Abstract
Purpose
The efficacy of peptide vaccines may be enhanced by stimulating immune cells with multiple peptides derived from distinct tumor-associated antigens. We have evaluated the heteroclitic XBP1 US184–192 (YISPWILAV), heteroclitic XBP1 SP367–375 (YLFPQLISV), native CD138260–268 (GLVGLIFAV), and native CS1239–247 (SLFVLGLFL) peptides, which have strong HLA-A2 affinity and immunogenicity in combination, for their ability to elicit multiple myeloma antigen-specific responses.
Experimental Design
Multipeptide-specific cytotoxic T lymphocytes (MP-CTL) were generated by the stimulation of CD3+ T lymphocytes from HLA-A2+ individuals with either autologous mature dendritic cells or T2 cells pulsed with a cocktail of these four peptides.
Results
The peptide cocktail did not compromise tumor antigen-specific activity of CTL. MP-CTL displayed increased total, effector memory (CCR7−CD45RO+), and activated (CD69+) CD3+CD8+ T lymphocytes. In addition, MP-CTL demonstrated IFN-γ production, cell proliferation, and cytotoxicity against HLA-A2+ multiple myeloma cells, including HLA-A2+ MM patients’ cells. Importantly, MP-CTL showed specific responses in functional assays to each relevant peptide, but not to an irrelevant HLA-A2 specific CMV pp65 (NLVPMVATV) peptide.
Conclusions
These results highlight the potential therapeutic application of vaccination with a cocktail of HLA-A2 specific peptides to induce CTL with a broad spectrum of immune responses against multiple myeloma antigens.
Keywords: Multiple myeloma, Multipeptide Vaccine, Cancer Immunotherapy
INTRODUCTION
Multiple myeloma (MM) is a malignant disorder characterized by a multifocal proliferation and clonal expansion of long-lived plasma cells within the bone marrow, associated with skeletal destruction, serum monoclonal gammopathy, immune suppression, and end-organ sequelae (1, 2). Despite aggressive chemotherapeutic regimens and novel therapies including immuno-modulatory drugs (thalidomide, lenalidomide) and the proteasome inhibitor (bortezomibe), development of acquired resistance to these agents is associated with refractory relapsed MM (3). Alternatively, active-specific immunotherapy may provide more durable responses through induction of cytotoxic T lymphocytes (CTL) targeting cancer cells (4, 5). Although immunotherapy has shown a therapeutic benefit in cancer (6–8), this option is suboptimal in MM and requires further improvement. The major challenges in developing a successful MM-specific immunotherapy include heterogeneity of tumor-associated antigens (TAA) expression, frequent mutations of specific TAA, tumor escape mechanisms, changes in immune cell function, and variability of the human T-cell repertoire. Thus, we hypothesized that use of immunogenic HLA-A2-specific epitopes from multiple TAA may enhance induction of antigens-specific CTL targeting malignant plasma cells and associated therapeutic efficacy in HLA-A2+ MM patients.
As target antigens, we selected XBP1 (X-box binding protein 1), CD138 (Syndecan-1), and CS1 (CD2 subset 1, CRACC, SLAMF7, CD319), which are associated with MM pathogenesis and are highly expressed on the tumor cells. First, we propose XBP1 as an attractive therapeutic target antigen since XBP1 is a basic leucine zipper-containing transcription factor, which is required for the terminal differentiation of B lymphocytes to plasma cells and is uniformly expressed in all MM patients’ cells and cell lines (9, 10). This antigen has been implicated in the proliferation of malignant plasma cells, and is differentially expressed between normal plasma cells and plasma cells from patients’ with monoclonal gammopathy of undetermined significance (MGUS) or MM (11, 12). High amounts of immunoglobulin produced by plasma cells evoke ER stress, which in turn activates IRE1-mediated XBP1 expression and subsequently mRNA splicing during plasma cell differentiation (10, 13, 14). As a consequence, the relative mRNA expression levels of spliced XBP1 compared to unspliced XBP1 are higher in MM compared with normal plasma cells (9), making XBP1 as a potential therapeutic target. The second potential target antigen we propose for the development of MM-specific immunotherapy is CD138, a transmembrane heparan sulfate–bearing proteoglycan expressed by most MM cells. CD138 is critical for the growth of tumor cells by mediating cell-cell adhesion, binding MM cells to molecules such as collagen and fibronectin in the extracellular matrix, as well as binding to growth factors and cytokines (15, 16). In patients with MM, shed syndecan-1 accumulates in the bone marrow, and soluble syndecan-1 facilitates MM tumor progression, angiogenesis, and metastasis in vivo. Therefore, targeting CD138 on malignant plasma cells to prevent or reduce high levels of syndecan-1 in the serum, an indicator of poor prognosis in MM (17–19), may have a direct clinical benefit. Finally, CS1 is a cell surface glycoprotein of the CD2 family, which is highly and uniformly expressed by malignant plasma cells and has restricted expression in normal tissues (20–23). CS1 localizes to the uropods of polarized MM cells, where it mediates adhesion of MM cells to bone marrow stroma and other human MM cells (24). Based on the universal expression of these functional antigens on MM cells, we hypothesized that development of an immunotherapeutic strategy targeting XBP1, CD138 as well as CS1 antigens could represent a novel treatment option for MM.
In previous studies, we have identified immunogenic HLA-A2-specific peptides derived from each of these target antigens including heteroclitic XBP1 unspliced (US)184–192 (YISPWILAV) (25), heteroclitic XBP1 spliced (SP)367–375 (YLFPQLISV) (25), native CD138260–268 (GLVGLIFAV) (26), and native CS1239–247 (SLFVLGLFL) peptides (27). These selected peptides were highly immunogenic in ex vivo studies, inducing antigen-specific CTL, which specifically responded against HLA-A2+ MM cells. In the current studies, we provide evidence that a cocktail of four HLA-A2-specific peptides derived from XBP1 US, XBP1 SP, CD138, and CS1 induces MP-specific CTL enriched for effector CD8+ T cells with distinct functional immunogenic properties against HLA-A2+ MM cells. The ability to induce CTL against multiple target epitopes using a combination of these four immunogenic peptides provides the framework for their potential use in targeted immunotherapy to improve outcome in patients with plasma cell related disorders.
MATERIALS AND METHODS
Cell lines
The MM cell lines, McCAR, MM1S and U266 were obtained from ATCC (Manassas, VA). The T2 cell line, a human B and T cell hybrid expressing HLA-A2 molecules, was provided by Dr. J. Molldrem (University of Texas M. D. Anderson Cancer Center, Houston, TX). All cell lines were cultured in RPMI-1640 medium (Gibco-Life Technologies, Rockville, MD) supplemented with 10% fetal calf serum (FCS; BioWhittaker, Walkersville, MD), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco-Life Technologies).
Reagents
Mouse anti-human CD3, CD4, CD8, CCR7, CD45RO, CD69, CD107a, IFN-γ, and HLA-A2 mAbs conjugated with FITC, PE, PerCP, PerCP-Cy5.5, APC, Pacific Blue, APC-H7 or PE-Cy7 were purchased from Becton Dickinson (BD)/Pharmingen or BD/Biosciences (San Diego, CA). Recombinant human IL-2, IL-4, IFN-α and TNF-α were purchased from R&D Systems (Minneapolis, MN), and GM-CSF was obtained from Immunex (Seattle, WA).
