Abstract
Systemic inflammation, as evidenced by elevated inflammatory cytokines, is a feature of advanced renal failure and predicts worse survival. Dialysate IL-6 concentrations associate with variability in peritoneal small solute transport rate (PSTR), which has also been linked to patient survival. Here, we determined the link between systemic and intraperitoneal inflammation with regards to peritoneal membrane function and patient survival as part of the Global Fluid Study, a multinational, multicenter, prospective, combined incident and prevalent cohort study (n=959 patients) with up to 8 years of follow-up. Data collected included patient demographic characteristics, comorbidity, modality, dialysis prescription, and peritoneal membrane function. Dialysate and plasma cytokines were measured by electrochemiluminescence. A total of 426 survival endpoints occurred in 559 incident and 358 prevalent patients from 10 centers in Korea, Canada, and the United Kingdom. On patient entry to the study, systemic and intraperitoneal cytokine networks were dissociated, with evidence of local cytokine production within the peritoneum. After adjustment for multiple covariates, systemic inflammation was associated with age and comorbidity and independently predicted patient survival in both incident and prevalent cohorts. In contrast, intraperitoneal inflammation was the most important determinant of PSTR but did not affect survival. In prevalent patients, the relationship between local inflammation and membrane function persisted but did not account for an increased mortality associated with faster PSTR. These data suggest that systemic and local intraperitoneal inflammation reflect distinct processes and consequences in patients treated with peritoneal dialysis, so their prevention may require different therapeutic approaches; the significance of intraperitoneal inflammation requires further elucidation.
Individual differences in peritoneal membrane function have been shown to influence clinical outcomes in patients undergoing peritoneal dialysis (PD). In particular a high peritoneal solute transport rate (PSTR) has been linked to worse survival.1 This association has been considered to be due to one of two main mechanisms: less efficient ultrafiltration and excess fluid reabsorption as a consequence of early loss of the glucose gradient during the dialysis dwell2 or because high PSTR is a manifestation of the systemic inflammation commonly seen in advanced kidney failure.3,4 The picture is further complicated by changes in PSTR due to acquired membrane injury with time on PD,5 where in addition to reducing ultrafiltration by the above mechanisms it can be associated with a reduction in membrane efficiency (reduced osmotic conductance).6,7
More recently it was shown that PSTR is associated with the amount of IL-6 in drained dialysate, which is present in higher concentrations than can be explained by diffusion from plasma, implying its local production.8,9 Furthermore, individuals with genetic polymorphisms associated with increased IL-6 production, both systemically and locally, have increased PSTR10,11 and worse survival.12 Suggesting that this association is not the main link between PSTR and survival is the observation that the association is confined to patients treated with continuous ambulatory PD,1 whereas in more recent cohorts where automated PD (APD) predominates the effect disappears13,14 or even reverses.15 To date no studies have linked dialysate cytokine profiles to survival, and only small studies have suggested that dialysate IL-6 appearance reflects a wider activation of the local cytokine network.9,16 The purpose of this first major analysis of the Global Fluid Study was to test the following hypotheses: (1) that intraperitoneal and systemic inflammation are distinct entities, (2) that local not systemic inflammation is associated with membrane function (PSTR), (3) that different clinical factors are associated with local and systemic inflammation, and (4) that systemic but not local inflammation predicts patient survival.
Results
Description of Incident and Prevalent Cohorts
The clinical characteristics of the 959 patients included in the analyses are shown in Table 1. In comparing the incident and prevalent groups, the latter used more icodextrin and APD, had greater total dialysate volumes, and had lower urine volume. Although the use of APD was relatively low, it was much more likely to be prescribed in patients with faster PSTR (0.78 versus 0.7; P=0.0005).
Table 1.
Study population characteristics
| Characteristic | Incident (n=575) | Center Effect (P Value) | Prevalent (n=384) | Center Effect (P Value) | Difference between Incident and Prevalent (P Value) |
|---|---|---|---|---|---|
| Age (yr) | 55.6±15.3 | 0.001 | 54.2±15.2 | 0.037 | NS |
| Women (%) | 38.4 | NS | 46.4 | NS | 0.05 |
| Korean (%) | 37.2 | 36.2 | NS | ||
| Body mass index (kg/m2) | 25.2±4.7 | <0.001 | 25.3±4.7 | <0.001 | NS |
| Total dialysate volume (L) | 7.96±1.29 | <0.001 | 8.38±1.87 | <0.001 | <0.001 |
| BP (mmHg) | 136±21/80±12 | <0.001 | 135±20/81±12 | NS | NS |
| Median duration of PD (d) | 40 (28, 55) | <0.001 | 360 (169, 609) | <0.001 | <0.001 |
| 4-hour PSTR | 0.71±0.12 | <0.001 | 0.71±0.12 | <0.001 | NS |
| Ultrafiltration capacity (ml) | |||||
| High (4% glucose) | 696.9±18.4 | <0.001 | 721.9±22.5 | 0.03 | <0.001 |
| Medium (2.5% glucose) | 229.3±17.6 | <0.001 | 248.6±17.0 | <0.001 | <0.001 |
| Albumin | 35.0±5.2 | <0.001 | 35.4±4.8 | 0.06 | NS |
| Hemoglobin | 11.0±2.2 | <0.001 | 11.2±1.8 | <0.001 | NS |
| Median urine volume (L) | 0.90 (0.46, 1.44) | <0.001 | 0.60 (0.19, 1.21) | 0.001 | <0.001 |
| Biocompatible solution use (%) | 19.3 | <0.001 | 16.1 | <0.001 | NS |
| Icodextrin solution use (%) | 19.1 | <0.001 | 28.0 | <0.001 | 0.002 |
| Comorbidity (low/intermediate/high) (%) | 35.6/56.8/7.6 | <0.001 | 43.9/48.5/7.6 | <0.001 | NS |
| Use of APD | 6.0 | <0.001 | 15.1 | <0.001 | <0.001 |
Values expressed with a plus/minus sign are the mean ± SD. Median values are accompanied by interquartile ranges in parentheses for skewed variable. NS, not significant.
For most patient characteristics and prescription practices, there were highly significant center effects (for intracluster correlations, see Supplemental Table 1) and for this reason all linear regression models used multilevel methods.
