pattern of injury.5,14 This general concept applicable to all glomerular diseases emphasizes the importance of urgent reduction of the rate of podocyte detachment as the major therapeutic strategy to prevent development of glomerulosclerosis and progression to ESRD.
Different glomerular diseases showed different relationships between proteinuria and the rate of podocyte depletion. For example, in membranous glomerulonephropathy no correlation between the rate of podocyte detachment and proteinuria was discerned. This observation is consistent with the clinical observation that progression in membranous nephropathy is not closely related to the degree of proteinuria and that all patients with membranous nephropathy do not progress.43,44 In contrast, there was a significant relationship between proteinuria and rate of podocyte detachment in diabetes, lupus nephritis, and other conditions where reduction in proteinuria has a well-established correlation with reduced progression rate.36,37 There was a significant correlation between the rate of podocyte detachment and proteinuria for all clinic patients, compatible with the concept that proteinuria is an expected consequence of glomerular filter dysfunction for any cause such that any marker of glomerular injury will correlate with proteinuria. Proteinuria is a nonspecific marker of many forms of glomerular and kidney injury. The data provided suggest that proteinuria will persist despite effective therapies that reduce the rate of podocyte detachment in many glomerular diseases. In contrast, podocyte detachment is one among many causes of proteinuria, but it defines the progression process and therefore can provide useful adjunctive information to the nephrologist to guide response to treatment and risk for progression in the clinic.
Concise Methods
This study was approved by the University of Michigan Institutional Review Board committee (#HUM00025707).
Normal Urine Samples
A total of 291 urine samples were collected from unidentified people aged 3–89 years who had no known kidney disease or hypertension. Each sample was associated with a short consent form filled out by the urine donor that included information about age, sex, race, medical conditions, and medications. Urine samples were collected from Pediatric and Geriatric clinics at the University of Michigan and from volunteers. Exclusion criteria included any kidney-related disease, antihypertensive medication, urine dipstick-positive for blood or protein, and UProtCR of >0.18. There were no statistical differences in mRNA between the excluded samples on the basis of the above criteria (n=37) as a group and the samples included in the normal group for subsequent analysis.
Clinical Sample Collection
Samples were prospectively collected from the adult and pediatric outpatient clinics and from inpatients at University of Michigan Hospitals over a 4-year period. Patients signed a consent form at the clinic visit. The samples used were the urine left over from routine urine collections provided by patients at their regular clinic visits. Some samples were midstream samples and some were not. All samples were assigned a study number that connected them to clinical information from the chart. Samples were otherwise de-identified. Clinical information used for analysis was prospectively collected for each sample from the clinical chart and used to populate a de-identified database. A total of 164 patients (45.8%) had a single sample, 75 (20.9%) had two samples, 51 (14.2%) had three samples, and the other 68 (19.0%) had four or more samples. The range was 1–23, the median was 2, and the interquartile range was 1–3.
Volume of Urine for Assay
In control samples, which contain low RNA levels, podocin mRNA was not detectable in 33% of samples starting with <20 ml urine and in 15% of samples starting with 30–50 ml urine. In clinic samples, podocin mRNA was undetectable in 12% of samples starting with >30 ml of urine. Therefore, whenever possible we used >30 ml urine.
Urine Processing
Urine (up to 50 ml in a sterile 50-ml plastic centrifuge tube) was centrifuged at 4°C for 15 minutes at 4000 rpm (3200 g) on a table-top centrifuge. Two 2-ml aliquots of the supernatant was removed and stored at −20°C for protein, creatinine, and other measurements. The urine pellet was suspended in 750 μl of cold diethylpyrocarbonate-treated PBS (pH, 7.4) at 4°C using a sterile disposable polystyrene transfer pipette and then transferred to a labeled 1.7-ml plastic centrifuge tube. A second 750 μl of PBS was used to wash the bottom of the 50-ml centrifuge tube to recover remaining pellet material, and added to the 1.7-ml tube. The transferred pellet material in 1.5 ml PBS was then centrifuged at 12,000 rpm in a mini-centrifuge for 5 minutes at 4°C. The supernatant was discarded. To the centrifuged material, washed pellet was added to 350 μl of RLT buffer containing β-mercaptoethanol at 10 μl/ml of RLT buffer according to the RNeasy Qiagen protocol (Germantown, MD). The pellet was suspended in RLT/β− mercaptoethanol buffer was then frozen at −80°C for assay.
