Abstract
There is a paucity of quality evidence regarding the effects of sodium restriction in patients with CKD, particularly in patients with pre-end stage CKD, where controlling modifiable risk factors may be especially important for delaying CKD progression and cardiovascular events. We conducted a double-blind placebo-controlled randomized crossover trial assessing the effects of high versus low sodium intake on ambulatory BP, 24-hour protein and albumin excretion, fluid status (body composition monitor), renin and aldosterone levels, and arterial stiffness (pulse wave velocity and augmentation index) in 20 adult patients with hypertensive stage 3–4 CKD as phase 1 of the LowSALT CKD study. Overall, salt restriction resulted in statistically significant and clinically important reductions in BP (mean reduction of systolic/diastolic BP, 10/4 mm Hg; 95% confidence interval, 5 to 15 /1 to 6 mm Hg), extracellular fluid volume, albuminuria, and proteinuria in patients with moderate-to-severe CKD. The magnitude of change was more pronounced than the magnitude reported in patients without CKD, suggesting that patients with CKD are particularly salt sensitive. Although studies with longer intervention times and larger sample sizes are needed to confirm these benefits, this study indicates that sodium restriction should be emphasized in the management of patients with CKD as a means to reduce cardiovascular risk and risk for CKD progression.
Cardiovascular disease (CVD) is the leading cause of premature mortality in the CKD population.1,2 CVD risk increases with only mild kidney impairment (estimated GFR [eGFR] <60 ml/min per 1.73 m2) and further escalates as CKD progresses,3 making early intervention to reduce CVD risk of utmost importance.4
Dietary sodium intake shows great promise as a modifiable risk factor for reducing the risks of cardiovascular disease and CKD progression.5,6 Extensive research has demonstrated the effect of sodium intake on fluid overload and hypertension,7,8 which are predictors of cardiac and vascular remodeling.9 Trials in sodium restriction recently showed significant reductions in proteinuria and albuminuria,7,10,11 which are strong predictors of CKD progression and CVD events.12 In addition, excessive sodium intake is thought to have direct toxic effects on blood vessels through mediating factors such as oxidative stress, inflammation, endothelial cell dysfunction, and vascular stiffness.13–15
The available evidence detailing the effects of sodium restriction in CKD patients is of poor quality, lacks randomization,16–18 a control group,17 or blinding,10,11 or does not use gold-standard measurement techniques (e.g., using clinic instead of ambulatory BP).10,11 Furthermore, several studies failed to either evaluate or adjust for the influence of key confounding factors, such as potassium intake or body weight,10,11,19–22 thereby making it difficult to assess whether the observed results can be solely attributed to dietary sodium.
The aim of this double-blind placebo-controlled randomized crossover study was to evaluate the effects of dietary sodium intake on BP, proteinuria, extracellular fluid volume, and arterial stiffness as markers of risks of cardiovascular and CKD progression. We hypothesized that a low sodium intake would decrease 24-hour BP, fluid volume, and 24-hour urinary protein and albumin compared with high sodium intake in patients with moderate-to-severe CKD.
Results
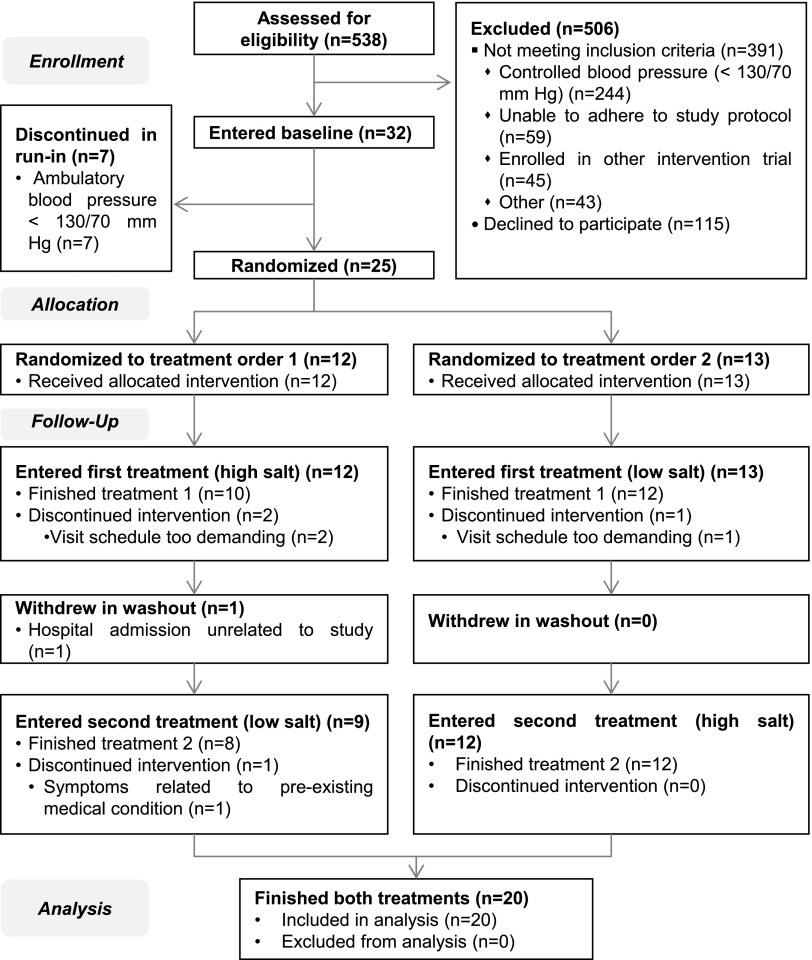
Figure 1 shows the Consolidated Standards of Reporting Trials flow diagram for phase 1 of the LowSALT CKD study. Twenty-five patients completed the run-in period and were randomized. Twenty patients completed the study and were included for analysis.