Demonstration That Local Peritoneal and Systemic Inflammation Is Uncoupled
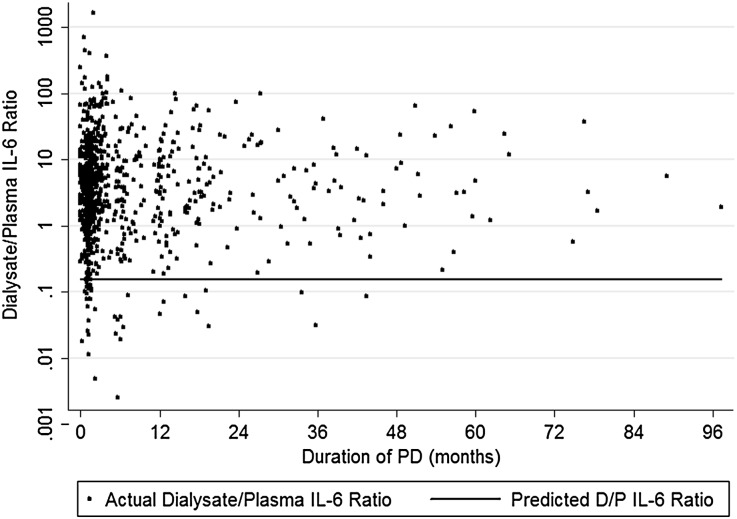
To establish that dialysate IL-6 is representative of a localized inflammatory process, it is necessary to demonstrate both local production and an association with other pro-inflammatory cytokines that is independent of plasma. Taking molecular size into account, 87% of patients had dialysate IL-6 concentrations higher than predicted by diffusion across the peritoneal membrane (Figure 1). Values for IL-1, TNF-α, and IFN-γ were 33.3%, 6.9%, and 45.7%, respectively. Within the peritoneal and circulatory compartments there were moderate to strong correlations between the measured cytokines reflecting localized activation of proinflammatory networks (Table 2). In contrast, correlations between dialysate and plasma were absent or weaker than those seen within blood or dialysate.
Figure 1.
Graph of dialysate-to-plasma (D/P) concentration ratio (y-axis) for IL-6 concentrations. The line represents the ratio predicted by the three-pore model (0.145), so all points above this line are predicted to represent local production, which occurs in the majority of patients (87%).
Table 2.
Correlation coefficients between cytokine concentrations
| Variable | Dialysate | Plasma | |||||
|---|---|---|---|---|---|---|---|
| IL-1β | IFN-γ | IL-6 | TNF-α | IL-1β | IFN-γ | TNF-α | |
| Incident | |||||||
| Dialysate (n=563) | |||||||
| IFN-γ | 0.65a | ||||||
| IL-6 | 0.29a | 0.29a | |||||
| TNF-α | 0.82a | 0.74a | 0.42a | ||||
| Plasma (n=557) | |||||||
| IL-1β | −0.004 | 0.005 | −0.01 | −0.07 | |||
| IFN-γ | 0.0002 | −0.01 | 0.11 | −0.007 | 0.1 | ||
| TNF-α | −0.05 | −0.01 | 0.15b | −0.03 | 0.08 | 0.5a | |
| IL-6 | 0.05 | 0.09 | 0.28a | 0.04 | 0.13b | 0.25a | 0.35a |
| Prevalent | |||||||
| Dialysate (n=378) | |||||||
| IFN-γ | 0.61a | ||||||
| IL-6 | 0.21a | 0.32a | |||||
| TNF-α | 0.76a | 0.75a | 0.4a | ||||
| Plasma (n=379) | |||||||
| IL-1β | 0.12 | 0.1 | −0.02 | 0.09 | |||
| IFN-γ | 0.04 | 0.05 | −0.001 | 0.05 | 0.12 | ||
| TNF-α | −0.07 | −0.06 | 0.11 | 0.01 | 0.14 | 0.43a | |
| IL-6 | −0.02 | 0.11 | 0.27a | 0.07 | 0.15 | 0.24a | 0.29a |
There were 548 and 374 common observations between dialysate and plasma samples for incident and prevalent, patients respectively.
Sidak adjusted P values ≤0.001.
P=0.01–0.05; otherwise P<0.05.
Local, Not Systemic, Inflammation Is the Main Determinant of PSTR
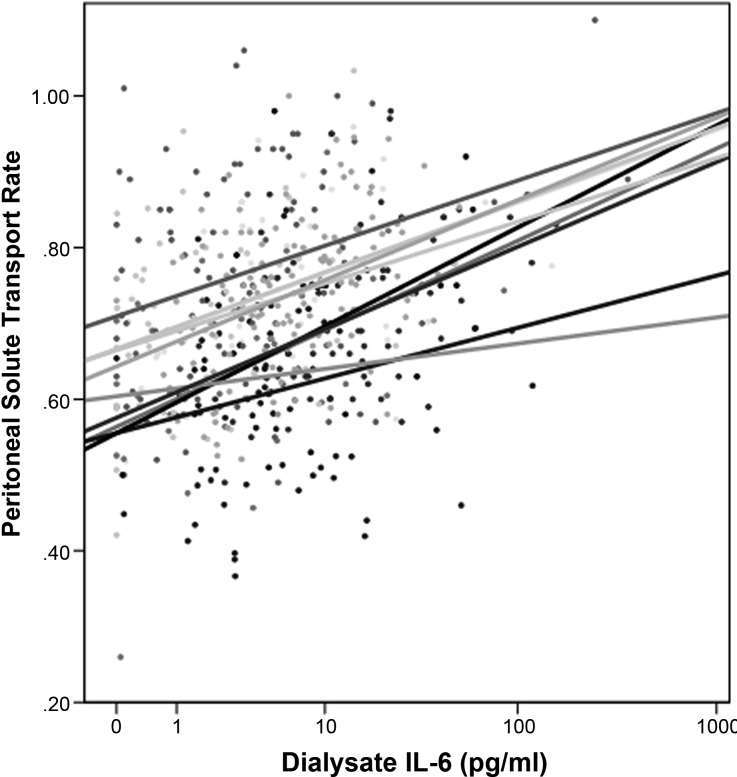
Results of the multivariable, multilevel, linear regression models showing the associations with PSTR are displayed in Table 3. Dialysate IL-6 concentration was the most significant association in both patient cohorts, a pattern observed in all of the participating centers (see Figure 2). This was independent of patient factors (sex, race, body mass index, BP, urine volume, diabetic status) and dialysis prescription, all of which had significant associations. For incident patients, the timing of the initial membrane function assessment had an effect that was not linear for tests done early (i.e., from baseline the PSTR rose for 2 months, increasing by 0.08, then fell to a total gain of 0.06 by 3 months). In prevalent patients, higher PSTR was associated in a linear fashion with longer time on treatment. Cytokine concentrations produced a better model than appearance rates (Δ−2 log likelihood = 36).