RNA Preparation and Quantitative RT-PCR Assay
The total urine pellet RNA was isolated using the protocol of the RNeasy Mini Kit (catalog no. 74106; Qiagen). Quantitation of the absolute nephrin, podocin, TGF-β1, and aquaporin-2 mRNA abundance was performed using the 7900 HT Fast Real-Time PCR System (Applied Biosystems) using TaqMan Fast Universal PCR Master Mix, with sample cDNA in a final volume of 25 μl per reaction. TaqMan Probes (Applied Biosystems) used were as follows: human NPHS1 (nephrin; catalog no. s00190446_m1) and NPHS2 (podocin; catalog no. Hs00922492_m1). All data were from 2-μl sample measured in duplicate. Standard curves were constructed for each assay using serially diluted cDNA standards. Assays were accepted only if the r2 was >0.97 for standard curves using SDS 2.2.2 software (Applied Biosystems). Human nephrin and podocin cDNA of known sequence and concentration were used as standards for each assay so that the data could be calculated on a molar basis for each probe. We previously reported analysis urine RNA quality, recovery, and stability. The coefficient of assay variation is 35%. Model systems demonstrate that proteinuria itself does not affect measurement of urine podocyte mRNA markers, as demonstrated by the fact that in a protein overload model induced by injecting albumin into the peritoneal cavity of rats to induce high-level proteinuria (UProtCR of >80), urine podocin mRNA excretion did not change,14 and in progression models urine aquaporin-2 mRNA (a kidney gene unrelated to glomerular injury) did not vary in the face of UProtCR >80.14
Stability of Urine mRNAs and Sample Handling
As previously reported, the urine mRNA markers decay over 4–6 hours after voiding; decay occurs more quickly if the urine is stored at room temperature than if it is stored at 4°C.15 After this initial period of decay, mRNA markers remain remarkably constant for prolonged periods of time—up to 48 hours—even at room temperature, presumably reflecting viable cells in the urine. For this study we collected urine samples at any time from 7 a.m. until 4 p.m. and stored the samples at 4°C. At 4:30 p.m. they were then collected for transport to the laboratory for processing.
Data Expression Method and Interpretation
All assays were performed against cDNA standards to allow data to be expressed in moles of cDNA. Because the urine sample volume varied from 5 to 50 ml, we first expressed data per ml of urine, and then, to compensate for urine concentration, we expressed data per g of urine creatinine. All data are therefore expressed as moles per g creatinine (e.g., UPodCR). The low-speed centrifugation conditions used do not pellet exosomes and other subcellular structures, so we assume that the urine pellet mRNAs measured are derived from whole cells and are therefore a measure of the podocyte detachment rate. Urine samples were collected from patients at clinic visits or from inpatients, so the interval between samples was not standardized. Therefore for comparisons between patient groups we used the averaged UPodCR value calculated from all available urine samples from each patient.
Imputation of Podocin and Nephrin mRNA Values Below the Limit of Detection for Control Sample Comparisons
For the comparisons among age, race, and sex for normal controls, we imputed missing values to ensure that undetectably low levels of urine mRNAs would not obscure differences between groups. For this analysis we first reassayed all samples that had missing podocin mRNA data to exclude a technical explanation for missing data, thereby confirming that all missing data points were due to there being a signal below the limit of detection. Only samples in which levels of aquaporin-2 and TGF-β1 mRNAs were both detectable but nephrin and podocin were not detectable were used for imputation of the nephrin and podocin mRNAs to exclude nondetectability occurring for technical reasons. Because the reason for doing imputation in the control group was to ensure that low levels of mRNAs were not present in particular groups of controls, we imputed the undetectable values by assigning them an average value for the 10 lowest levels measured in the control group. Imputed mRNA values were not used for any comparisons between control and clinic samples.
Definitions of Clinical and Pathologic Groupings
Progressor groups were defined as patients with biopsy-proven glomerulonephritides. Three progressor groups were defined: (1) An eGFR-progressor group, whose eGFR decreased by at least 50% during the observation period (n=8); (2) an NS/FSGS-progressor group (n=9), who reached ESRD requiring RRTs (dialysis or kidney transplantation) during the observation period and had diffuse foot process effacement by ultrastructural analysis (seven had FSGS lesions, one had mesangial expansion, and one had a pathologic diagnosis of focal global glomerulosclerosis); and (3) a non-FSGS progressor group (n=5), who reached ESRD requiring RRTs but whose pathologic diagnoses comprised MPGN of undetermined type, MPGN type II, anti-GBM disease, IgA nephropathy, and Alport syndrome.