Figure 1.
Consolidated Standards of Reporting Trials diagram. Of the 25 patients who were randomized, 75% (n=20/25) completed the study and were included for analysis. Reasons for withdrawal were: visit schedule too demanding (n=3), hospital admission unrelated to study (n=1), and symptoms related to pre-existing condition (n=1).
Patient Characteristics and Study Compliance
Baseline characteristics of participants who completed the study are shown in Table 1. Those who withdrew from the study did not differ in age or sex (data not shown), but had significantly higher weight and body mass index (BMI) values compared with those who completed the study (101±18 versus 86±13 kg [P<0.01] and 36.2±7.1 versus 29.3±4.1 kg/m2 [P<0.01], respectively). Estimations of dietary intake indicated good compliance with sodium intake targets, and showed that energy and potassium intakes were stable between the interventions (Table 2). Pill counts validated study medication compliance, with 100% of patients classified as adherent in the high salt period (salt tablets) and 90% of patients (n=18 of 20) classed as adherent in the low salt period (placebo tablets).
Table 1.
Baseline characteristics of LowSALT CKD study participants (n=20)
| Characteristic | Value |
|---|---|
| Age (yr) | 68.5±11.0 |
| Men | 15 (75) |
| Diabetes mellitus | 9 (40) |
| Number of antihypertensive medications | 3.15±1.09 |
| Renin-angiotensin system blockade | 6 (30) |
| α-blocker | 16 (80) |
| β-blocker | 7 (35) |
| Calcium antagonist | 14 (70) |
| Diuretic | 8 (40) |
| Weight (kg) | 85.8±12.9 |
| BMI (kg/m2) | 29.3±4.1 |
| BP (mmHg) | |
| 24-h systolic BP | 151.3±13.3 |
| 24-h diastolic BP | 81.7±7.8 |
| 24-h mean arterial pressure | 104.5±8.1 |
| Maximum systolic BP | 206.2±24.8 |
| Urinary sodium (mmol/24 h) | 126 (78, 188) |
| Proteinuria (mg/24 h) | 586 (136, 1600) |
| Albuminuria (mg/24 h) | 327 (22, 1100) |
Data are presented as n (%), mean ± SD, or median (interquartile range).
Table 2.
Values during low salt and high salt periods (n=20)
| Characteristics | n | High Salt | Low Salt | ∆ (High Salt – Low Salt) | P |
|---|---|---|---|---|---|
| 24-h systolic BP (mmHg) | 20 | 154.6±11.9 | 144.9±13.1 | 9.7 [4.5 to 14.8] | <0.001 |
| 24-h diastolic BP (mmHg) | 20 | 83.3±9.0 | 79.4±9.4 | 3.9 [1.3 to 6.4] | <0.01 |
| 24-h mean arterial pressure (mmHg) | 20 | 106.7±8.7 | 100.9±9.7 | 5.8 [2.6 to 9.1] | <0.01 |
| Maximum systolic BP (mmHg) | 20 | 212.7±25.7 | 198.9±26.6 | 13.8 [0.5 to 27.1] | 0.04 |
| Extracellular volume (L) | 18 | 20.0±3.7 | 19.2±3.7 | 0.8 [0.4 to 1.2] | <0.01 |
| Weight (kg) | 20 | 86.4±12.6 | 86.0±12.2 | 0.4 [0.0 to 0.8] | 0.03 |
| Plasma renin (pmol/L) | 20 | 16.5 (8.5–47.0) | 64.5 (32.0–117.5) | −48 (−70.5, −23.5) | <0.001 |
| Plasma aldosterone (mU/L) | 20 | 33.3 (25.0–58.8) | 87.1 (29.8–133.5) | −53.8 (−74.7, −4.8) | <0.001 |
| Proteinuria (mg/24 h) | 19 | 835 (185–1600) | 493 (123–1300) | 342 (62, 300) | <0.01 |
| Albuminuria (mg/24 h) | 19 | 291 (40–1000) | 143 (16–889) | 148 (24, 111) | <0.001 |
| PWV (m/s) | 16 | 11.1±2.3 | 10.5±2.5 | 0.5 [−0.2 to 1.2] | 0.13 |
| AI (%) | 19 | 28.9±8.8 | 27.2±11.5 | 1.7 [−2.6 to 6.0] | 0.42 |
| Sodium excretion (mmol/24 h) | 19 | 168 (146–219) | 75 (58–112) | 93 (88, 107) | <0.001 |
| Potassium excretion (mmol/24 h) | 19 | 57±18 | 57±21 | 0 [−6 to 6] | 0.91 |
| Energy intake (from diet history) | 20 | 6600±1400 | 6800±1500 | −200 [−700 to 300] | 0.32 |
| Sodium intake (from diet history)a | 20 | 53 (43–66) | 56 (48–63) | 3 (−5, 3) | 0.31 |
Data are presented as the mean ± SD, median (interquartile range), or mean [95% confidence interval]. The change between interventions was analyzed using the paired t test with normally distributed data and the Wilcoxon signed-rank test for non-normal data.