Table 3.
Predictors of PSTR
| Variable | Incident | Prevalent | ||
|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| Age (per decade) | 0.001 (−0.005 to 0.008) | 0.7 | −0.004 (−0.012 to 0.004) | 0.4 |
| Body mass index | −0.002a (−0.005 to −0.0001) | 0.04 | −0.0009 (−0.004 to 0.002) | 0.5 |
| APD use | −0.02 (−0.06 to 0.02) | 0.3 | −0.008 (−0.04 to 0.03) | 0.7 |
| Systolic BP (per 10 mmHg) | 0.005a (0.0002 to 0.009) | 0.04 | 0.001 (−0.004 to 0.007) | 0.6 |
| Male sex | 0.02a (0.003 to 0.04) | 0.02 | 0.02a (0.002 to 0.05) | 0.04 |
| Duration of PD | 0.08×monthb (0.03 to 0.13) | <0.001 | 0.01×yearb (0.004 to 0.02) | 0.003 |
| 0.02×montha (0.04 to 0.003) | ||||
| Biocompatible solution use | −0.005 (−0.02 to 0.02) | 0.7 | −0.04a (−0.07 to (−0.004) | 0.03 |
| Icodextrin use | 0.06b (0.03 to 0.09) | <0.001 | 0.04a (0.01 to 0.07) | 0.01 |
| Average glucose concentration (per g/L) | 0.005b (0.002 to 0.007) | <0.001 | 0.005b (0.001 to 0.008) | 0.004 |
| Dialysate IL-6 | 0.08b (0.06 to 0.11) | <0.001 | 0.09b (0.07 to 0.12) | <0.001 |
| Dialysate TNF-α | 0.04 (−0.03 to 0.10) | 0.3 | −0.03 (−0.1 to 0.06) | 0.6 |
| Dialysate IFN-γ | −0.009 (−0.04 to 0.02) | 0.6 | 0.008 (−0.03 to 0.04) | 0.6 |
| Plasma IL-6 | −0.02 (−0.06 to 0.01) | 0.2 | 0.006 (−0.04 to 0.05) | 0.8 |
| Plasma TNF-α | 0.02 (−0.04 to 0.09) | 0.5 | −0.05 (−0.12 to 0.02) | 0.2 |
| Plasma IFN-γ | −0.009 (−0.04 to 0.02) | 0.6 | −0.02 (−0.06 to 0.03) | 0.4 |
| Plasma IL-1β | 0.02 (−0.06 to 0.11) | 0.6 | 0.001 (−0.09 to 0.09) | 0.98 |
| Diabetic | 0.02a (0.001 to 0.05) | 0.04 | 0.004 (−0.03 to 0.03) | 0.8 |
| Comorbidity | 0.0005 (−0.01 to 0.01) | 0.9 | 0.003 (−0.01 to 0.02) | 0.7 |
| Urine volume (per L) | 0.0b (0.01 to 0.04) | <0.001 | 0.02a (0.005 to 0.04) | 0.01 |
| Korean | 0.08a (0.01 to 0.15) | 0.02 | 0.05 (−0.005 to 0.11) | 0.07 |
Data are presented so that coefficients represent the change in dialysate-to-plasma creatinine ratio. A total of 499 and 307 patients were in the final models for incident and prevalent patients, respectively. Cytokine coefficients are per log10 changes in concentration. CI, confidence interval.
P=0.01–0.05.
P<0.01.
Figure 2.
Scatterplot of PSTR measured as 4-hour dialysate-to-plasma creatinine ratio with dialysate IL-6 demonstrating a center effect. Each line represents a center-specific linear regression slope. Dialysate IL-6 is measured on a logarithmic scale. Despite the center effects seen for many of the measurements in GLOBAL the correlation between dialysate IL-6 and PSTR was always observed.
Factors Associated with Local and Systemic Inflammation
Before proceeding to survival analyses, it was necessary to determine the clinical associations with local (Table 4) and systemic (Table 5) inflammation as defined by the dialysate and plasma IL-6 concentrations, respectively.
Table 4.