Synthetic Peptides
Heteroclitic XBP1 US184–192 (YISPWILAV), heteroclitic XBP1 SP367–375 (YLFPQLISV), native CD138260–268 (GLVGLIFAV), and native CS1239–247 (SLFVLGLFL) peptides were derived from XBP1 unspliced (US), XBP1 spliced (SP), CD138, and CS1 antigens, respectively. Influenza virus matrix protein58–66 (GILGFVFTL) and CMV pp65 (NLVPMVATV) were selected as HLA-A2-specific control peptides. All peptides were synthesized by standard fmoc (9-fluorenylmethyl-oxycarbonyl) chemistry, purified to >90% using reverse-phase chromatography, and validated by mass-spectrometry for molecular weight (Biosynthesis, Lewisville, TX). Lyophilized peptides were dissolved in DMSO (Sigma, St. Louis, MO), diluted in AIM-V medium (Gibco-Life Technologies), and stored at −140°C.
Peptide Binding Assay
A cocktail of four HLA-A2 peptides including heteroclitic XBP1 US184–192, heteroclitic XBP1 SP367–375, CD138260–268 and CS1239–247, was evaluated for binding affinity using the T2 cell line, as described elsewhere (28). In brief, T2 cells were pulsed overnight with the MP cocktail (0 μg/ml, 6.25 μg/ml, 12.5 μg/ml, 25 μg/ml, 50 μg/ml) plus 3 μg/ml human β2-microglobulin (Sigma, St Louis, MO). Following incubation, cells were stained with anti-human HLA-A2-FITC mAb and analyzed using a FACSCanto™ II flow cytometer (Becton Dickinson, San Jose, CA).
Peptide Stability Assay
The MP cocktail was examined for HLA-A2 stability, as described elsewhere (29). Briefly, T2 cells were pulsed overnight with the MP cocktail (25 µg/ml) plus 3 µg/ml human β2-microglobulin, and the peptide/HLA-A2 complex stability was measured at 0, 2, 4, 6 and 14 hours post-BFA treatment by staining cells with mouse anti-human HLA-A2-FITC mAb and flow cytometric analysis.
Generation of Monocytes-derived Dendritic Cells
Monocytes-derived dendritic cells (DC) were generated as described elsewhere (30), with minor modifications. Briefly, monocytes isolated from peripheral blood mononuclear cells (PBMC) were cultured for 7 days in the presence of 1,000 U/ml GM-CSF and 1,000 U/ml IL-4 in RPMI-1640 medium (Gibco-Life Technologies) supplemented with 10% FCS. Fresh media plus GM-CSF and IL-4 was added to the cultures every other day. Mature DC (mDC) were obtained by adding 1,000 U/ml IFN-α plus 10 ng/ml TNF-α, along with fresh GM-CSF and IL-4 in 10% FCS-RPMI, on day 7 and then incubating for an additional three days.
Isolation of CD3+ T cells from Normal PBMC
CD3+ T cells were obtained from HLA-A2+ normal donors by negative selection using the EasySep® magnet and Robosep® from StemCell Technologies (Vancouver, Canada). In brief, PBMC were depleted of B cells, monocytes, NK cells, erythroid cells, platelets, and basophils using a cocktail of bispecific tetrameric antibody complexes. After the removal of magnetically labeled unwanted cells, the enriched CD3+ T cells were washed and examined by flow cytometry.
Isolation of Primary CD138+ cells from Bone Marrow Mononuclear Cells of MM Patients
Primary CD138+ cells were isolated from bone marrow mononuclear cells obtained from both HLA-A2+ and HLA-A2− MM patients using RoboSep® CD138 positive immunomagnetic selection technology (StemCell Technologies), after appropriate informed consent.
Induction of Multipeptide-specific CTL
MP-CTL were generated ex vivo by repeated MP stimulation of CD3+ T lymphocytes obtained from HLA-A2+ normal donors. In brief, APC (mDC or T2 cells) pulsed overnight with a cocktail of heteroclitic XBP1 US184–192, heteroclitic XBP1 SP367–375, CD138260–268 and CS1239–247 peptides (25 μg/ml total; 6.25 μg/ml/peptide) were irradiated (20 Gy) and then used to prime autologous CD3+ T cells at a 1:20 APC/MP-to-CD3+ T cell ratio in AIM-V medium supplemented with 10% human AB serum. Cultures were restimulated every seven days with irradiated APC/MP for a total of 4 cycles to generate MP-CTL. IL-2 (50 U/ml) was added to the cultures two days after the second stimulation and was replenished until the culture was completed.
Phenotypic Analysis of MP-CTL and Identification of T cell Subtypes
One week after the fourth stimulation, MP-CTL and control T cells were evaluated for total CD3+CD8+ T cells or naïve, effector memory, and activated CD3+CD8+ T cells by staining with CD3-PacBlue, CD8-APC-H7, CCR7-PeCy7, CD45RO-PE and/or CD69-PerCP mAbs. After staining, the cells were washed, fixed in 2% paraformaldehyde-PBS and analyzed by flow cytometry.
CD107a Upregulation and Intracellular IFN-γ Production
CD107a degranulation and IFN-γ producing CD8+ CTL were identified by cell surface marker and intracellular cytokine staining by flow cytometry. Briefly, MP-CTL or control T cells were stimulated with HLA-A2+ McCAR or U266 MM cell lines, or with K562-A*0201 cells pulsed with respective peptide in the presence of CD107a mAb. After 1 hour incubation, CD28/CD49d mAb (BD), as well as protein transport inhibitors Brefeldin A (BD) and Monensin (BD), were added to the cultures and incubated for an additional 5 hours. As a baseline control, MP-CTL were cultured in media with CD28/CD49d mAb, Brefeldin A, and Monensin alone. After incubation, cells were stained with CD3-PacBlue and CD8-APC-H7, CCR7-PeCy7, CD45RO-PE, and/or CD69-PerCP anti-human mAbs, followed by fixation/permeabilization (Cytofix/Cytoperm, BD) and stained with anti-IFN-γ FITC mAb to detect intracellular cytokine production. Lastly, cells were washed with Perm/Wash solution (BD), fixed in 2% paraformaldehyde, and analyzed by flow cytometry.
Cell Proliferation by Carboxy Fluorescein Succinimidyl Ester (CFSE) Labeling
Proliferation of MP-CTL was evaluated using CFSE, as described elsewhere (31) with minor modifications. In brief, MP-CTL labeled with CFSE (Molecular Probes, Eugene, OR) were incubated with stimulator cells (HLA-A2+ CD138+ MM patients’ cells or MM cell lines). As a control, MP-CTL labeled were cultured in media alone. After a 5-day incubation, cells were harvested and stained with anti-CD3 and anti-CD8 mAbs, and evaluated by flow cytometry to measure proliferation.
Cytotoxicity by Calcein-Release Assay
The cytotoxic activity of MP-CTL was measured using a calcein-release cytotoxicity assay, as described previously (32) with minor modifications. Briefly, target cells were labeled with calcein-AM (Molecular Probes) and incubated for 4 hours with MP-CTL at various effector:target cell ratios in 96-well U-bottom microtiter plates (triplicate wells/sample). The fluorescence of each supernatant was monitored at 490 nm excitation and 520 nm emission wavelengths using a VICTOR2-1420 multilabel counter (PerkinElmer, Waltham, MA). Cytotoxicity of CTL was calculated as follows: % Specific Lysis = [(experimental release – spontaneous release) ÷ (maximum release – spontaneous release)].
Statistical Analysis
Results are presented as mean ± SE. Groups were compared using unpaired Student’s t-test. Differences were considered significant when p < 0.05.
