SUMMARY
Proper cell-cycle transitions are driven by waves of ubiquitin-dependent degradation of key regulators by the anaphase-promoting complex (APC) and Skp1-Cullin1-F-box (SCF) E3 ubiquitin ligase complexes. But precisely how APC and SCF activities are coordinated to regulate cell-cycle progression remains largely unclear. We previously showed that APC/Cdh1 earmarks the SCF component Skp2 for degradation. Here, we continue to report that SCFβ-TRCP reciprocally controls APC/Cdh1 activity by governing Cdh1 ubiquitination and subsequent degradation. Furthermore, we define both cyclin A and Plk1, two well-known Cdh1 substrates, as upstream modifying enzymes that promote Cdh1 phosphorylation to trigger Cdh1 ubiquitination and subsequent degradation by SCFβ-TRCP. Thus, our work reveals a negative repression mechanism for SCF to control APC, thereby illustrating an elegant dual repression system between these two E3 ligase complexes to create the ordered cascade of APC and SCF activities governing timely cell-cycle transitions.
INTRODUCTION
As defective regulation of the cell cycle leads to genomic instability and ultimately cancer development (Harper et al., 2002; Nakayama et al., 2001), identification of key regulatory mechanisms may shed light on mechanisms that initiate and promote carcinogenesis. There is mounting evidence suggesting that proper cell-cycle transitions are driven by coordinated waves of ubiquitin-dependent degradation of key cell-cycle regulators by the anaphase-promoting complex (APC) and Skp1-Cullin1-F-box (SCF) E3 ubiquitin ligase complexes (Harper et al., 2002; Nakayama et al., 2001; Nakayama and Nakayama, 2005). However, molecular mechanisms mediating ordered SCF and APC activities have not been fully characterized. Previously, we and others demonstrated that APC/Cdh1 ubiquitinates and thus targets the SCF component Skp2 for degradation in early G1 phase (Bashir et al., 2004; Wei et al., 2004). This was the first illustration of the crosstalk between SCF and APC, which has shed light on the tight regulatory mechanisms of coordinated cell-cycle progression, gaining important insight as to why SCF and APC activities are mutually exclusive during the cell cycle (Nakayama et al., 2001).
Cdh1 is one of the substrate adaptors for the APC E3 ligase complex that directly interacts with APC/Cdh1 substrates via Destruction Boxes (D-box) or KEN Boxes (Harper et al., 2002; Peters, 2006). Because Cdh1 is a crucial regulator of cell-cycle progression, its function is tightly controlled in vivo. Cdh1 associates with the APC core complex in the late M phase to activate APC/Cdh1, which plays a critical role in mitosis exit by completing the degradation of Securin and cyclin B (Peters, 2006). On the other hand, APC/Cdh1 activity is terminated by multiple mechanisms including cyclin-A-dependent phosphorylation of Cdh1 that dissociates Cdh1 from the APC core complex to ensure a timely transition from G1 to S phase (Lau et al., 2013; Lukas et al., 1999; Sørensen et al., 2001). Notably, Cdh1 protein levels were significantly reduced in the late G1 phase (Benmaamar and Pagano, 2005), suggesting that its stability might be under tight regulation during the G1 to S cell-cycle transition. Although Cdh1 undergoes self-ubiquitination and degradation (Listovsky et al., 2004), this seems to not account for the low Cdh1 abundance during the late G1 phase; as in this specific cell-cycle phase, Cdh1 is inactivated by Cdk2/cyclin-A- and/or Cdk2/cyclin-E-dependent phosphorylation (Keck et al., 2007; Lau et al., 2013; Lukas et al., 1999). Therefore, the molecular mechanism(s) underlying the timely degradation of Cdh1 at the G1-S boundary still remains largely elusive.
Given that APC negatively regulates the SCF complex (Bashir et al., 2004; Wei et al., 2004), it is an intriguing possibility that SCF may reciprocally repress APC, thereby generating a negative feedback loop. In support of this theory, emerging evidence has demonstrated that Cdh1 is unstable, and Cullin-1 is involved in its degradation (Benmaamar and Pagano, 2005). However, the identity of the specific F-box protein that binds Cullin-1 and promotes Cdh1 degradation remains elusive. To date, whereas the F-box protein, Emi-1, has been shown to repress APC/Cdh1 activity (Hsu et al., 2002), it does so not by affecting Cdh1 stability but rather by blocking the ability of Cdh1 to degrade its physiological substrates (Miller et al., 2006). Here, we report that the F-box protein, β-TRCP, regulates Cdh1 degradation. We further defined that sequential phosphorylation of Cdh1 by cyclin A and Plk1 triggers β-TRCP-mediated Cdh1 ubiquitination and subsequent degradation. Therefore, in addition to APC-mediated suppression of SCF by targeting Skp2 for degradation, there also exists a negative repression mechanism by which the SCF complex controls APC E3 ligase activity.