Predictors of log dialysate IL-6
| Variable | Incident | Prevalent | ||
|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| Age (per decade) | 0.04a (0.01 to 0.06) | 0.002 | 0.05a (0.01 to 0.08) | 0.01 |
| BMI | 0.0009 (−0.007 to 0.009) | 0.8 | 0.008 (−0.003 to 0.02) | 0.2 |
| APD use | −0.06 (−0.21 to 0.10) | 0.5 | 0.2 (−0.004 to 0.3) | 0.06 |
| Systolic BP (per 10 mmHg) | − 0.02b (− 0.03 to − 0.002) | 0.03 | -0.03a (−0.05 to −0.002) | 0.03 |
| Male sex | 0.04 (−0.04 to 0.11) | 0.3 | 0.1 (−0.0003 to 0.2) | 0.051 |
| Duration of PD (per yr) | 0.1 (−0.6 to 0.8) | 0.7 | 0.02 (−0.02 to 0.05) | 0.4 |
| Biocompatible solution use | 0.0007 (−0.09 to 0.09) | 0.99 | 0.1 (−0.04 to 0.3) | 0.1 |
| Icodextrin use | 0.3a (0.2 to 0.4) | <0.001 | 0.2a (0.07 to 0.3) | 0.003 |
| Average glucose concentration (per g/L) | 0.01a (0.004 to 0.02) | 0.006 | −0.004 (−0.02 to 0.01) | 0.5 |
| Dialysate TNF-α | 0.8a (0.6 to 1.0) | <0.001 | 0.7a (0.3 to 1.0) | 0.001 |
| Dialysate IFN-γ | 0.006 (−0.1 to 0.1) | 0.9 | 0.02 (−0.1 to 0.2) | 0.8 |
| Plasma IL-6 | 0.3a (0.2 to 0.4) | <0.001 | 0.3a (0.1 to 0.5) | 0.001 |
| Plasma TNF-α | −0.2 (−0.4 to 0.06) | 0.1 | 0.2 (−0.1 to 0.5) | 0.2 |
| Plasma IFN-γ | 0.06 (−0.06 to 0.19) | 0.3 | −0.1 (−0.3 to 0.03) | 0.1 |
| Plasma IL-1β | −0.1 (−0.3 to 0.3) | 0.9 | −0.05 (−0.5 to 0.4) | 0.8 |
| Diabetic | 0.01 (− 0.08 to 0.10) | 0.8 | 0.05 (−0.08 to 0.2) | 0.4 |
| Comorbidity | 0.02 (−0.03 to 0.06) | 0.5 | −0.004 (−0.06 to 0.05) | 0.9 |
| Urine volume (per L) | 0.03 (−0.02 to 0.08) | 0.2 | -0.1b (−0.2 to −0.02) | 0.01 |
| Korean | −0.02 (−0.2 to 0.2) | 0.8 | −0.2 (−0.5 to 0.1) | 0.3 |
A total of 497 and 327 patients were in the final models for incident and prevalent groups, respectively. Log10 transformations of dialysate IL-6 were used as the dependent variable. Cytokine coefficients are per log10 changes in concentration. Using diabetes status rather than comorbidity was not a significant predictor for either group. CI, confidence interval.
P<0.01.
P=0.01–0.05.
Table 5.
Predictors of log plasma IL-6
| Variable | Incident | Prevalent | ||
|---|---|---|---|---|
| Coefficient | P Value | Coefficient | P Value | |
| Age (per decade) | 0.02a (0.007 to 0.04) | 0.004 | 0.01 (−0.009 to 0.03) | 0.3 |
| Body mass index | 0.0001 (−0.005 to 0.005) | 0.96 | 0.004 (−0.002 to 0.01) | 0.2 |
| APD use | 0.04 (−0.06 to 0.14) | 0.4 | 0.003 (−0.08 to 0.09) | 0.9 |
| Systolic BP (per 10 mmHg) | 0.003 (−0.007 to 0.014) | 0.5 | −0.009 (−0.02 to 0.004) | 0.2 |
| Male sex | 0.05 (−0.001 to 0.09) | 0.06 | 0.02 (−0.03 to 0.07) | 0.5 |
| Duration of PD (per yr) | −0.2 (−0.6 to 0.2) | 0.4 | 0.02b (0.0008 to 0.03) | 0.04 |
| Biocompatible solution use | 0.003 (−0.05 to 0.06) | 0.9 | −0.02 (−0.10 to 0.06) | 0.6 |
| Icodextrin use | 0.04 (−0.02 to 0.11) | 0.3 | −0.02 (−0.09 to 0.05) | 0.6 |
| Average glucose concentration (per g/L) | 0.003 (−0.004 to 0.009) | 0.4 | −0.002 (−0.01 to 0.005) | 0.5 |
| Dialysate IL-6 | 0.13a (0.07 to 0.18) | <0.001 | 0.09b (0.03 to 0.15) | 0.002 |
| Dialysate IFN-γ | 0.07 (−0.003 to 0.15) | 0.06 | 0.04 (−0.04 to 0.1) | 0.3 |
| Dialysate TNF-α | −0.2b (−0.3 to −0.005) | 0.04 | −0.1 (−0.4 to 0.06) | 0.2 |
| Plasma TNF-α | 0.4a (0.2 to 0.5) | <0.001 | 0.4a (0.2 to 0.5) | <0.001 |
| Plasma IFN-γ | 0.05 (−0.03 to 0.13) | 0.2 | 0.2a (0.07 to 0.3) | 0.001 |
| Plasma IL-1β | 0.2b (0.001 to 0.4) | 0.049 | 0.3b (0.07 to 0.5) | 0.01 |
| Diabetic | −0.05 (−0.1 to 0.007) | 0.09 | 0.07b (0.002 to 0.15) | 0.045 |
| Comorbidity | 0.05a (0.02 to 0.08) | 0.001 | 0.02 (−0.01 to 0.05) | 0.2 |
| Urine volume (per L) | −0.02 (−0.05 to 0.01) | 0.3 | −0.009 (−0.05 to 0.03) | 0.7 |
| Korean | 0.03 (−0.05 to 0.11) | 0.4 | −0.04 (−0.1 to 0.1) | 0.4 |
A total of 497 and 310 patients were in the final models for incident and prevalent groups, respectively. Log10 transformations of plasma IL-6 were used as the dependent variable. Cytokine coefficients are per log10 changes in concentration. Using diabetes status rather than comorbidity was not a significant predictor in the incident group, but for the prevalent group diabetes was highly significant and there was a trend toward a better model with diabetes rather than comorbidity (Δ−2 log likelihood = 2.19). CI, confidence interval.
P<0.01.
P=0.01–0.05.
Local membrane inflammation was associated with older age, lower systolic BP, use of icodextrin, local TNF-α, and systemic IL-6 concentrations in incident and prevalent patients. Factors associated with systemic inflammation were similar where a reciprocal effect might be expected (e.g., the plasma and dialysate cytokines) but also included a relationship with comorbidity; in prevalent patients this was with the overall comorbid burden whereas in prevalent patients it was especially evident with patients with diabetes (although the IL-6 levels were still different between the grades of comorbidity, one-way between-subjects ANOVA, P=0.006).
Sensitivity analyses excluding one center with marginally worse data quality increased the significance of the association between plasma IL-6 and age (P=0.02) in prevalent patients.