RESULTS
A multipeptide (MP) cocktail of XBP1 unspliced, XBP1 spliced, CD138, and CS1-specific peptides display high HLA-A2 binding affinity and stability
The four immunogenic peptides including heteroclitic XBP1 US184–192 (YISPWILAV), heteroclitic XBP1 SP367–375 (YLFPQLISV), native CD138260–268 (GLVGLIFAV), and native CS1239–247 (SLFVLGLFL) (Table 1) have been individually demonstrated to induce an immune response. Here we have evaluated the peptides as a MP cocktail. The HLA-A2-specific binding and stability of the MP cocktail was evaluated by measuring upregulation of HLA-A2 molecules on T2 cells by flow cytometry, and compared with affinity of the HLA-A2-specific control influenza virus matrix protein (IVMP)58–66 peptide. The peptide binding assay demonstrated an increase in HLA-A2 mean fluorescence intensity (MFI) on T2 cells in a dose-dependent manner (0 – 50 μg/ml), reaching a plateau at a total peptide concentration of 25 μg/ml (6.25 μg/peptide/ml; MFI: 10,787.33 ± 2,371.71), which was similar to the highest total peptide concentration tested, 50 μg/ml (MFI: 10,889.33 ± 2,888.48) (Figure 1a). Therefore, a total MP concentration of 25 μg/ml (6.25 μg/peptide/ml) was selected for evaluation of HLA-A2 binding stability.
Table 1.
| TAA | IDENTIFICATION | TYPE | SEQUENCE |
|---|---|---|---|
| XBP1 Unspliced | XBP1184–192 | Heteroclitic | YISPWILAV |
| XBP1 Spliced | XBP1 SP367–375 | Heteroclitic | YLFPQLISV |
| CD138 | CD138260–268 | Native | GLVGLIFAV |
| CS1 | CS1239–247 | Native | SLFVLGLFL |
Figure 1. HLA-A2-specific binding affinity and stability of XBP1 US, XBP1 SP, CD138, and CS1 multipeptide.
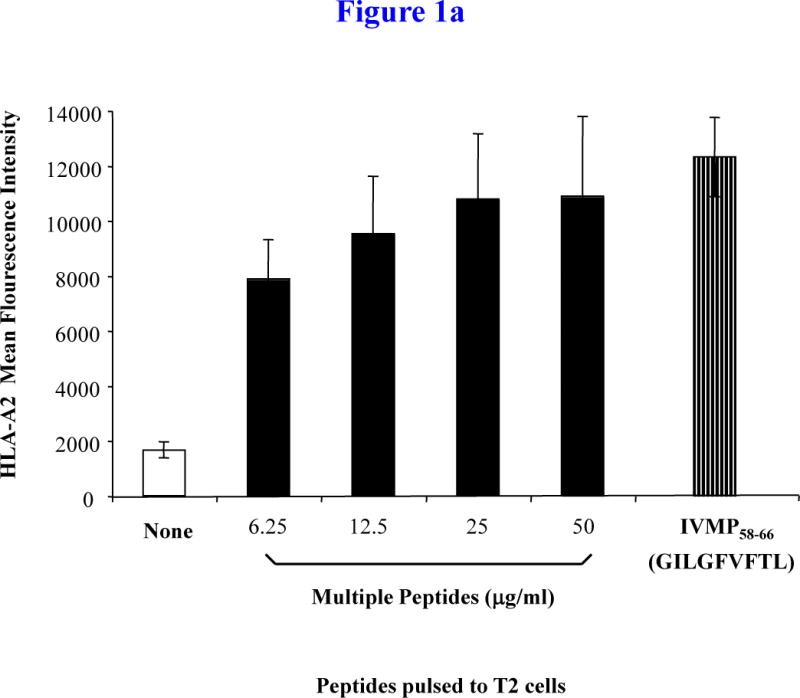
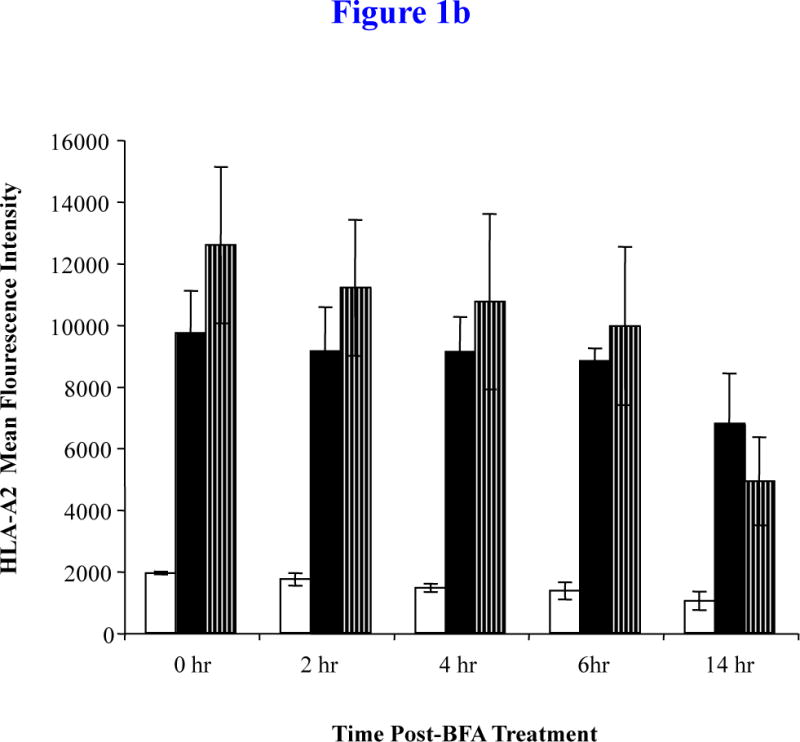
Figure 1a. HLA-A2 binding capacity of multipeptide (MP) cocktail
T2 cells were pulsed overnight with a cocktail of heteroclitic XBP1 US184–192 (YISPWILAV), heteroclitic XBP1 SP367–375 (YLFPQLISV), native CD138260–268 (GLVGLIFAV) and native CS1239–247 (SLFVLGLFL) peptides in serum-fee AIM-V media at total peptide concentrations ranging from 0 μg/ml to 50 μg/ml. Influenza virus matrix protein58–66 (IVMP58–66; GILGFVFTL) was used as an HLA-A2-specific positive control peptide. Following overnight peptide pulsing, T2 cells were harvested, washed, and stained with HLA-A2-FITC mAb for flow cytometric analyses. HLA-A2-specificity of the MP cocktail is shown as an increase in HLA-A2 mean fluorescence intensity (MFI) on T2 cells. The HLA-A2 binding was dose-dependent with the MFI plateau observed at the concentration of 25 μg/ml. The values represent the mean MFI ± SE of three separate experiments.
Figure 1b. HLA-A2 stability of MP cocktail
The MP cocktail (25 μg/ml; 6.25 μg/peptide) pulsed T2 cells were washed and incubated with Brefeldin A (BFA) to block the protein transport of newly synthesized HLA-A2 molecules. The binding stability of MP was measured on T2 cells at 0, 2, 4, 6 and 14 hrs post-BFA treatment and analyzed for HLA-A2 MFI by flow cytometry. An increase in the HLA-A2 MFI was observed at each time point on T2 cells pulsed with the MP from T2 cells alone. The binding of MP was highly stable for up to 6 hours post-BFA treatment. At 14 hrs post-BFA treatment, the stability of MP cocktail was greater than the control IVMP58–66 peptide. The values represent the mean MFI ± SE of three separate experiments.