Does not include sodium from study medication.
Effect of Dietary Sodium Restriction on Ambulatory BP and Other Outcomes
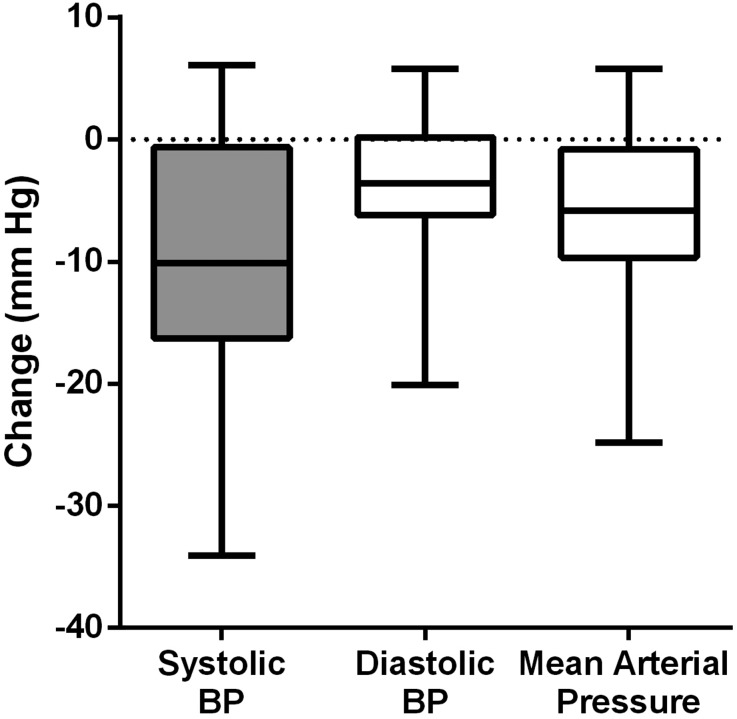
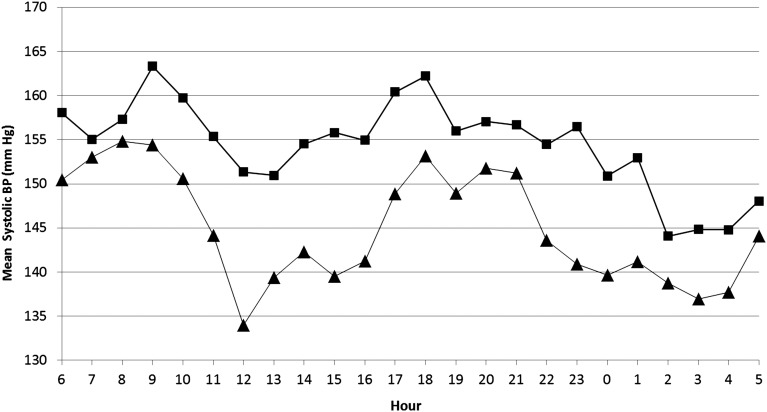
Ambulatory BP changes are shown in Table 2 and Figure 2. A mean reduction of 9.7/3.9 mmHg (systolic BP [SBP]/diastolic BP [DBP]) was achieved from the high salt period to the low salt period. BP reductions were consistent over the 24-hour period, with no significant difference between reductions in the daytime (6 am to 10 pm) and night-time (10 pm to 6 am) periods (P=0.75) (Figure 3).
Figure 2.
Effect of high sodium versus low sodium intake on BP values using 24-hour ambulatory BP monitoring. The central line denotes the median with the box indicating the interquartile range and whiskers indicating the range of change in BP measures from the high salt to the low salt period.
Figure 3.
Comparison of mean systolic BP values over 24 hours during high (▪) and low (▲) salt periods. Systolic BP was reduced consistently during the day (06:00–22:00 hours) and night (22:00–6:00 hours) periods.