The definitions for MCD, FSGS, SSNS, SDNS, and SRNS groups were as follows: If a patient had previously had a biopsy specimen showing MCD and later had a biopsy specimen showing FSGS, the patient was assigned to the FSGS group. If the patient had a biopsy specimen showing MCD, he or she was assigned to the MCD group except as above. SSNS was defined as nephrotic syndrome with a complete remission in response to steroid treatment. SDNS was defined as nephrotic syndrome that required steroid treatment to maintain remission or partial remission. SRNS was defined as nephrotic syndrome that had become or was de novo unresponsive to steroid treatment but may or may not have been responsive to other agents.
Diabetes-associated kidney disease was defined as any patient in the kidney clinics who had a diagnosis of diabetes mellitus. Many of these patients were also hypertensive. Hypertension-associated kidney disease was defined as any patient referred to the kidney clinic who was nondiabetic and in whom hypertension was assumed to be the principal cause of kidney injury. Acute postinfectious GN was a clinical diagnosis based on serologic and clinical measures. The diagnosis of HSP, membranous GN, IgA nephropathy, SLE–associated GN, MPGN, and pauci-immune GN were all biopsy-based pathologic diagnoses. An autosomal dominant polycystic kidney disease diagnosis was based on family history and presence of polycystic kidneys. Uncategorized patients included all of those with diagnoses in inadequate numbers for a statistically valid subgroup.
Medications
The clinic group was taking a wide range of medications, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and other antihypertensive medications. Immunosuppressive medications included prednisone, methylprednisolone, mycophenolate mofetil, cyclosporine, tacrolimus, azathioprine, methotrexate, cyclophosphamide, and rituximab.
Statistical Analyses
For descriptive purposes, mean ±SD, median and interquartile range (for skewed variables), and range were used to show the distributions of continuous variables. Count with relative frequency was applied as the descriptive statistic for categorical variables. All the continuous skewed variables underwent log transformation before comparison. Distribution of categorical variables across different subgroups was compared using chi-squared test. Means of variables in two and more than two groups were compared by t test and ANOVA, respectively. K-way ANOVA was applied whenever there was more than one factor. The Bonferroni adjustment was applied in multiple comparisons. Pearson correlation coefficient was calculated to explore the linear correlation between variables. Linear regression models were applied to quantify rates of changes in Log UProtCR by levels of Log UPodCR across the entire sample group as well as the defined subgroups. The slopes of different regression lines were compared across different subgroups as well as entire samples. SPSS software, version 20 (Chicago, IL), was used for statistical analyses.
Disclosures
None.
Acknowledgments
We are grateful to Dr. Matthias Kretzler for helpful discussion. We are grateful to the Vrishin Chandra Research Foundation and the National Kidney Foundation of Michigan for help with sample collection.
The project described was supported by the National Center for Research Resources, grant UL1RR024986, and is now at the National Center for Advancing Translational Sciences, grant UL1TR000433. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). The work was also supported by grants from the Renal Research Institute, funding from D.B.K. through the Robert C. Kelsch Collegiate Professorship. R.W. was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants DK R0146073 and P30 DK081943. L.W. was supported by NIH T32DK065517 training grant and by a NEPTUNE Career Development Award U54 DK083912 from the NIDDK and the NIH Office of Rare Diseases Research (ORDR)/NCATS. F.A. was supported by NIH grant 5T32DK7378-34. An abstract was presented at the American Society of Nephrology 2012 Annual Meeting in San Diego.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Steffes MW, Schmidt D, McCrery R, Basgen JM, International Diabetic Nephropathy Study Group : Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int 59: 2104–2113, 2001 [DOI] [PubMed] [Google Scholar]
- 9.White KE, Bilous RW, Marshall SM, El Nahas M, Remuzzi G, Piras G, De Cosmo S, Viberti G: European Study for the Prevention of Renal Disease in Type I diabetes (ESPRIT): Podocyte number in normotensive type I diabetic patients with albuminuria. Diabetes 51: 3083–3089, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD: Podocytopenia and disease severity in IgA nephropathy. Kidney Int 61: 1475–1485, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P: Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52: 1031–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Lai FM, Kwan BC, Lai KB, Chow KM, Li PK, Szeto CC: Podocyte loss in human hypertensive nephrosclerosis. Am J Hypertens 22: 300–306, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa I, Ma J, Motojima M, Matsusaka T: Podocyte damage damages podocytes: Autonomous vicious cycle that drives local spread of glomerular sclerosis. Curr Opin Nephrol Hypertens 14: 205–210, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC: Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC: Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC: Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant 27: 