In the peptide binding stability assay, T2 cells were pulsed overnight with 25 μg/ml of the MP cocktail, washed to remove unbound peptides, and then treated with Brefeldin A (BFA) to block cell surface expression of newly synthesized HLA-A2 molecules. T2 cells were then evaluated for their HLA-A2 MFI at 0, 2, 4, 6 or 14 hrs post-BFA treatments. Flow cytometric analysis demonstrated that stability of the MP cocktail was highly maintained up to 6 hrs post-BFA treatment (MFI: 0 hr = 9,726.00 ± 1,373.24, 2 hr = 9,132.33 ± 1,435.51, 4 hr: 9,125.33 ± 1,130.62, 6 hr: 8,818.67 ± 413.50) (Figure 1b). At 14 hr post-BFA treatment, HLA-A2-specific affinity of MP cocktail was decreased, but was still greater (MFI: 6,793.67 ± 1,617.01) than affinity of the control IVMP58–66 peptide (MFI: 4,921.33 ± 1,428.16). Based on these results, we confirmed a high level of HLA-A2-specific affinity and stability of the MP cocktail and then proceeded to further evaluate the cocktail for its immunogenicity and ability to induce MM-specific CTL.
Multipeptide-specific CTL display a distinct phenotype representing specific T cell subtypes
Flow cytometric analyses showed that MP-CTL contained a higher proportion of CD3+CD8+ T cells (donor 1: 86%, donor 2: 74%) compared to control T cell cultures (donor 1: 25%, donor 2: 25%) (Figure 2). We also observed distinct phenotypic changes in the CD3+CD8+ T cell subset within the MP-CTL. The frequency of effector memory T cells (EM: CD45RO+CCR7−/CD3+CD8+) was increased (Donor 1: Control 5% vs. MP-CTL 44%, Donor 2: Control 4% vs. MP-CTL 35%), associated with a corresponding decrease in naïve T cells (CD45RO−CCR7+/CD3+CD8+) (Donor 1: Control 74% vs. MP-CTL 8%, Donor 2: Control 60% vs. MP-CTL 6%). In addition, we observed an increase in the frequency of activated CD69+/CD3+CD8+ T cells within the MP-CTL as compared to the control T cell cultures (Donor 1: Control 3% vs. MP-CTL 39%, Donor 2: Control 5% vs. MP-CTL 13%) (Figure 2). Thus, these results demonstrate that repeated stimulation of CD3+ T cells with the MP cocktail comprised of XBP1-US, XBP1-SP, CD138, and CS1-specific peptides results in distinct phenotypic changes and expansion of CD3+/CD8+ T cell subsets characteristic of antigen-specific CTL.
Figure 2. Distinct phenotype of multipeptide-specific CTL (MP-CTL).
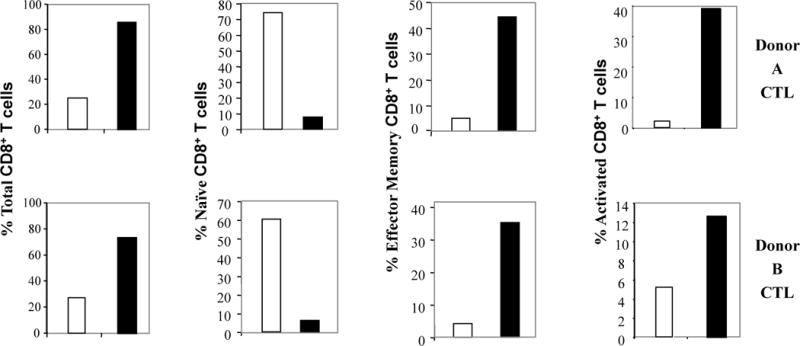
MP-CTL were generated from HLA-A2+ normal donors’ CD3+ T cells by repeated stimulation with irradiated APC pulsed with a cocktail of peptides (25 μg/ml; 6.25 μg/peptide) derived from XBP1 US, XBP1 SP, CD138, and CS1 antigen. One week after their fourth stimulation, the MP-CTL were evaluated for their phenotypic profile by flow cytometry. The MP-CTL (■) obtained from two different donors (Donor A, Donor B) showed a higher percentage of total CD8+ T cells as compared to the control unstimulated CD3+ T cells (□). The MP-CTL were further characterized as having higher frequencies of effector memory cells (CD45RO+CCR7−), higher activated (CD69+) cells and a corresponding lower percentage of naïve cells (CD45RO−CCR7+) within the CD3+CD8+ T cell subset.
Multipeptide-specific CTL include a high proportion of CD8+ CTL producing IFN-γ in response to HLA-A2+ MM cells
In previous studies, we have demonstrated HLA-A2-restricted and MM-specific IFN-γ production by CTL generated specifically with XBP1 US, XBP1 SP, CD138 or CS1 peptide in response to patient MM cells or MM cell lines (25, 26, 27). In the current study, MP-CTL were analyzed by flow cytometry for their ability to produce intracellular IFN-γ upon stimulation with the HLA-A2+ MM cell lines. Both EM (CD45RO+CCR7−) and activated (CD69+) CD3+CD8+ T cells within the MP-CTL produced IFN-γ in response to HLA-A2+ MM cell lines (Figure 3). The frequency of IFN-γ producing cells was increased upon stimulation with either McCAR cells [Donor 1: Control vs. MP-CTL - 0% vs. 4.7% EM cells, 0.8% vs. 6.1% activated cells; Donor 2: 0.2% vs. 2.7% EM cells, 1% vs. 3.9% activated cells] or U266 cells [Donor 1: Control vs. MP-CTL - 0% vs. 8% EM cells, 0% vs. 11.2% activated cells; Donor 2: 0.4% vs. 2.9% EM cells, 1.3% vs. 3.0% activated cells]. The naïve (CD45RO−CCR7+) CD3+CD8+ T cells within the MP-CTL showed a minimum level of IFN-γ-production when stimulated with the HLA-A2+ MM cell lines (data not shown).
Figure 3. IFN-γ production by MP-CTL.
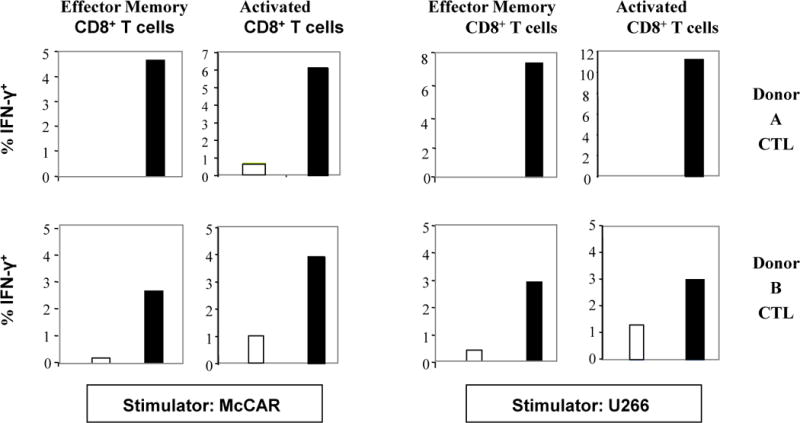
Intracellular IFN-γ production was measured by flow cytometry after stimulation of the MP-CTL with HLA-A2+ MM cell lines. The effector memory (CD45RO+/CCR7−) and activated (CD69+) CD3+CD8+ T cells within the MP-CTL (■) demonstrated increased IFN-γ production (% IFN-γ+ cells) in response to the HLA-A2+ MM cell lines (McCAR, U266). In contrast, control T cells (□) did not produce IFN-γ in response to the MM cell lines.
Multipeptide-specific CTL proliferate in response to HLA-A2+ MM cells
The function of the MP-CTL was analyzed using a CFSE-proliferation assay. MP-CTL proliferation was measured on day 5, evidenced by a decrease in fluorescence of the CFSE-labeled MP-CTL (Q1-gated cells) following stimulation with HLA-A2+ MM primary cells or cell lines (Figure 4). The MP-CTL proliferated in response to CD138+ MM cells from three HLA-A2+ MM patients (proliferating cells: 33%, 29% or 41%). In addition, MP-CTL also proliferated in response to McCAR (proliferating cells: 57%) and U266 (proliferating cells: 49%) HLA-A2+ MM cell lines. MP-CTL cultured in media alone displayed only a low level (5%) proliferation. Taken together, these data demonstrate the proliferation of MP-CTL in stimulation with either HLA-A2+ patient MM cells or MM cell lines.