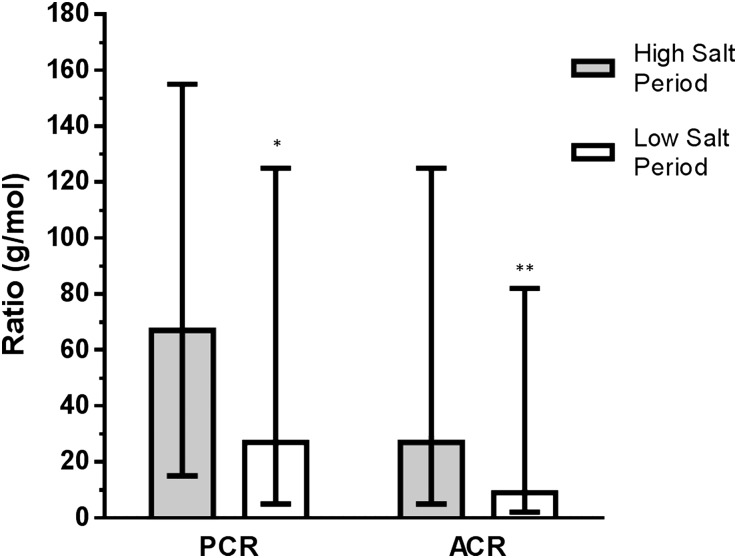
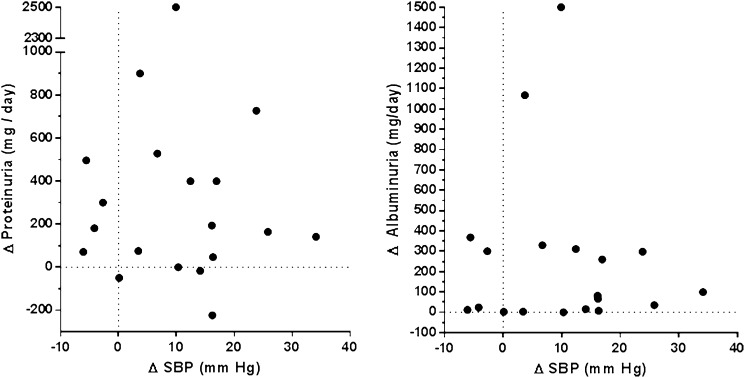
Fluid volume, body weight, proteinuria, and albuminuria were also reduced in the low salt period. Plasma renin and plasma aldosterone increased, whereas the pulse wave velocity (PWV) and the augmentation index (AI) did not change (Table 2). Figure 4 shows changes in the protein/creatinine ratio and the albumin/creatinine ratio. Changes in proteinuria and albuminuria were not significantly correlated with SBP change (r=−0.03 [P=0.09] and r=−0.10 [P=0.69], respectively) (Figure 5).
Figure 4.
Median urinary protein/creatinine ratio (PCR) and albumin/creatinine ratio (ACR) during the high salt and low salt periods. Error bars indicate interquartile range.*P<0.01; **P<0.001 for difference from high salt period. PCR and ACR were significantly reduced on a low-sodium diet compared with a high-sodium diet.
Figure 5.
Relationship between change in systolic BP (SBP) and change in proteinuria (left) or albuminuria (right) from the high salt period to the low salt period.
One patient had missing data for urinary measures (sample not collected, n=1), and two for fluid measurement (presence of a pacemaker contraindicating measurement [n=1]; data loss [n=1]). PWV and AI measurements of acceptable quality could not be recorded in four patients and one patient, respectively.
Sensitivity analyses using analysis of covariance were performed (see Supplemental Table 1). All treatment effects were independent of treatment order.
Discussion
This is the first double-blind randomized controlled trial to assess the effect of sodium restriction on ambulatory BP and other cardiovascular risk factors in nondialyzed, nontransplanted CKD patients. We found that dietary sodium restriction significantly decreased ambulatory BP by 10/4 mmHg SBP/DBP over the 24-hour period. In addition, considerable reductions in extracellular volume, proteinuria, and albuminuria were found, with proteinuria and albuminuria changes occurring independent of BP changes.
The effect of sodium intake on BP is traditionally thought to be driven primarily through changes in fluid volume, mediated by the renin-angiotensin-aldosterone system,23 although recent research indicates that other mediators (e.g., vascular stiffness or inflammation) may play a more pronounced role than once thought.11 Fluid overload is a strong predictor of cardiovascular events and renal function decline in CKD.9 This study found that reducing dietary sodium intake by 100 mmol reduced extracellular volume by 0.8 L with concurrent BP reductions of approximately 10/4 mmHg SBP/DBP, a considerable magnitude of change comparable with that expected from the addition of an antihypertensive medication.24 If such a gradient were found to be sustainable, this potentially translates into significantly reduced heart disease risk, based on the results of a recent meta-analysis (not specific to renal disease) concluding that lowering SBP by 10 mmHg or DBP by 5 mmHg corresponds to a 22% reduction in risk of coronary heart disease events and a 41% reduction in stroke risk.25 In a study of 217 veterans with CKD, Agarwal and Andersen found that a 1 SD increase of SBP (16.3 mmHg) increased the risk of death by 43% (hazard ratio, 1.43; 95% confidence interval, 1.08 to 1.90), whereas risk of progression to ESRD was increased 3-fold (hazard ratio, 3.04; 95% confidence interval, 2.13 to 4.35).26
There are few randomized controlled trials of salt restriction in CKD to which the results of this study can be compared. Fine et al. evaluated the effect of adding 60 mmol of supplementary sodium to the usual diet of 20 peritoneal dialysis patients and found increases of 9/5 mmHg SBP/DBP after 6 weeks.19 Increases were 13/5 mmHg in a subgroup of hypertensive patients, although this did not reach statistical significance, most likely due to insufficient power, with a small sample size (n=10).19 The Holland Nephrology Study (HONEST) found reductions of 8/7 mmHg in patients with nondiabetic nephropathy and background treatment with an angiotensin converting enzyme inhibitor,10 and Vogt et al. found reductions of 6/3 SBP/DBP in 33 proteinuric patients without diabetes.11 These BP reductions are comparable with those seen in this study, although differences in CKD stage make it difficult to make direct comparisons. In addition, these studies used clinic BP rather than ambulatory BP measurements.27 Nevertheless, these studies indicate a degree of generalizability of the BP results of this study to the larger CKD population.