Systemic Not Local Inflammation Predicts Patient Survival
A total of 427 patients died in the two cohorts (241 and 186 in the incident and prevalent groups, respectively, during median follow-up times of 5.25 and 5.06 years) during the 8-year follow-up period. Survival of incident patients was independently predicted by age, cumulative comorbidity, plasma albumin, and systemic inflammation (IL-6 and TNF-α), whereas dialysate cytokines levels and PSTR had no effect (Table 6). Survival analysis in the prevalent group also found age, comorbidity, and systemic IL-6, but not peritoneal inflammation, to predict death, with some additional differences. Plasma albumin did not predict survival whereas residual renal function was protective and a faster PSTR was associated with increased mortality. Sensitivity analyses excluding one center with higher levels of missing data increased the significance of PSTR in prevalent patients (P=0.02) and excluding patients with previous episodes of PD treatment from the prevalent group made no difference.
Table 6.
Predictors of survival
| Variable | Incident | Prevalent | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Dialysate TNF-α | 0.99 (0.34 to 2.89) | 0.98 | 0.86 (0.22 to 3.43) | 0.8 |
| Dialysate IL-6 | 0.93 (0.66 to 1.31) | 0.7 | 0.96 (0.65 to 1.44) | 0.9 |
| Dialysate IFN-γ | 1.18 (0.69 to 2.00) | 0.5 | 1.20 (0.65 to 2.19) | 0.6 |
| Plasma IL-1β | 0.56 (0.15 to 2.15) | 0.4 | 0.52 (0.16 to 1.74) | 0.3 |
| Plasma TNF-α | 3.39a (1.26–9.16) | 0.02 | 2.03 (0.52 to 7.93) | 0.3 |
| Plasma IL-6 | 2.15b (1.22 to 3.78) | 0.008 | 2.68b (1.28 to 5.58) | 0.009 |
| Plasma IFN-γ | 0.89 (0.49 to 1.60) | 0.7 | 1.16 (0.62 to 2.16) | 0.6 |
| Age (per yr) | 1.06b (1.05 to 1.08) | <0.001 | 1.06b (1.04 to 1.07) | <0.001 |
| Male sex | 0.94 (0.69 to 1.29) | 0.7 | 1.28 (0.92 to 1.78) | 0.1 |
| Comorbidity (per disease) | 1.68b (1.44 to 1.96) | <0.001 | 1.37b (1.18 to 1.58) | <0.001 |
| Urine volume (per L) | 0.95 (0.76 to 1.19) | 0.7 | 0.65b (0.48 to 0.87) | 0.004 |
| Duration of PD (per mo) | 1.17 (0.05 to 29.16) | 0.9 | 1.14b (1.04 to 1.24) | 0.005 |
| Albumin (per 1 g/dl) | 0.94b (0.91 to 0.97) | <0.001 | 0.99 (0.95 to 1.03) | 0.6 |
| PSTR (per 0.1 increase in dialysate-to-plasma creatinine ratio) | 1.10 (0.98 to 1.23) | 0.1 | 1.18a (1.003 to 1.41) | 0.049 |
| Body mass index | 1.01 (0.97 to 1.05) | 0.6 | 1.01 (0.98 to 1.04) | 0.6 |
Models stratified by center. Cytokine hazard ratios are for each 1×log10 change in concentration. CI, confidence interval.
P=0.01–0.05.
P<0.01.
Discussion
This analysis of the Global Fluid Study clearly shows that systemic and local peritoneal inflammatory cytokine networks are uncoupled and that they have different consequences for patient survival. Local, subclinical peritoneal inflammation is demonstrated to be the strongest known factor associated with between-patient variability in PSTR, independent of center effects, and the lack of an association with survival refutes the prior hypothesis that fast PSTR increases mortality through its association with systemic inflammation. If anything, evidence points to intraperitoneal inflammation being a contributor to systemic inflammation without influencing its association with mortality.
Although the association between local inflammation and PSTR has been found in prior studies,8,9,16 none of these has had the power or the degree of detailed clinical data to show its relative importance compared with previously demonstrated, much weaker, clinical associations. As with CANUSA (Canada-USA),17 ANZDATA (Australian and New Zealand Dialysis and Transplantation),18 and the Stoke PD Study,4,19 patients with diabetes and men were found to have higher PSTR, whereas the association with increasing age, overall comorbidity, and inverse relationship to body mass index were not seen. This is also the first study to identify important center effects and adjust for these in the analytic approach. These center effects will reflect differences, such as case mix and race, practice patterns related to dialysis, and erythropoietin prescription, which could largely be adjusted for, but also differences in PSTR that are likely to reflect local variations in exactly how the peritoneal equilibration test is performed (e.g., volume infused) or biochemically analyzed (including correction for glucose), as well as unknown factors. Timing of the initial peritoneal equilibration test showed a complex relationship from which it is possible to infer an early increase in PSTR within the first 4 weeks of treatment with a subsequent fall before a longer-term increase, in keeping with previous reports.19–21 Given the ANZDATA finding that race influenced PSTR,18 it is interesting that this was found to be higher in Korean patients, independent of IL-6 levels, suggesting that other genetic factors might be important.
It is difficult to disentangle the observed association between use of icodextrin or higher glucose concentration solutions with higher dialysate IL-6 concentrations given that they are also associated with PSTR and thus there may be some confounding by indication. However, icodextrin in combination with other solutions has been associated with increased solute transport,22,23 as have other biocompatible solutions at the commencement of treatment with PD.24 One possible explanation is that more biocompatible dialysate improves local cell viability and thus facilitates the local production of cytokines or vasoactive mediators.25,26 In light of the recently published balANZ study, in which use of a biocompatible solution was associated with disappearance of the increase in solute transport with time on PD,24 it is interesting to note in this study that prevalent patients using these solutions had lower PSTR.
The associations between plasma IL-6, other systemic inflammatory cytokines, and comorbidity were to be expected and are in keeping with the previously described relationship between IL-6 polymorphisms, comorbidity, and survival in hemodialysis and PD patients.12,27 More surprising is the association between plasma and dialysate IL-6. This could reflect the fact that genetically high IL-6 producers more readily synthesize more of this cytokine in any of the body compartments.10 Alternatively, the high concentrations in dialysate, which in some of these patients was >1000 times that of plasma despite the diluting effects of 2 L of instilled solution, reflects peritoneal membrane concentrations that could spill over into plasma.