Figure 4. Induction of MP-CTL proliferation in stimulation with HLA-A2+MM cells including primary cells and cell lines.
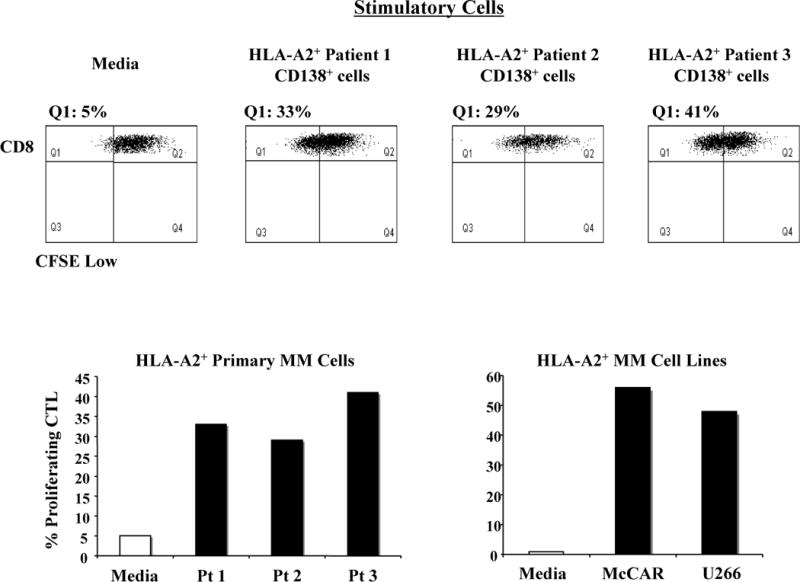
One week after fourth stimulation, MP-CTL were evaluated by flow cytometry for their cell proliferation by culture of the CFSE-labeled MP-CTL with HLA-A2+ primary MM cells or cell lines. The cell proliferation was measured as the percent decrease in CFSE expression (Q1-gated) on day 5 of culture after gating on the CD3+CD8+ population. The MP-CTL cultured in media alone was used to determine background cell proliferation. MP-CTL demonstrated an increase in cell proliferation in stimulation with primary CD138+ cells isolated obtained from three different HLA-A2+ MM patients (top). The percentage of proliferating CTL is summarized in response to the HLA-A2+ primary MM cells (bottom left) or HLA-A2+ McCAR and U266 MM cell lines (bottom right).
Multipeptide-specific CTL induce specific lysis of HLA-A2+ MM cells
Next, we evaluated the cytotoxic activity of MP-CTL using a 4-hour calcein-release assay. MP-CTL generated from different HLA-A2+ donors’ CD3+ T cells were evaluated for their cytotoxic activity against HLA-A2+ MM patient cells or MM cell lines (Figure 5). The HLA-A2+ MM patients’ cells were effectively lysed by MP-CTL at various Effector:Target cell ratios (Donor A MP-CTL; Patient #1: 0 – 48%, Patient #2: 9 – 42%), (Donor B MP-CTL; Patient #1: 0 – 45%, Patient #2: 1 – 35%). In addition, MP-CTL similarly demonstrated a high level of cytotoxic activity against McCAR cells (Donor A MP-CTL: 8 – 36%, Donor B MP-CTL: 0 – 74%:) and U266 (Donor A MP-CTL: 0 – 83%, Donor B MP-CTL: 2 – 43%) MM cell lines. Compared to MP-CTL, control CD3+ T cells from the same donors showed a significantly lower level of cytotoxicity against HLA-A2+ MM patient cells or MM cell lines. In addition, MP-CTL did not lyse MHC mismatched tumor cells, including the HLA-A2− MM cell line (MM1S) or HLA-A2− MM cells from three different patients (data not shown). Taken together, these data confirm the HLA-A2-restricted cytotoxic activity of MP-CTL against HLA-A2+ MM cells.
Figure 5. Cytotoxic activity of MP-CTL against HLA-A2+MM cells including primary MM cells and cell lines.
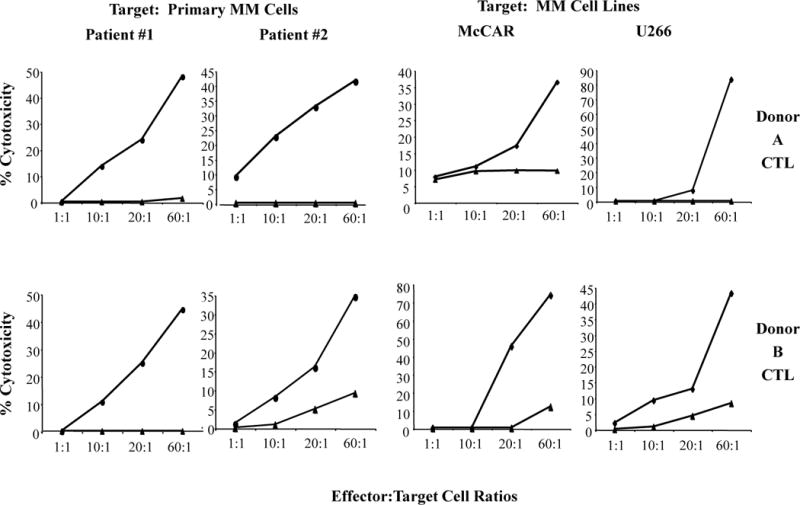
Specific cytotoxic activity of the MP-CTL was analyzed one week after their fourth stimulation using a 4-hour calcein-release assay. MP-CTL (●) generated from T cells of Donor A (top) and Donor B (bottom) demonstrated effective lysis of both primary cells from two HLA-A2+ MM patients and HLA-A2+ U266 and McCAR MM cell lines. Control unstimulated T cells (▲) did not induce significant lysis of primary MM cells or cell lines.