Although it is comparable with other studies in CKD, the magnitude of BP reduction seen in this study is larger than that usually found in sodium restriction studies in populations without renal impairment. A recent meta-analysis of sodium-restriction trials in diabetes found reductions of 5/3 mmHg with a 130 mmol sodium reduction in hypertensive patients with diabetes.7 Other meta-analyses in hypertensive patients or individuals without CKD indicated that SBP reductions of 4–5 mmHg and DBP reductions of 2–3 mmHg were commonly found with an approximately 100 mmol change in sodium intake.28–30 Although it is difficult to directly compare studies without taking the many potential confounding factors into consideration, BP reductions found in the LowSALT CKD study were nearly double those seen in the aforementioned meta-analyses, providing support for the hypothesis that CKD patients may have increased susceptibility to the adverse effects of excessive dietary sodium.31,32 Nevertheless, further research with larger sample sizes is needed to confirm this.
A potential contributor toward the large degree of BP reduction observed is the high use of antihypertensive medication. With a mean use of 3–4 antihypertensive medications, our study population was likely to have a considerable proportion of individuals with resistant hypertension, a disorder characterized by persistently high BP despite multiple antihypertensive medications.33 Pimenta et al. examined the effects of sodium restriction in 12 patients with resistant hypertension who were taking 3.4±0.5 medications and found that SBP/DBP was 23/9 mmHg lower after 1 week on the low-sodium diet compared with the high-sodium diet (50 versus 250 mmol). The authors concluded that patients with resistant hypertension are extremely salt sensitive.34
Reduced diurnal variation in BP (reduced or absent BP reduction at night compared with day) has been identified as a risk factor for CVD and renal disease progression in CKD patients.27,35 We found no shift in diurnal variation, with SBP reduced consistently over the 24-hour period. The effect of dietary sodium restriction on diurnal variation of BP has not previously been examined in CKD patients, but studies in other populations have also found that reducing dietary sodium does not change diurnal variation.34,36
In the low salt period, 24-hour proteinuria and albuminuria were substantially reduced by 40%–50% compared with the high salt period, which is comparable with the proteinuria reduction found in the HONEST study (49%).10 In a meta-analysis of patients with diabetes or vascular disease, a 50% decrease in the urinary albumin/creatinine ratio was associated with a 15% lower risk of all-cause mortality, a 16% lower risk of cardiovascular event, and a 27% lower risk of renal outcomes.12 The relationship between sodium intake and proteinuria is thought to be mediated by increased glomerular capillary pressure.5 Changes in proteinuria and albuminuria were independent of BP changes, suggesting that clinical benefits of sodium restriction may be over and above those related to systemic BP reduction. Vogt et al. also found that changes in proteinuria with sodium restriction were independent of the effect on BP, concluding that the relationship between sodium intake and proteinuria may have been driven by changes in glomerular structure or function.11
Excess sodium may have direct toxic effects on blood vessels through mediating factors such as oxidative stress, inflammation, and endothelial cell dysfunction as well as increasing vascular stiffness.13,14 Further research into the link between sodium intake and mediators such as marinobufagenin, a vasoconstrictor linked to sodium loading37 and shown to cause renal ischemia in rats,38 may be warranted. Arterial stiffness was reduced in the low salt period compared with the high salt period, but these changes were not statistically significant. Pimenta et al. also found a trend for reduced arterial stiffness (measured using the same techniques).34 Larger studies of sodium restriction in CKD patients with longer follow-up are warranted.
In this study, reduction of risk factors was achieved through dietary sodium restriction without significant adverse effects, except for symptomatic hypotension, which was resolved by modification of the antihypertensive regimen. Despite efforts to keep antihypertensive medications stable during the trial, four patients required medication changes during the study. This could have been avoided by ceasing medications before baseline, but this was outside the scope of this study. Of these four patients, three patients had a medication ceased in the low salt period due to symptomatic hypotension, and one had a dose reduction of a medication in the high salt period for reasons unrelated to the study. For the three patients who had medication cessation in the low salt period, this would have underestimated the effect of sodium restriction on BP; however, BP reductions were still considerable (SBP reduction of 16–24 mmHg and DBP reduction of 4–11 mmHg). The reduction of dosage that occurred in the high salt period for one patient could have overestimated the magnitude of BP change for this patient; however, when the analyses were repeated with this patient excluded, the results did not change. Therefore, we conclude that this did not introduce bias.