The relationship between systemic inflammation and survival, independent of age and comorbidity was as anticipated, although previous studies have not reported independent effects of TNF-α and IL-6 as observed here in incident patients.28,29 There were other potentially important differences between the incident and prevalent cohorts, partly because prevalent patients are by definition a self-selected cohort. As would be expected, longer duration of PD was a risk factor for worse survival. Relative preservation of residual urine volume, in keeping with prior studies, is more important than for incident patients, whereas it is likely that the patients with the lowest plasma albumin concentrations will have already died; this would explain the lack of association with survival. It is interesting to note that in these prevalent patients increased solute transport was associated with reduced survival; this may be because the relative importance of membrane function would be expected to increase as residual function becomes more critical. Although the use of APD at the start of PD in the Global Fluid Study cohort is relatively low, APD was used preferentially in patients with high PSTR and with double the frequency in prevalent compared with incident patients. In contrast to most published cohorts, icodextrin use was high.
This study has several limitations. Despite the depth and completeness of the clinical data collected and attempts to account for important observed center effects, it must be acknowledged that there are likely to be practice patterns and local factors that remain poorly understood or unmeasured. Although the study used 10 centers from three countries, a degree of selection bias might be present because the selected centers had better data quality. As with any observational study, direction of causality must always be questioned. The genetic associations between high producing IL-6 polymorphisms and membrane function, effectively Mendelian randomization experiments, strongly suggest that activation of local cytokine networks are the cause rather than the consequence of increased PSTR, which is also biologically plausible. However, many of the statistical associations demonstrated do not have clear biologic explanations. For example, a lower systolic BP was associated with higher dialysate but not plasma IL-6 concentration. These require reproduction in separate cohorts and further investigation. Despite clear evidence of local production in some patients, average dialysate TNF-α levels were less than predicted by the three-pore model, but the results were biologically plausible and compatible with previous studies.30 Cost and feasibility dictated that we limit our inflammatory cytokine profiles to just four; other studies using larger panels of biomarkers confirm that these are representative of activation of the inflammatory pathway in general.9,16,31 Controversy exists as to whether dialysate biomarkers should be expressed as absolute concentrations or appearance rates. In our multivariable analysis, dialysate IL-6 concentrations produced better models than appearance rates, suggesting that biologic effects are determined by concentration, mediated by changes over log orders. Correcting for appearance rates produced worse models, probably because the dialysate samples were all standardized to 4-hour dwells; a recent study has shown a linear increase in IL-6 concentrations with time.32
In conclusion, dialysate IL-6 concentration, representing local subclinical intraperitoneal inflammation, is the most significant known predictor of PSTR but does not determine patient survival. Intraperitoneal and systemic inflammation is largely independent. Independent of inflammation, higher PSTR may still be associated with worse survival in prevalent patients. The clinical implications of these findings are that attention to membrane function in dialysis prescription rather than switching off membrane inflammation per se is important for patient survival. The relevance of membrane inflammation is yet to be determined.
Concise Methods
Study Design
The Global Fluid Study is an international, multicenter, prospective, observational cohort study designed to answer a series of research questions seeking to relate peritoneal membrane function to local and systemic biomarkers as predictors of predefined clinical endpoints (e.g., patient survival, membrane injury). In was open to any center worldwide as advertised at international meetings. Ten centers from the United Kingdom, Korea, and Canada were finally included (see Supplemental Table 2) in this analysis. An additional six centers (comprising 247 patients) were excluded on the basis of a preanalysis assessment indicating poor data quality (more than 10 variables were missing for more than 10% of data or the center failed to enlist a representative number of eligible patients during the recruitment window) and it was judged unlikely that this could be improved upon because of logistic issues. Recruiting incident (within first 90 days of PD) and prevalent patients, enrollment commenced in June 2002 and finished in December 2008 (with some centers stopping before then), with follow-up censored at center-specific dates in December 2010. Any patient on peritoneal dialysis was eligible for inclusion provided they could give informed consent. The sample size was the maximum logistically feasible, as determined by each center. Dialysate sampling was from a 4-hour peritoneal equilibration test, with some centers also collecting samples from an overnight dwell. Simultaneous clinical data were collected and stored in a purpose built Peritoneal Dialysis Access database. Ethical approval was obtained from the Multi-Centre Research Ethics Committee for Wales covering the United Kingdom, while local country ethics were obtained for other contributing countries.
Prospective Collection of Routine Clinical Measurements
Routine demographic characteristics were recorded and comorbidity documented using the validated Stoke Comorbidity Index, which both categorizes patients into low-risk (score 0), intermediate-risk (score 1–2), and high-risk (score >2) groups and enables analysis by individual comorbid conditions within the index. Patient-level ethnicity was not available and thus was recorded as non-Korean versus Korean on the basis of center. Routine blood, urine, and dialysate tests were performed locally and, if necessary, converted into standardized SI units.
PD-related measurements included residual renal function (mean of urea and creatinine clearances), dialysis regimen and dose, and peritoneal membrane function using the peritoneal equilibration test (solute transport rate: dialysate-to-plasma creatinine ratio (PSTR) and net ultrafiltration capacity at 4 hours with 2.27% or 3.86% glucose, corrected for flush volume, if included in the measurement). The glucose exposure rate was calculated as total grams of glucose within the daily dialysate, and the average daily glucose concentration was the total daily dialysate glucose/total daily dialysate volume (g/L). Biocompatible solutions are defined as those with low glucose degradation products.
Sample Analysis
Dialysate and plasma samples were stored locally at −80°C, then transferred frozen to a central laboratory in the United Kingdom. Plasma and 4-hour dialysate samples (overnight collections excluded) were assayed for IL-1β, TNF-α, IFN-γ, and IL-6 by electrochemiluminescence immune assay using the quality-controlled commercially available Pro-Inflammatory I 4-plex (Meso-Scale Discovery, Gaithersburg, MD). Triplicate measurements were made, the mean of which was used; all assays were run with internal controls to assure assay fidelity.