Multipeptide-specific CTL generate individual immune responses to each relevant peptide
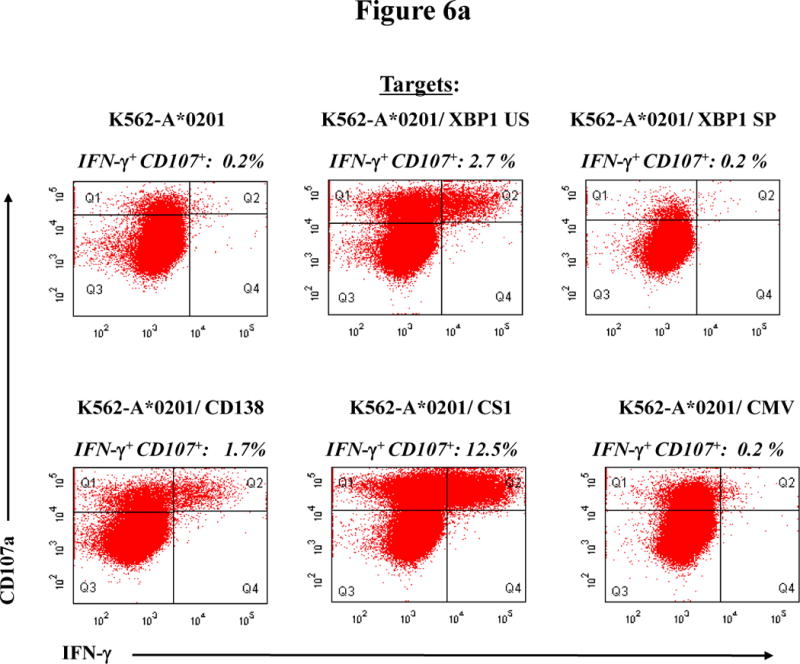
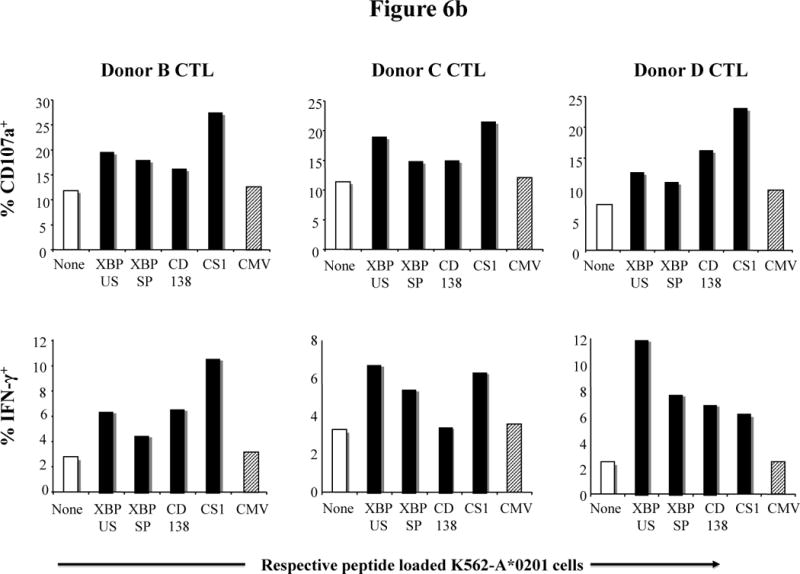
Finally, we evaluated whether CTL generated using MP cocktail responds to each of the peptides individually. MP-CTL were analyzed for their ability to degranulate (CD107a expression) and produce intracellular IFN-γ in response to heteroclitic XBP1 US184–192 (YISPWILAV), heteroclitic XBP1 SP367–375 (YLFPQLISV), native CD138260–268 (GLVGLIFAV), and native CS1239–247 (SLFVLGLFL) peptides. The analyses were performed by measuring the specific MP-CTL response to K562-A*0201 cells (33) pulsed with the respective peptide. As controls, we used non peptide-pulsed K562-A*0201 cells or K562-A*0201 cells pulsed with an irrelevant HLA-A2-specific CMV pp65 (NLVPMVATV) peptide. Figure 6a shows a representative flow cytometric analysis of the peptide-specific response from Donor A MP-CTL. The MP-CTL showed a high proportion of CD107a+IFNγ+/CD3+CD8+ T cells (gated Q2) in response to XBP1 US (2.7%), CD138 (1.7%), and CS1 (12.5%) peptides, but not to XBP1 SP (0.2%) peptide. No response was observed to the irrelevant CMV pp65 peptide (0.2%) or to the non peptide (0.2%) controls. Further analyses were performed using MP-CTL generated from three additional HLA-A2+ donors (Donor B, Donor C, Donor D) for their CD107a degranulation or IFN-γ production in response to K562-A*0201 cells presenting each individual peptide (Figure 6b). Specific responses were detected in MP-CTL generated from each of these donors against all the relevant peptides, but not to irrelevant HLA-A2 specific CMV pp65 peptide. However, variations were detected among the CTL generated from different individuals in the level of specific response in degranulation and IFN-γ production to each relevant peptide. Therefore, these studies indicate that the MP cocktail including XBP1 US, XBP1 SP, CD138, and CS1 epitopes can induce response to respective peptides, with specific CTL targeting multiple antigens on HLA-A2+ MM cells.
Figure 6. Peptide-specific activities of MP-CTL in degranulation and IFN-γ production.
MP-CTL generated with a cocktail of XBP1 US, XBP1 SP, CD138, and CS1 peptides were evaluated for their respective peptide-specific responses. Specific activities of MP-CTL were measured in response to the individual peptide pulsed APC by detecting the increase of CD107a+ or IFN-γ+ cells.
Figure 6a. Peptide-specific activities of MP-CTL generated from Donor A
Representative flow cytometric analyses of CD107a degranulation and IFN-γ production (Q2 gated) in CD3+CD8+ T cells within MP-CTL generated from a single donor (Donor A). The MP-CTL demonstrate peptide-specific responses to the XBP1 US, CD138 or CS1 peptide presented in K562-A*0201 cells. In contrast, the MP-CTL did not show specific responses to the XBP1 SP, irrelevant HLA-A2-specific CMV pp65 (NLVPMVATV) or no peptide pulsed K562-A*0201 cells.
Figure 6b. Peptide-specific activities of MP-CTL generated from Donor B, Donor C and Donor D
Peptide-specific CD107a degranulation and IFN-γ production was further investigated in MP-CTL generated from three additional HLA-A2+ donors (Donor B, Donor C and Donor D). The MP-CTL generated from each of these donors demonstrated specific responses to all the relevant XBP1 US, XBP1 SP, CD138, and CS1 peptides. Their responses show an increase in the frequency of total CD107a upregulation (top panel) and IFN-γ production (bottom panel).
DISCUSSION
The discovery and application of novel immunogenic peptides offers a potentially new immunotherapeutic option, either as a vaccine or cellular therapy. The XBP1, CD138 and CS1 antigens have been implicated in MM pathogenesis, and all are more highly expressed on patient MM cells compared to normal plasma cells. Indeed, the therapeutic potential of targeting these antigens has been evaluated with promising preclinical and clinical studies (34, 35). However, immunologic tolerance to antigens as self-proteins may inhibit development of an effective immune response and therefore be detrimental to developing an effective therapeutic strategy (36–38). In order to bypass tolerance and enhance peptide immunogenicity, we designed heteroclitic peptides YISPWILAV or YLFPQLISV from non-spliced or spliced XBP1 protein, respectively, which have higher HLA-A2 affinities than their originally identified native XBP1 US184–192 (NISPWILAV) or XBP1 SP367–375 (ELFPQLISV) peptides (25). In previous studies, we and others have demonstrated that heteroclitic peptides can generate functional CTL against tumor cells with cross-reactivity to their corresponding native peptides, suggesting their clinical applicability (29, 39, 40). In addition, the native peptides from CD138 and CS1 antigens, CD138260–268 (GLVGLIFAV) and CS1239–247 (SLFVLGLFL), were highly specific to the HLA-A2 locus, maintained a strong MHC/peptide stability complex, and induced functional anti-MM CTL (26, 27). Therefore, the CD138260–268 and CS1239–247 peptides were used in their native form and were not further modified. These peptides, especially in combination, may be useful for the development of a vaccine strategy to treat MM-related diseases.
In the current studies, we evaluated the ability of a cocktail of these four HLA-A2 peptides to induce specific CTL responses against the respective MM target antigens. We hypothesized that a multi-epitope vaccine would allow for a wider repertoire of tumor-associated peptides to be presented, thereby inducing a more robust immune response against tumor cells compared to vaccines specific to a single antigen, which may lose activity following specific antigen mutation or deletion on tumor cells. Moreover, this approach can also overcome the variation or absence of the appropriate T-cell repertoire, which can result in the lack of peptide-specific CTL induction to a single antigen-based vaccine. Therefore, we propose that a using a cocktail of immunogenic peptides capable of generating CTL to multiple MM-associated antigens represents a more promising immunotherapeutic strategy.