Given that this study was designed as a proof of concept, limitations to translating these findings to practice include the short intervention duration, difference in baseline characteristics between completers and withdrawers, and small sample size. Phase 2 of this study will entail follow-up to 6 months and will assess long-term effects of sodium restriction on the outcomes presented in this article as well as assessment of dietary compliance to determine the translation of benefits outside of the clinical trial setting.39
The difference between weight and BMI between those who completed and those who withdrew from the study may have affected the study results; however, reasons for attrition were not related to the treatment but rather to the intensity of the data collection schedule and to medical reasons unrelated to the study. Although the small sample size may have limited the generalizability of the results to other CKD populations, it was sufficient to afford an observed power of 80%. Moreover, our results are consistent with those found in other studies of salt restriction in CKD.10,11,19,40 This study differs from these previous studies by blinding participants and investigators to allocation, using 24-hour ambulatory (rather than clinic) BP to measure BP changes, comprehensively measuring dietary and medication adherence, and using sodium supplements versus placebo control to facilitate stability of other dietary factors between the interventions. Furthermore, we assessed potential confounding factors, such as body weight, energy intake, and potassium intake, to ensure that the observed results were attributable to a change in sodium intake.
This study represents the first double-blind randomized controlled trial assessing the effect of salt restriction on ambulatory BP and 24-hour proteinuria in hypertensive, nondialyzed, nontransplanted CKD patients. We found that sodium restriction reduced extracellular volume, produced sizeable BP reductions, and halved proteinuria and albuminuria without significant adverse effects in CKD patients. These reductions in proteinuria and albuminuria were found even in patients who did not achieve clinically significant BP reductions. This study indicates that CKD patients may be particularly susceptible to the adverse effects of excessive sodium intake, and provides high-quality evidence to support sodium restriction in CKD.
Concise Methods
This study was a 6-week single-center double-blind randomized crossover trial and comprises the first phase of the LowSALT CKD study (total duration of 6 months). A detailed description of the LowSALT CKD study was published elsewhere.39 Trial registration numbers for the LowSALT CKD study are as follows: Universal Trial Number U1111-1125-2149, and Australian New Zealand Clinical Trials Registry Number ACTRN12611001097932.
Participants
Hypertensive patients with stage 3 or stage 4 CKD (GFR 15–59 ml/min per 1.73 m2 nondialyzed, nontransplanted) aged ≥18 years with systolic BP of 130–169 mmHg and diastolic BP ≥70 mmHg were recruited through the outpatient clinics of a large metropolitan hospital. Exclusion criteria included salt-wasting CKD, pregnant or breastfeeding, current prescription of medications providing >20 mmol sodium per day, life expectancy <6 months, current involvement in another intervention study, or insufficient mental or physical capacity to adhere to the study protocol. Ethical approval was granted by the institution’s human research ethics committees. All participants provided informed consent before trial participation. This trial adhered to the ethical principles set by the Declaration of Helsinki.
Design
After baseline measurements were taken, participants were counseled to follow a low-sodium diet (goal 60–80 mmol) for the 6-week study. Dietary education was individualized to the participant’s food preferences and was provided by an accredited practicing dietitian. Participants were also offered a variety of low-sodium foods (one preprepared meal per day, snack foods, breakfast cereal, cheese, and bread) throughout the study. After a 1-week run-in period on a low-sodium diet, participants were randomized to high sodium intake (low-sodium diet with a goal of 60–80 mmol, plus 120 mmol sodium per day via slow-release sodium tablets) or low sodium intake (low-sodium diet with a goal of 60–80 mmol, plus a matched quantity of placebo capsules) for 2 weeks. After a 1-week washout (continued low-sodium diet), patients crossed over to the other intervention. The target total sodium intakes (dietary intake plus study medication) were 180–200 mmol in the high salt period and 60–80 mmol in the low salt period. Randomization was performed by an external statistical consultant, with the study medication packaged offsite and labeled with the study numbers and intervention order. Participants, investigators, and outcome assessors were blinded to the allocation.
Outcome Measurements
The TM-2430 ambulatory blood pressure monitor (A&D Medical, Thebarton, Australia) was used to perform 24-hour ambulatory BP measurements at baseline and at the end of each intervention. Measurements were recorded every 20 minutes during the day (6 am to 10 pm) and 30 minutes at night (10 pm to 6 am) for a 24-hour period. A 24-hour urine sample was collected concurrently with ambulatory BP measurements and analyzed for protein, albumin, creatinine, and sodium concentration.
In addition, fluid status, body weight, arterial stiffness, and plasma renin and aldosterone were measured at baseline and the end of each intervention. Blood samples were collected after an overnight fast. Fluid status was assessed by measuring extracellular volume using a body composition monitor (Fresenius Medical Care, Hamburg, Germany). Body weight was recorded using a standard protocol. Arterial stiffness was assessed by carotid-femoral PWV and by radial pulse wave analysis (PWA) (used to measure AI) measured using the SphygmoCor CPV (AtCor Medical, Sydney, Australia). Both PWV and PWA were measured in duplicate at each time point. A SEM value ≤20% of the PWV value was used to indicate an acceptable quality PWV measurement. Quality of PWA measurements was assessed as recommended in the SphygmoCor manual.41 Participants, investigators, and outcome assessors were blinded to the results of all outcomes.