4079–4087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara M, Yanagihara T, Itoh M, Matsuno M, Kihara I: Immunohistochemical and urinary markers of podocyte injury. Pediatr Nephrol 12: 43–48, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Hara M, Yanagihara T, Takada T, Itoh M, Matsuno M, Yamamoto T, Kihara I: Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis. Am J Nephrol 18: 35–41, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H: The urinary podocyte as a marker for the differential diagnosis of idiopathic focal glomerulosclerosis and minimal-change nephrotic syndrome. Am J Nephrol 20: 175–179, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Hara M, Yanagihara T, Kihara I: Urinary podocytes in primary focal segmental glomerulosclerosis. Nephron 89: 342–347, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Pätäri A, Forsblom C, Havana M, Taipale H, Groop PH, Holthöfer H: Nephrinuria in diabetic nephropathy of type 1 diabetes. Diabetes 52: 2969–2974, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV: Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 285: F40–F48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petermann AT, Pippin J, Krofft R, Blonski M, Griffin S, Durvasula R, Shankland SJ: Viable podocytes detach in experimental diabetic nephropathy: Potential mechanism underlying glomerulosclerosis. Nephron, Exp Nephrol 98: e114–e123, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T: Apical cell membranes are shed into urine from injured podocytes: A novel phenomenon of podocyte injury. J Am Soc Nephrol 16: 408–416, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Szeto CC, Lai KB, Chow KM, Szeto CY, Yip TW, Woo KS, Li PK, Lai FM: Messenger RNA expression of glomerular podocyte markers in the urinary sediment of acquired proteinuric diseases. Clin Chim Acta 361: 182–190, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J: Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 16: 1733–1741, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hara M, Yanagihara T, Kihara I: Cumulative excretion of urinary podocytes reflects disease progression in IgA nephropathy and Schönlein-Henoch purpura nephritis. Clin J Am Soc Nephrol 2: 231–238, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Camici M: Urinary detection of podocyte injury. Biomed Pharmacother 61: 245–249, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Lai FM, Tam LS, Li KM, Lai KB, Chow KM, Li KT, Szeto CC: Messenger RNA expression of podocyte-associated molecules in urinary sediment of patients with lupus nephritis. J Rheumatol 34: 2358–2364, 2007 [PubMed] [Google Scholar]
- 31.Wang G, Lai FM, Lai KB, Chow KM, Kwan BC, Li PK, Szeto CC: Urinary messenger RNA expression of podocyte-associated molecules in patients with diabetic nephropathy treated by angiotensin-converting enzyme inhibitor and angiotensin receptor blocker. Eur J Endocrinol 158: 317–322, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Skoberne A, Konieczny A, Schiffer M: Glomerular epithelial cells in the urine: what has to be done to make them worthwhile? Am J Physiol Renal Physiol 296: F230–F241, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Hara M, Yamagata K, Tomino Y, Saito A, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, Yan K: Urinary podocalyxin is an early marker for podocyte injury in patients with diabetes: Establishment of a highly sensitive ELISA to detect urinary podocalyxin. Diabetologia 55: 2913–2919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asao R, Asanuma K, Kodama F, Akiba-Takagi M, Nagai-Hosoe Y, Seki T, Takeda Y, Ohsawa I, Mano S, Matsuoka K, Kurosawa H, Ogasawara S, Hirayama Y, Sekine S, Horikoshi S, Hara M, Tomino Y: Relationships between levels of urinary podocalyxin, number of urinary podocytes, and histologic injury in adult patients with IgA nephropathy. Clin J Am Soc Nephrol 7: 1385–1393, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craici IM, Wagner SJ, Bailey KR, Fitz-Gibbon PD, Wood-Wentz CM, Turner ST, Hayman SR, White WM, Brost BC, Rose CH, Grande JP, Garovic VD: Podocyturia predates proteinuria and clinical features of preeclampsia: longitudinal prospective study. Hypertension 61: 1289–1296, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T: Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 54: 205–226, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Wiggins JE, Patel S, Shedden K, Goyal M, Wharram B, Martini S, Kretzler M, Wiggins RC: NFkB in the pro-inflammatory, pro-coagulant, pro-fibrotic aging glomerulus. J Am Soc Nephrol 21: 587–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M, Study Group Members of the Gesellschaft für Pädiatrische Nephrologie : Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81: 494–501, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ: Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 20: 333–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romagnani P: Kidney regeneration: Any prospects? Contrib Nephrol 170: 228–236, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Troyanov S, Roasio L, Pandes M, Herzenberg AM, Cattran DC: Renal pathology in idiopathic membranous nephropathy: A new perspective. Kidney Int 69: 1641–1648, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Heeringa SF, Branten AJ, Deegens JK, Steenbergen E, Wetzels JF: Focal segmental glomerulosclerosis is not a sufficient predictor of renal outcome in patients with membranous nephropathy. Nephrol Dial Transplant 22: 2201–2207, 2007 [DOI] [PubMed] [Google Scholar]