We recognize the potential concern of epitope dominance and competition among these peptides specific to the same HLA molecules, which may impair or block the full spectrum of immune response against all of the target antigens (41). When using a mixture of peptides specific to the same MHC locus, a specific concern arises whether the lower-affinity peptides will effectively bind and present in MHC molecules to induce T cell responses in the presence of higher-affinity peptides. In clinical trials of multipeptide vaccines, with each peptide has been administered at a different injection site to avoid this possibility of competition among peptides (42–44). However, this requirement might limit feasibility of this approach, since many novel peptides specific to TAAs have been defined. Here, we investigated whether the four peptides selected can be applied in combination to induce MP-CTL. In these studies, we generated MP-CTL ex vivo by stimulating HLA-A2+ normal donors’ CD3+ T cells with APC pulsed with a cocktail of the four immunogenic peptides. To avoid potential competition in HLA-A2 affinity among the specific peptides, we avoided excess concentrations of an individual peptide by using a minimum concentration of MP (25 μg/ml total; 6.25 μg/peptide) to pulse the APC during CTL generation. Our data showed that simultaneous pulsing with a four peptide cocktail did not compromise the functional immune activity of resultant MP-CTL. Importantly, MP-CTL demonstrated specific functional activities, IFN-γ production, CD107 degranulation, and cell proliferation triggered by each relevant peptide, but not to an irrelevant HLA-A2-specific CMV pp65 (NLVPMVATV) peptide. The XBP1 SP367–375 and CS1239–247 peptides, which have relatively lower HLA-A2 affinity, induced CTL whose immunological function was comparable to CTL generated with the higher HLA-A2 affinity XBP1 US184–192 and CD138260–268 contained within the cocktail. Thus, we demonstrate that the possible competition among MP, which bind to the same MHC class I molecules, does not compromise immunogenicity of lower-affinity peptides.
These data suggest that immunogenic peptides administered in a mixture may generate functional CTL in patients. In addition, several reports using a mixture of peptides with different HLA-A2 affinities further support our observation. For example, a prior study showed that CTL generated by ex vivo stimulation with a peptide mixture demonstrated reactivity to three different peptides at a level comparable to that obtained by stimulation with each individual peptide separately (45). This study also demonstrated that CTL recognition of lower-affinity peptides specific to HLA-A2 molecules was maintained when target cells were co-pulsed with higher affinity peptides. Other investigators have also reported that competition among peptides for MHC binding does not significantly inhibit T-cell induction or activities (46, 47). Our in vitro studies utilized normal donor T cells and DC in order to optimize vaccine development. Importantly, defects in both T cells and DC have been described in MM patients. Thus, we are currently in evaluation of the MP for their ability to elicit tumor-specific immune response using patients’ T cells and DC. In addition, their functional capacity to respond to MP vaccine, as well as its clinical relevance, will be assessed in a clinical trial.
Other considerations that influence the success of vaccine trials include: selection of an optimal adjuvant, inclusion of MHC class II-specific peptides, selection of the appropriate patient population, as well as concomitant chemotherapy, monoclonal antibody therapy, or immunomodulatory drug therapy. For example, a previous phase III randomized trial did not show a superior clinical response to the gp100 peptide vaccine combined with ipilimumab (anti-CTLA4) compared to ipilimumab alone (48). However, the gp100 peptide vaccine demonstrated an improved clinical response in a more recent phase III trial reported, when it was co-administered with IL-2 (49). This difference both highlights the need for further validation of these results trials, and suggests important differences in adjuvant therapies administered with the vaccine. Besides anti-CTLA4 and IL-2, the efficacy of other adjuvants such as CpG, GM-CSF, IFN-α and Montanide ISA51 has been reported in various studies (7, 50–52). Importantly, combination studies with vaccines must be designed very carefully since patients may already have received chemotherapy with long-lasting negative effects on their immune systems, thus weakening the potential benefit of a therapeutic vaccine. In our separate studies, we have demonstrated that conventional chemotherapy used in MM is detrimental to the function of immune-mediated responses (53, 54). However, our and other recent studies have shown that the immunomodulatory agent lenalidomide increases immune stimulatory properties and inhibits regulatory T-cells in MM (55); thus, the efficacy of a vaccine may be enhanced when used in combination with lenalidomide. In addition, induction of CD4+ T cell response using MHC class II peptides may be critical for establishing more long-term immunity to the HLA-A2-specific peptides (56, 57).
In summary, we have developed an immunotherapy targeting multiple TAA using a cocktail comprised of XBP1-US, XBP1-SP, CD138 and CS1-specific epitopes, which may be applied in MM and other plasma cell disorders. This proposed novel vaccine-based therapy will first be evaluated as an individual immunotherapy, but may require additional incorporation of optimal adjuvants, MHC Class II peptides, and/or immunomodulatory agents in suitable patient populations.
Statement of Translational Relevance.
In these studies, we provide evidence that a cocktail of four HLA-A2-specific peptides derived from the XBP1 unspliced, XBP1 spliced, CD138, and CS1 antigens induces multipeptide-specific CTL with a characteristic phenotypic profile enriched for CD8+ effector memory T cells and distinct functional immunogenic properties against HLA-A2+ multiple myeloma cells. These results suggest the potential therapeutic application of this cocktail of peptides to induce CTL with a broad spectrum of immune responses against antigens-associated with MM pathogenesis. This proposed multipeptide vaccine therapy might provide for targeted immunotherapy, alone or with optimal adjuvants and/or combinational drug therapies, to improve outcome in patients with plasma cell related disorders.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health Grants RO1-124929 to Dr. Nikhil C. Munshi, P50-100007, PO1-78378 and PO1155258 to Drs. Kenneth C. Anderson and Nikhil C. Munshi, and RO1-50947 to Dr. Kenneth C. Anderson. This research was also supported in part by a kind donation from Mr. and Mrs. Stewart Nagler. Dr. Kenneth C. Anderson is an American Cancer Society Clinical Research Professor.