Study Compliance
Dietary intake was measured using a combination of objective and self-reported measures to enhance validity.42 The 24-hour urinary sodium excretion was assessed, with participants and investigators blinded to the results. In addition, information about dietary intake at baseline and over the intervention periods was collected using an in-depth diet history interview facilitated by the study dietitian. These data were entered into FoodWorks 7 (version 7.0.2915; Xyris Software, New York, NY) using the Nuttab 2010 (for general unbranded foods and known recipes), AusBrands 2012 (where specific brand was known), and AusFoods 2012 (for convenience or preprepared foods in which the recipe is unknown) databases.
Medication compliance was measured by pill counts, as well as a patient completed daily record of doses taken/missed. Patients were considered compliant to the study medication if ≤10% tablets were missed over the entire intervention (equal to ≤12 mmol lower sodium intake per day from study medication).
Statistical Analyses
The primary outcome of interest was the change in 24-hour SBP from matched pairs of study participants (by crossover design) from the high salt period to the low salt period. Prior data from matched pairs indicated a mean response to sodium reduction of a 5 mmHg SBP decrease with a SD of 7 mmHg (Todd et al., unpublished observations). This study required 23 participants across both treatments to achieve 90% power, whereas 17 participants were required to achieve 80% power with an error of 0.05.
Results are presented as the mean ± SD for normally distributed data and the median (interquartile range) for data with non-normal distribution. Data analysis was initially performed blinded to treatment order and then was performed unblinded to confirm treatment order. Change from the high salt period to the low salt period was analyzed using paired t test with normally distributed data and the Wilcoxon signed-rank test for non-normal data. To test for independence of outcomes, variation due to treatment order or regression to the mean effects, analysis of covariance was conducted: treatment type, baseline values of the outcome parameter of interest and treatment order were included in the model and observations were clustered by study number to account for correlation of within-patient results. To test for predictors of salt sensitivity and differences between baseline characteristics in those who completed the study and those who withdrew, the t test was used for continuous outcomes with normal distribution, the Wilcoxon rank-sum test was used for continuous outcomes with non-normal distribution, and the chi-squared test was used for categorical outcomes. All statistical analyses were performed using STATA software (version 12) with probability values <0.05 considered to be significant.
Disclosures
None.
Acknowledgments
The authors acknowledge Fresenius Medical Care for providing the body composition monitor; Freedom Foods, Norco, Real Foods, Carman’s Fine Foods, Sanitarium Health & Wellbeing Company, Rosella, and Diego’s for donating food for the trial; Dr. Eduardo Pimenta for providing consultation on the design of the trial; the study nurse, Rachael Hale, for serving as the trial coordinator; Dr. Paul Taylor (University of Queensland) for providing the aldosterone assay; and the Princess Alexandra Hospital Renal Outpatient Department for providing organizational support.
This study was funded by research grants from the Princess Alexandra Hospital Private Practice Trust Fund and Kidney Health Australia. E.M. is funded by an Australian Postgraduate Association scholarship through University of Queensland. K.C. is a current recipient of a Queensland Government Health Research Fellowship and Lions Senior Medical Research Fellowship. D.J. is a current recipient of a Queensland Government Health Research Fellowship.
The results of this trial were presented in abstract form at the World Congress of Nephrology, May 31–June 4, 2013, in Hong Kong.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sodium Reduction in CKD: Suggestively Hazardous or Intuitively Advantageous?,” on pages 1931–1933.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013030285/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Agrawal V, Marinescu V, Agarwal M, McCullough PA: Cardiovascular implications of proteinuria: An indicator of chronic kidney disease. Nat Rev Cardiol 6: 301–311, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N, European Uremic Toxin Work Group : Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Basile JN: Recognizing the link between CKD and CVD in the primary care setting: Accurate and early diagnosis for timely and appropriate intervention. South Med J 100: 499–505, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Thijssen S, Kitzler TM, Levin NW: Salt: Its role in chronic kidney disease. J Ren Nutr 18: 18–26, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Wright JA, Cavanaugh KL: Dietary sodium in chronic kidney disease: A comprehensive approach. Semin Dial 23: 415–421, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suckling RJ, He FJ, Macgregor GA: Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev 12: CD006763, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Graudal NA, Hubeck-Graudal T, Jürgens G: Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens 25: 1–15, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Essig M, Escoubet B, de Zuttere D, Blanchet F, Arnoult F, Dupuis E, Michel C, Mignon F, Mentre F, Clerici C, Vrtovsnik F: Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant 23: 239–248, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, Navis G, Laverman GD, Holland Nephrology Study Group : Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 343: d4366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmieder RE, Mann JF, Schumacher H, Gao P, Mancia G, Weber MA, McQueen M, Koon T, Yusuf S, ONTARGET Investigators : Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 22: 1353–1364, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM: Low-sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens 23: 826–835, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rugale C, Delbosc S, Cristol JP, Mimran A, Jover B: Sodium restriction prevents cardiac hypertrophy and oxidative stress in angiotensin II hypertension. Am J Physiol Heart Circ Physiol 284: H1744–H1750, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, Mann JI, Walker RJ: Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr 91: 557–564, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Cianciaruso B, Bellizzi V, Minutolo R, Colucci G, Bisesti V, Russo D, Conte G, De Nicola L: Renal adaptation to dietary sodium restriction in moderate renal failure resulting from chronic glomerular disease. J Am Soc Nephrol 7: 306–313, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Bellizzi V, Di Iorio BR, De Nicola L, Minutolo R, Zamboli P, Trucillo P, Catapano F, Cristofano C, Scalfi L, Conte G, ERIKA Study Group : Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int 71: 245–251, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Koomans HA, Roos JC, Dorhout Mees EJ, Delawi IM: Sodium balance in renal failure. A comparison of patients with normal subjects under extremes of sodium intake. Hypertension 7: 714–721, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Fine A, Fontaine B, Ma M: Commonly prescribed salt intake in continuous ambulatory peritoneal dialysis patients is too restrictive: Results of a double-blind crossover study. J Am Soc Nephrol 8: 1311–1314, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Keven K, Yalçin S, Canbakan B, Kutlay S, Sengül S, Erturk S, Erbay B: The impact of daily sodium intake on posttransplant hypertension in kidney allograft recipients. Transplant Proc 38: 1323–1326, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, Kishida M, Hitomi H, Kiyomoto H, Miyashita T, Mori N, Urushihara M, Kobori H, Imanishi M: Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension 58: 205–211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruilope LM, Casal MC, Guerrero L, Alcázar JM, Férnandez ML, Lahera V, Rodicio JL: Sodium intake does not influence the effect of verapamil in hypertensive patients with mild renal insufficiency. Drugs 44[Suppl 1]: 94–98, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Bie P, Sandgaard NC: Determinants of the natriuresis after acute, slow sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R1–R10, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Heran BS, Wong MM, Heran IK, Wright JM: Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev (4): CD003823, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law MR, Morris JK, Wald NJ: Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338: b1665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal R, Andersen MJ: Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 69: 1175–1180, 2006 [DOI] [PubMed] [Google Scholar]
- 27.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P, European Society of Hypertension Working Group on Blood Pressure Monitoring : European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 21: 821–848, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Jürgens G, Graudal NA: Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride. Cochrane Database Syst Rev (1): CD004022, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Geleijnse JM, Kok FJ, Grobbee DE: Blood pressure response to changes in sodium and potassium intake: A metaregression analysis of randomised trials. J Hum Hypertens 17: 471–480, 2003 [DOI] [PubMed] [Google Scholar]
- 30.He FJ, MacGregor GA: Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev (3): CD004937, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Kimura G, Dohi Y, Fukuda M: Salt sensitivity and circadian rhythm of blood pressure: The keys to connect CKD with cardiovascular events. Hypertens Res 33: 515–520, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Koomans HA, Roos JC, Boer P, Geyskes GG, Mees EJ: Salt sensitivity of blood pressure in chronic renal failure. Evidence for renal control of body fluid distribution in man. Hypertension 4: 190–197, 1982 [DOI] [PubMed] [Google Scholar]
- 33.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM: Resistant hypertension: Diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51: 1403–1419, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, Calhoun DA: Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension 54: 475–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal R: Ambulatory blood pressure and cardiovascular events in chronic kidney disease. Semin Nephrol 27: 538–543, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization: Effect of Reduced Sodium Intake on Blood Pressure, Renal Function, Blood Lipids and Other Potential Adverse Effects, Geneva, Switzerland, World Health Organization, 2012 [Google Scholar]
- 37.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY: Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J Hypertens 23: 1515–1523, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Fedorova LV, Raju V, El-Okdi N, Shidyak A, Kennedy DJ, Vetteth S, Giovannucci DR, Bagrov AY, Fedorova OV, Shapiro JI, Malhotra D: The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: Implication of epithelial-to-mesenchymal transition. Am J Physiol Renal Physiol 296: F922–F934, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Hale RE, Campbell KL: The effect of lowering salt intake on ambulatory blood pressure to reduce cardiovascular risk in chronic kidney disease (LowSALT CKD study): Protocol of a randomized trial. BMC Nephrol 13: 137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konishi Y, Okada N, Okamura M, Morikawa T, Okumura M, Yoshioka K, Imanishi M: Sodium sensitivity of blood pressure appearing before hypertension and related to histological damage in immunoglobulin a nephropathy. Hypertension 38: 81–85, 2001 [DOI] [PubMed] [Google Scholar]
- 41.SphymoCor: SphymoCor CMV Research Applications Manual, Sydney, Australia, AtCor Medical Pty. Ltd., 2010
- 42.McMahon EJ, Campbell KL, Mudge DW, Bauer JD: Achieving salt restriction in chronic kidney disease. Int J Nephrol 2012: 720429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]