Statistical Analyses
Demographic features were compared with independent sample t tests, Mann-Whitney U tests, or chi-squared tests, depending on whether the variable was normally distributed, skewed, or categorical. Similarly, for center effects, a one-way ANOVA or Kruskal-Wallis was used (Table 1).
The Pearson R was used for cytokine correlations, with Sidak adjustment for multiple comparisons and a P value of 0.05 for statistical significance. The three-pore model was used to predict 4-hour cytokine dialysate-to-plasma ratios based on the predicted molecular radius.33 For plasma values of 0 with detectable dialysate cytokine, a ratio >1 was assumed; if both dialysate and plasma cytokine were undetectable, a ratio of 0 was assumed.
Three multilevel linear models for predictors of the continuous variables PSTR, and log10 transformations of dialysate and plasma IL-6 concentrations in three separate models were run to account for the observed center effects by introducing a center-level residual as well as the usual person-level residual. As an exploratory analysis, no adjustment of significance levels was made for multiple hypotheses tested. Random intercept models were fitted (random slopes models were attempted but did not converge). The variable selection method was to include all cytokine measures and all the important clinical and available demographic variables. Dialysate IL-1β was dropped and only one measure of BP was included because of multi-colinearity. Diabetes and comorbidity were included in separate models because existing literature suggests diabetic effects may be important independently of the comorbidity score, despite being highly correlated.18 The duration of PD was included as a linear or linear plus quadratic term in the incident group, as suggested by existing literature.20 The iterative generalized least-squares method was used for coefficient estimation and residuals were checked for normality. For clarity of interpretation, 23 patients with a previous episode of PD treatment were excluded from the prevalent group multilevel modeling.
We included cytokine results in the PSTR model as concentrations (as shown) or appearance rates, and we selected the type that provided the best goodness of fit as measured by −2 log likelihood values. The selected variable type was then used in all other analyses.
Cox modeling, stratified by center, was used for survival analysis, with robust SEMs. Hazard ratios for cytokines quoted are for a log10 change in concentration. Proportional hazards were checked with log-log plots, scaled Schoenfeld residual plots, and significance testing. Dialysate IL-1β was excluded because of high collinearity.
MLWin, version 2.26, was used for the multilevel modeling.34 All other analyses were run using Stata IC, version 12.1 (Stata Corp., College Station, Texas). Missing data, which ranged between 0% and 4.8% for different variables, were considered missing at random and complete case analysis was used. Loss to follow-up was trivial (16 incident and 8 prevalent patients, with 22 from one center and all patients from that center being dropped in a sensitivity analysis).
Disclosures
S.J.D. currently receives research funding and honoraria from Baxter Healthcare and Fresenius AG. N.T. has in the past received honoraria and speaker fees from Baxter Healthcare and Fresenius AG.
Acknowledgments
The authors would like to acknowledge the support of Anna-Clare Smith, Kathryn Craig, Maureen Fallon, and Charlotte James in the coordination of the GLOBAL study and the following clinical staff in the centers in coordination of sample and clinical data collection: Hilary Huxtable, SRN (Renal Unit, Morriston Hospital, Swansea, United Kingdom); Gill Gilbert, RGN (Hons) (Ipswich Hospital NHS Trust); Catherine Jones, RGN, and Jane Hollis, RGN (Hons) (Cambridge University Hospitals, NHS Foundation Trust); Jung-Ju Seo (Kyungpook National University Hospital, Daegu, South Korea); Kei-Lim Shin (Yeungnam University Hospital, Daegu, South Korea); Sung Hee Chung (Hyonam Kidney Laboratory, Soon Chun Hyang University, Seoul, South Korea); Joanne Leblanc-Chiasson, Rachel Belliveau, and Régina Arsenault (research assistant) (Dr. Georges-L.-Dumont Hospital, Moncton, New Brunswick, Canada); Kathy Yetzer and Donna Hackman (Division of Nephrology and Immunology, Department of Medicine, University of Alberta, Edmonton, Canada); and the nursing team on the PD Unit at the University Hospital of Wales, Cardiff, United Kingdom.
Infrastructure support for the establishment of the GLOBAL fluid study was provided as unrestricted educational grants from the International Society for Peritoneal Dialysis and Baxter Healthcare Renal Division, and we are grateful for their continued financial support, including sample analysis (special thanks to Dr. Cliff Holmes). The study was officially endorsed by the International Society for Peritoneal Dialysis (www.ispd.org) and by the British Renal Society (www.britishrenal.org).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013030314/-/DCSupplemental.