References
- 1.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KC, Carrasco RD. Pathogenesis of myeloma. Annu Rev Pathol. 2011;6:249–74. doi: 10.1146/annurev-pathol-011110-130249. [DOI] [PubMed] [Google Scholar]
- 3.Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silk AW, Finn OJ. Cancer vaccines: a promising cancer therapy against all odds. Future Oncol. 2007;3:299–306. doi: 10.2217/14796694.3.3.299. [DOI] [PubMed] [Google Scholar]
- 5.Speiser DE, Romero P. Molecularly defined vaccines for cancer immunotherapy, and protective T cell immunity. Semin Immunol. 2010;22:144–54. doi: 10.1016/j.smim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies FE, Dring AM, Li C, Rawstron AC, Shammas MA, O’Connor SM, et al. Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood. 2003;102:4504–11. doi: 10.1182/blood-2003-01-0016. [DOI] [PubMed] [Google Scholar]
- 10.Bagratuni T, Wu P, Gonzalez de Castro D, Davenport EL, Dickens NJ, Walker BA, et al. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood. 2010;116:250–3. doi: 10.1182/blood-2010-01-263236. [DOI] [PubMed] [Google Scholar]
- 11.Liou HC, Boothby MR, Finn PW, Davidon R, Nabavi N, Zeleznik-Le NJ, et al. A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science. 1990;247:1581–4. doi: 10.1126/science.2321018. [DOI] [PubMed] [Google Scholar]
- 12.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 14.Mori K. Frame switch splicing and regulated intramembrane proteolysis: key words to understand the unfolded protein response. Traffic. 2003;4:519–28. doi: 10.1034/j.1600-0854.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 15.Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 16.Wolowiec D, Dybko J, Wrobel T, Urbaniak-Kujda D, Jazwiec B, Tomaszewska-Toporska B, et al. Circulating sCD138 and some angiogenesis-involved cytokines help to anticipate the disease progression of early-stage B-cell chronic lymphocytic leukemia. Mediators Inflamm. 2006;2006:42394–9. doi: 10.1155/MI/2006/42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989;1:27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100:610–7. doi: 10.1182/blood.v100.2.610. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson RD, Yang Y. Syndecan-1: a dynamic regulator of the myeloma microenvironment. Clin Exp Metastasis. 2008;25:149–59. doi: 10.1007/s10585-007-9125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, et al. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol Cancer Ther. 2009;8:2616–24. doi: 10.1158/1535-7163.MCT-09-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy JJ, Hobby P, Vilarino-Varela J, Bishop B, Iordanidou P, Sutton BJ, et al. A novel immunoglobulin superfamily receptor (19A) related to CD2 is expressed on activated lymphocytes and promotes homotypic B-cell adhesion. Biochem J. 2002;361:431–6. doi: 10.1042/0264-6021:3610431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumaresan PR, Lai WC, Chuang SS, Bennett M, Mathew PA. CS1, a novel member of the CD2 family, is homophilic and regulates NK cell function. Mol Immunol. 2002;39:1–8. doi: 10.1016/s0161-5890(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 24.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–37. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC, et al. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: a potential therapeutic application in multiple myeloma. Leukemia. 2011;25:1610–9. doi: 10.1038/leu.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae J, Tai YT, Anderson KC, Munshi NC. Novel epitope evoking CD138 antigen-specific cytotoxic T lymphocytes targeting multiple myeloma and other plasma cell disorders. Br J Haematol. 2011;155:349–61. doi: 10.1111/j.1365-2141.2011.08850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae J, Song W, Smith R, Daley J, Tai YT, Anderson KC, et al. A novel immunogenic CS1-specific peptide inducing antigen-specific cytotoxic T lymphocytes targeting multiple myeloma. Br J Haematol. 2011 doi: 10.1111/j.1365-2141.2012.09111.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, Fukushima P, Barrett AJ. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–7. [PubMed] [Google Scholar]
- 29.Bae J, Martinson JA, Klingemann HG. Identification of novel CD33 antigen-specific peptides for the generation of cytotoxic T lymphocytes against acute myeloid leukemia. Cell Immunol. 2004;227:38–50. doi: 10.1016/j.cellimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Toujas L, Delcros JG, Diez E, Gervois N, Semana G, Corradin G, Jotereau F. Human monocyte-derived macrophages and dendritic cells are comparably effective in vitro in presenting HLA class I-restricted exogenous peptides. Immunology. 1997;91:635–42. doi: 10.1046/j.1365-2567.1997.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angulo R, Fulcher DA. Measurement of Candida-specific blastogenesis: comparison of carboxyfluorescein succinimidyl ester labelling of T cells, thymidine incorporation, and CD69 expression. Cytometry. 1998;34:143–51. [PubMed] [Google Scholar]
- 32.Roden MM, Lee KH, Panelli MC, Marincola FM. A novel cytolysis assay using fluorescent labeling and quantitative fluorescent scanning technology. J Immunol Methods. 1999;226:29–41. doi: 10.1016/s0022-1759(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 33.Britten CM, Meyer RG, Kreer T, Drexler I, Wolfel T, Herr W. The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8(+) T lymphocytes in IFN-gamma ELISPOT assays. J Immunol Methods. 2002;259:95–110. doi: 10.1016/s0022-1759(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 34.Lutz RJ, Whiteman KR. Antibody-maytansinoid conjugates for the treatment of myeloma. MAbs. 2009;1:548–51. doi: 10.4161/mabs.1.6.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–4. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borset M, Hjertner O, Yaccoby S, Epstein J, Sanderson RD. Syndecan-1 is targeted to the uropods of polarized myeloma cells where it promotes adhesion and sequesters heparin-binding proteins. Blood. 2000;96:2528–36. [PubMed] [Google Scholar]
- 37.Lotz C, Mutallib SA, Oehlrich N, Liewer U, Ferreira EA, Moos M, et al. Targeting positive regulatory domain I-binding factor 1 and X box-binding protein 1 transcription factors by multiple myeloma-reactive CTL. J Immunol. 2005;175:1301–9. doi: 10.4049/jimmunol.175.2.1301. [DOI] [PubMed] [Google Scholar]
- 38.Kim JR, Mathew SO, Patel RK, Pertusi RM, Mathew PA. Altered expression of signalling lymphocyte activation molecule (SLAM) family receptors CS1 (CD319) and 2B4 (CD244) in patients with systemic lupus erythematosus. Clin Exp Immunol. 2010;160:348–58. doi: 10.1111/j.1365-2249.2010.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Roberts W, Scheinberg DA. Synthetic peptide analogs derived from bcr/abl fusion proteins and the induction of heteroclitic human T-cell responses. Haematologica. 2005;90:1324–32. [PubMed] [Google Scholar]
- 40.May RJ, Dao T, Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Zhang RH, et al. Peptide epitopes from the Wilms’ tumor 1 oncoprotein stimulate CD4+ and CD8+ T cells that recognize and kill human malignant mesothelioma tumor cells. Clin Cancer Res. 2007;13:4547–55. doi: 10.1158/1078-0432.CCR-07-0708. [DOI] [PubMed] [Google Scholar]
- 41.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–13. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powell DJ, Jr, Rosenberg SA. Phenotypic and functional maturation of tumor antigen-reactive CD8+ T lymphocytes in patients undergoing multiple course peptide vaccination. J Immunother. 2004;27:36–47. doi: 10.1097/00002371-200401000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–77. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 44.Slingluff CL, Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–44. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson LW, Garbee CF, Hibbitts S, Brinckerhoff LH, Pierce RA, Chianese-Bullock KA, et al. Competition among peptides in melanoma vaccines for binding to MHC molecules. J Immunother. 2004;27:425–31. doi: 10.1097/00002371-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 47.Mullins DW, Engelhard VH. Limited infiltration of exogenous dendritic cells and naive T cells restricts immune responses in peripheral lymph nodes. J Immunol. 2006;176:4535–42. doi: 10.4049/jimmunol.176.8.4535. [DOI] [PubMed] [Google Scholar]
- 48.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N, et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15:2166–73. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 51.Giannopoulos K, Dmoszynska A, Kowal M, Rolinski J, Gostick E, Price DA, et al. Peptide vaccination elicits leukemia-associated antigen-specific cytotoxic CD8+ T-cell responses in patients with chronic lymphocytic leukemia. Leukemia. 2010;24:798–805. doi: 10.1038/leu.2010.29. [DOI] [PubMed] [Google Scholar]
- 52.Kuball J, de Boer K, Wagner E, Wattad M, Antunes E, Weeratna RD, et al. Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909. Cancer Immunol Immunother. 2011;60:161–71. doi: 10.1007/s00262-010-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bae J, Mitsiades C, Tai YT, Bertheau R, Shammas M, Batchu RB, et al. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol. 2007;178:7730–7. doi: 10.4049/jimmunol.178.12.7730. [DOI] [PubMed] [Google Scholar]
- 54.Song W, Tai YT, Tian Z, Hideshima T, Chauhan D, Nanjappa P, et al. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia. 2011;25:161–8. doi: 10.1038/leu.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–90. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 56.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitamura H, Sedlik C, Jacquet A, Zaragoza B, Dusseaux M, Premel V, Sastre-Garau X, Lantz O. Long peptide vaccination can lead to lethality through CD4+ T cell-mediated cytokine storm. J Immunol. 2010;185:892–901. doi: 10.4049/jimmunol.1000933. [DOI] [PubMed] [Google Scholar]


