Abstract
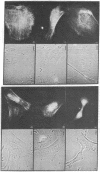
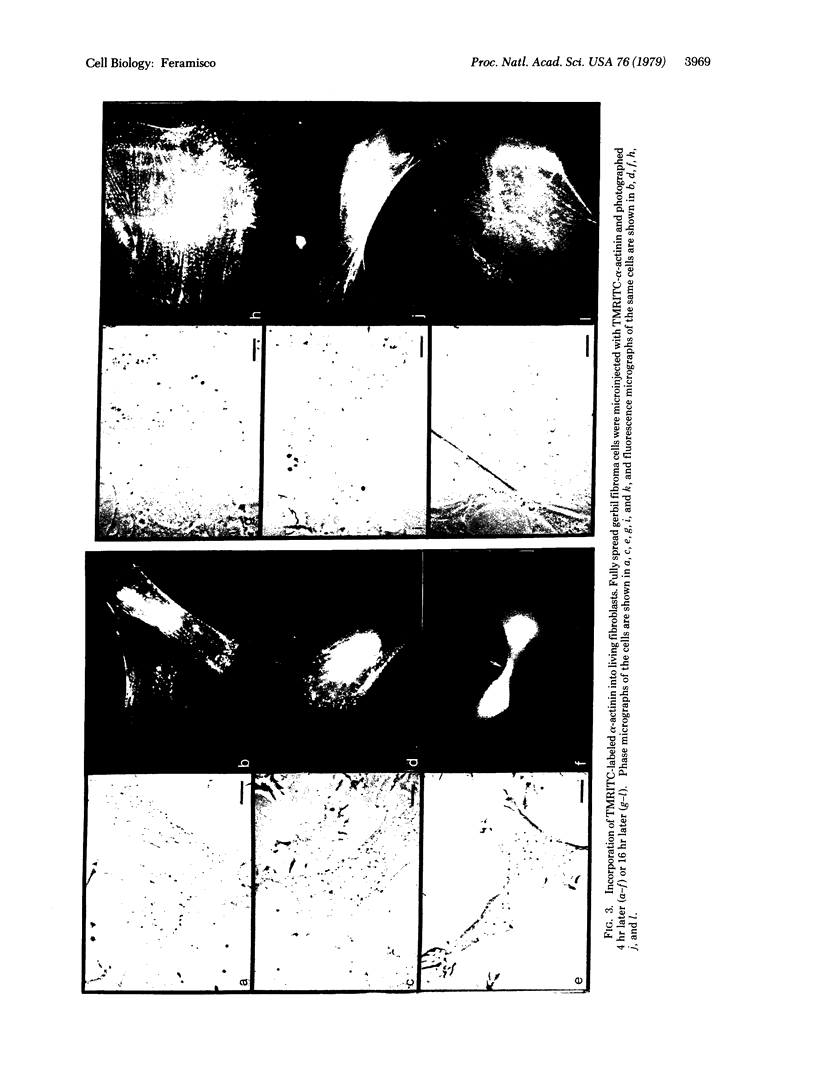
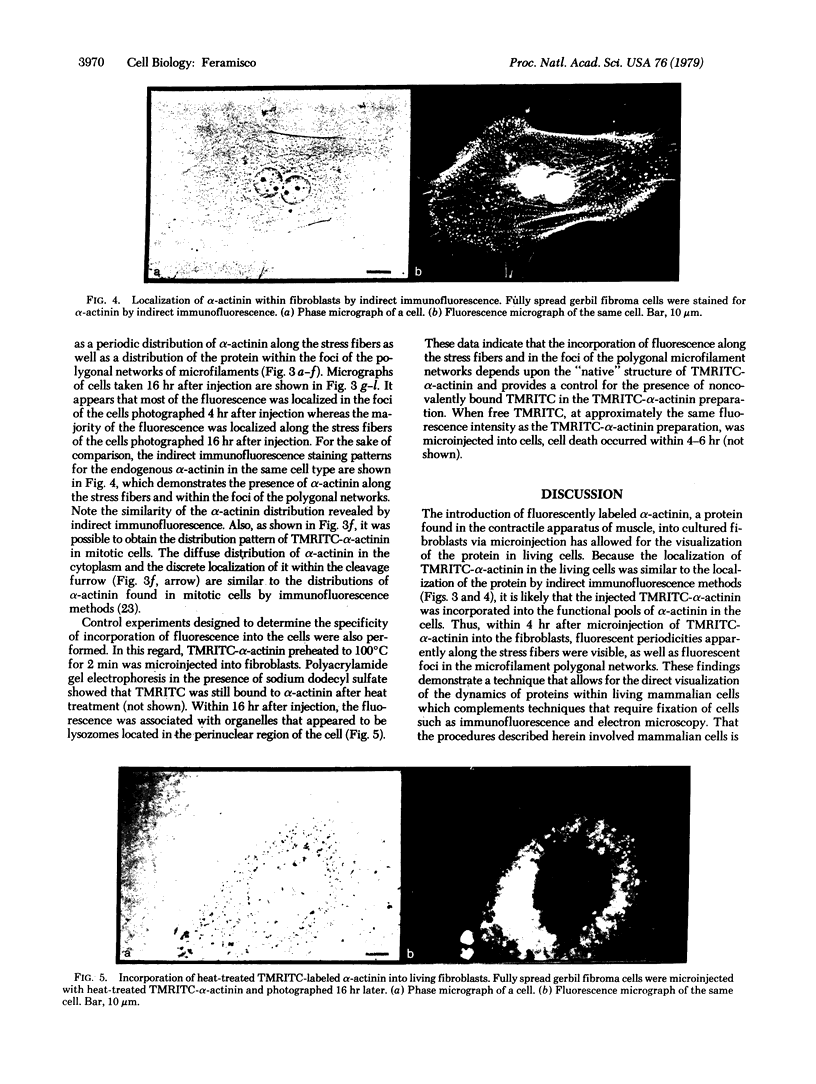
α-Actinin from chicken gizzard labeled with tetramethylrhodamine isothiocyanate has been incorporated into living fibroblast cells by microinjection. Fluorescent labeling of α-actinin was carried out such that the conjugated protein was functional in vitro as shown by its ability to bind to F-actin. Within 1-2 hr after injection, diffuse fluorescence was observed throughout the cytoplasm and only faint fluorescence was apparently associated with the stress fibers. During the ensuing 2-15 hr, however, most of the fluorescence was seen as periodicities along the stress fibers and as foci of the microfilament polygonal networks. This distribution of α-actinin in the living cells was strikingly similar to that found by indirect immunofluorescence localization of endogenous α-actinin in fixed samples of the same cell type. Control studies in which heat-treated (100°C, 2 min) fluorescent α-actinin or tetramethylrhodamine isothiocyanate alone was injected into the cells indicated that the stress fiber and polygonal network labeling was specific for “native” fluorescently labeled α-actinin. These results suggest that the dynamic properties of proteins and structures in cultured mammalian cells can be studied with the use of microinjection and fluorescence microscopic techniques.
Keywords: cytoskeleton, α-actinin
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Porter M. E., Pollard T. D. Alpha-actinin localization in the cleavage furrow during cytokinesis. J Cell Biol. 1978 Oct;79(1):268–275. doi: 10.1083/jcb.79.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goli D. E., Suzuki A., Temple J., Holmes G. R. Studies on purified -actinin. I. Effect of temperature and tropomyosin on the -actinin-F-actin interaction. J Mol Biol. 1972 Jun 28;67(3):469–488. doi: 10.1016/0022-2836(72)90464-0. [DOI] [PubMed] [Google Scholar]