References
- 1.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG: Meta-analysis: Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 17: 2591–2598, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Asghar RB, Davies SJ: Pathways of fluid transport and reabsorption across the peritoneal membrane. Kidney Int 73: 1048–1053, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Chung SH, Heimbürger O, Stenvinkel P, Qureshi AR, Lindholm B: Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol Dial Transplant 18: 590–597, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Davies SJ, Phillips L, Naish PF, Russell GI: Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 17: 1085–1092, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Davies SJ, Bryan J, Phillips L, Russell GI: Longitudinal changes in peritoneal kinetics: The effects of peritoneal dialysis and peritonitis. Nephrol Dial Transplant 11: 498–506, 1996 [PubMed] [Google Scholar]
- 6.Parikova A, Smit W, Struijk DG, Krediet RT: Analysis of fluid transport pathways and their determinants in peritoneal dialysis patients with ultrafiltration failure. Kidney Int 70: 1988–1994, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Lambie ML, John B, Mushahar L, Huckvale C, Davies SJ: The peritoneal osmotic conductance is low well before the diagnosis of encapsulating peritoneal sclerosis is made. Kidney Int 78: 611–618, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Pecoits-Filho R, Carvalho MJ, Stenvinkel P, Lindholm B, Heimbürger O: Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit Dial Int 26: 53–63, 2006 [PubMed] [Google Scholar]
- 9.Oh KH, Jung JY, Yoon MO, Song A, Lee H, Ro H, Hwang YH, Kim DK, Margetts P, Ahn C: Intra-peritoneal interleukin-6 system is a potent determinant of the baseline peritoneal solute transport in incident peritoneal dialysis patients. Nephrol Dial Transplant 25: 1639–1646, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Gillerot G, Goffin E, Michel C, Evenepoel P, Biesen WV, Tintillier M, Stenvinkel P, Heimbürger O, Lindholm B, Nordfors L, Robert A, Devuyst O: Genetic and clinical factors influence the baseline permeability of the peritoneal membrane. Kidney Int 67: 2477–2487, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hwang YH, Son MJ, Yang J, Kim K, Chung W, Joo KW, Kim Y, Ahn C, Oh KH: Effects of interleukin-6 T15A single nucleotide polymorphism on baseline peritoneal solute transport rate in incident peritoneal dialysis patients. Perit Dial Int 29: 81–88, 2009 [PubMed] [Google Scholar]
- 12.Verduijn M, Maréchal C, Coester AM, Sampimon DE, Boeschoten EW, Dekker FW, Goffin E, Krediet RT, Devuyst O: The -174G/C variant of IL6 as risk factor for mortality and technique failure in a large cohort of peritoneal dialysis patients. Nephrol Dial Transplant 27: 3516–3523, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Perl J, Huckvale K, Chellar M, John B, Davies SJ: Peritoneal protein clearance and not peritoneal membrane transport status predicts survival in a contemporary cohort of peritoneal dialysis patients. Clin J Am Soc Nephrol 4: 1201–1206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Fang W, Bargman JM, Oreopoulos DG: High peritoneal permeability is not associated with higher mortality or technique failure in patients on automated peritoneal dialysis. Perit Dial Int 28: 82–92, 2008 [PubMed] [Google Scholar]
- 15.Johnson DW, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Badve SV: Superior survival of high transporters treated with automated versus continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant 25: 1973–1979, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Martikainen T, Ekstrand A, Honkanen E, Teppo AM, Grönhagen-Riska C: Do interleukin-6, hyaluronan, soluble intercellular adhesion molecule-1 and cancer antigen 125 in dialysate predict changes in peritoneal function? A 1-year follow-up study. Scand J Urol Nephrol 39: 410–416, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D, The Canada-USA (CANUSA) Peritoneal Dialysis Study Group : Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. J Am Soc Nephrol 9: 1285–1292, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Rumpsfeld M, McDonald SP, Purdie DM, Collins J, Johnson DW: Predictors of baseline peritoneal transport status in Australian and New Zealand peritoneal dialysis patients. Am J Kidney Dis 43: 492–501, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Davies SJ: Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int 66: 2437–2445, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Struijk DG, Krediet RT, Koomen GCM, Boeschoten EW, Hoek FJ, Arisz L: A prospective study of peritoneal transport in CAPD patients. Kidney Int 45: 1739–1744, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Johnson DW, Mudge DW, Blizzard S, Arndt M, O’Shea A, Watt R, Hamilton J, Cottingham S, Isbel NM, Hawley CM: A comparison of peritoneal equilibration tests performed 1 and 4 weeks after PD commencement. Perit Dial Int 24: 460–465, 2004 [PubMed] [Google Scholar]
- 22.Lui SL, Yung S, Yim A, Wong KM, Tong KL, Wong KS, Li CS, Au TC, Lo WK, Ho YW, Ng F, Tang C, Chan TM: A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: A randomized clinical trial. Am J Kidney Dis 60: 966–975, 2012 [DOI] [PubMed] [Google Scholar]
- 23.le Poole CY, Welten AG, ter Wee PM, Paauw NJ, Djorai AN, Valentijn RM, Beelen RH, van den Born J, van Ittersum FJ: A peritoneal dialysis regimen low in glucose and glucose degradation products results in increased cancer antigen 125 and peritoneal activation. Perit Dial Int 32: 305–315, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, Jones B, Kulkarni H, Langham R, Ranganathan D, Schollum J, Suranyi MG, Tan SH, Voss D: on behalf of the balANZ Trial Investigators. The effect of low glucose degradation product, neutral pH versus standard peritoneal dialysis solutions on peritoneal membrane function: The balANZ trial. Nephrol Dial Transplant 27: 4445–4453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topley N, Jörres A, Luttmann W, Petersen MM, Lang MJ, Thierauch KH, Müller C, Coles GA, Davies M, Williams JD: Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int 43: 226–233, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Opatrna S, Lysak D, Trefil L, Parker C, Topley N: Intraperitoneal IL-6 signaling in incident patients treated with icodextrin and glucose bicarbonate/lactate-based peritoneal dialysis solutions. Perit Dial Int 32: 37–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao M, Guo D, Perianayagam MC, Tighiouart H, Jaber BL, Pereira BJ, Balakrishnan VS: Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 45: 324–333, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Tripepi G, Mallamaci F, Zoccali C: Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modeling. J Am Soc Nephrol 16[Suppl 1]: S83–S88, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Meuwese CL, Snaedal S, Halbesma N, Stenvinkel P, Dekker FW, Qureshi AR, Barany P, Heimburger O, Lindholm B, Krediet RT, Boeschoten EW, Carrero JJ: Trimestral variations of C-reactive protein, interleukin-6 and tumour necrosis factor-α are similarly associated with survival in haemodialysis patients. Nephrol Dial Transplant 26: 1313–1318, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Zemel D, Imholz AL, de Waart DR, Dinkla C, Struijk DG, Krediet RT: Appearance of tumor necrosis factor-α and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int 46: 1422–1430, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Yu Z, Tan BK, Dainty S, Mattey DL, Davies SJ: Hypoalbuminaemia, systemic albumin leak and endothelial dysfunction in peritoneal dialysis patients. Nephrol Dial Transplant 27: 4437–4445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes Barreto D, Coester AM, Noordzij M, Smit W, Struijk DG, Rogers S, de Waart DR, Krediet RT: Variability of effluent cancer antigen 125 and interleukin-6 determination in peritoneal dialysis patients. Nephrol Dial Transplant 26: 3739–3744, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Rippe B: A three-pore model of peritoneal transport. Perit Dial Int 13[Suppl 2]: S35–S38, 1993 [PubMed] [Google Scholar]
- 34.Rasbash J, Charlton C, Browne WJ, Healy M, Cameron B: MLwiN Version 2.1, Centre for Multilevel Modelling, University of Bristol, 2009 [Google Scholar]