- Gordon W. E., 3rd, Bushnell A. Immunofluorescent and ultrastructural studies of polygonal microfilament networks in respreading non-muscle cells. Exp Cell Res. 1979 May;120(2):335–348. doi: 10.1016/0014-4827(79)90393-8. [DOI] [PubMed] [Google Scholar]
- Gordon W. E., 3rd Immunofluorescent and ultrastructural studies of "sarcomeric" units in stress fibers of cultured non-muscle cells. Exp Cell Res. 1978 Dec;117(2):253–260. doi: 10.1016/0014-4827(78)90138-6. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Rathke P. C., Hülsmann N., Franke W. W., Wohlfarth-Bottermann K. E. Cytoplasmic actomyosin fibrils in tissue culture cells: direct proof of contractility by visualization of ATP-induced contraction in fibrils isolated by laser micro-beam dissection. Cell Tissue Res. 1976 Feb 27;166(4):427–443. doi: 10.1007/BF00225909. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. G., Sheetz M., Singer S. J. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraydarian K., Briskey E. J., Mommaerts W. F. The modification of actomyosin by alpha- actinin. I. A survey of experimental conditions. Biochim Biophys Acta. 1967 Apr 11;133(3):399–411. doi: 10.1016/0005-2795(67)90544-2. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G. Evidence for the autophagy of microinjected proteins in HeLA cells. J Cell Biol. 1977 Dec;75(3):807–817. doi: 10.1083/jcb.75.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromer M. H., Goll D. E. Studies on purified -actinin. II. Electron microscopic studies on the competitive binding of -actinin and tropomyosin to Z-line extracted myofibrils. J Mol Biol. 1972 Jun 28;67(3):489–494. doi: 10.1016/0022-2836(72)90465-2. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L. Molecular cytochemistry: incorporation of fluorescently labeled actin into living cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):857–861. doi: 10.1073/pnas.75.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]