RESULTS
Cullin 1 Controls Cdh1 Stability
Using synchronization methods, we found that, consistent with a previous report (Benmaamar and Pagano, 2005), Cdh1 expression levels fluctuate over the cell cycle with lower expression levels during the S phase (Figures 1A and 1B). Because the APC/Cdh1 E3 ubiquitin ligase activity is inhibited by Cdk2/cyclin A in the late G1/S phase, these results indicate that an as-yet-unidentified E3 ligase, other than Cdh1 itself (Listovsky et al., 2004), might be responsible for mediating the degradation of Cdh1 during this period. In keeping with this notion, compared to wild-type (WT)-Cdh1, ΔFizzy-Cdh1, and ΔC-box-Cdh1 that are deficient in associating with the APC core complex (Figures S1A–S1C), thereby impaired in self-ubiquitination, are still relatively unstable during the G1 phase (Figure S1D). As multisubunit Cullin-Ring complexes comprise the largest known family of E3 ligases (Petroski and Deshaies, 2005), we first examined whether a specific Cullin-Ring complex was involved in regulating Cdh1 degradation. Consistent with a previous report (Benmaamar and Pagano, 2005), inactivation of Cullin 1, by overexpressing a dominant-negative Cullin 1 mutant, significantly increased the abundance of endogenous Cdh1 (Figure 1C). More importantly, ectopic overexpression of other dominant-negative forms of Cullins did not notably affect Cdh1 expression in HeLa cells (Figure 1D), arguing for a specific role of Cullin 1 in governing Cdh1 stability (Figure S1E). Consistently, we detected a specific interaction between Cdh1 and Cullin 1, but not other Cullin family members (Figure 1E). Furthermore, we detected an interaction between endogenous Cdh1 and Cullin 1 (Figure 1F). In further support of a physiological role for Cullin 1 in Cdh1 stability control, depletion of endogenous Cullin 1, but not endogenous Cullin 2 that does not associate with Cdh1 in vivo (Figure 1E), led to a significant increase in Cdh1 abundance (Figures 1G and S1F). These results altogether suggest the involvement of the SCF type of E3 ligase complex in Cdh1 protein stability control.
Figure 1. Cullin 1 Controls Cdh1 Stability.
(A and B) Cdh1 expression levels fluctuate across the cell cycle. (A) Immunoblot (IB) analysis of U2OS cells synchronized by growth in hydroxyurea and then released for the indicated periods of time. (B) IB analysis of HeLa cells arrested in the M phase by nocodazole treatment and then released back into the cell cycle. FACS analysis revealed that cells entered the S phase approximately 12 hr postnocodazole release (see also Figures 6B, 6C, 6E, and 6F).
(C) Induced overexpression of a dominant-negative form of Cullin 1 (N252-Cullin 1) results in upregulation of Cdh1 and other known SCF substrates.
(D) Expression of a dominant-negative (DN) Cullin 1 results in an increase in Cdh1 abundance. IB analysis of whole-cell lysates (WCL) derived from HeLa cells transfected with the indicated constructs encoding various dominant-negative (DN) forms of Cullin family of proteins.
(E and F) Cullin 1 interacts with Cdh1 in vivo. (E) IB analysis of WCL and immunoprecipitates (IP) derived from 293T cells transfected with HA-Cdh1 and the indicated Myc-tagged Cullin family of proteins. Thirty hours posttransfection, cells were pretreated with the proteasome inhibitor MG132 (10 µM) for 10 hr before harvesting to detect the HA-Cdh1 and Myc-Cullin interaction. (F) IB analysis of WCL and anti-Cdh1 IP derived from HeLa cells. Cells were treated with the proteasome inhibitor MG132 (10 µM) overnight before harvesting to detect the interaction of endogenous Cdh1 with the indicated proteins.
(G) Depletion of endogenous Cullin 1 results in an increase in Cdh1 protein levels. IB analysis of WCL derived from HeLa cells infected with the indicated lentiviral shRNA constructs. Twenty hours postinfection, cells were selected with 1 µg/ml puromycin for 72 hr to eliminate noninfected cells.
See also Figure S1.
SCFβ-TRCP Negatively Regulates the Stability of Cdh1
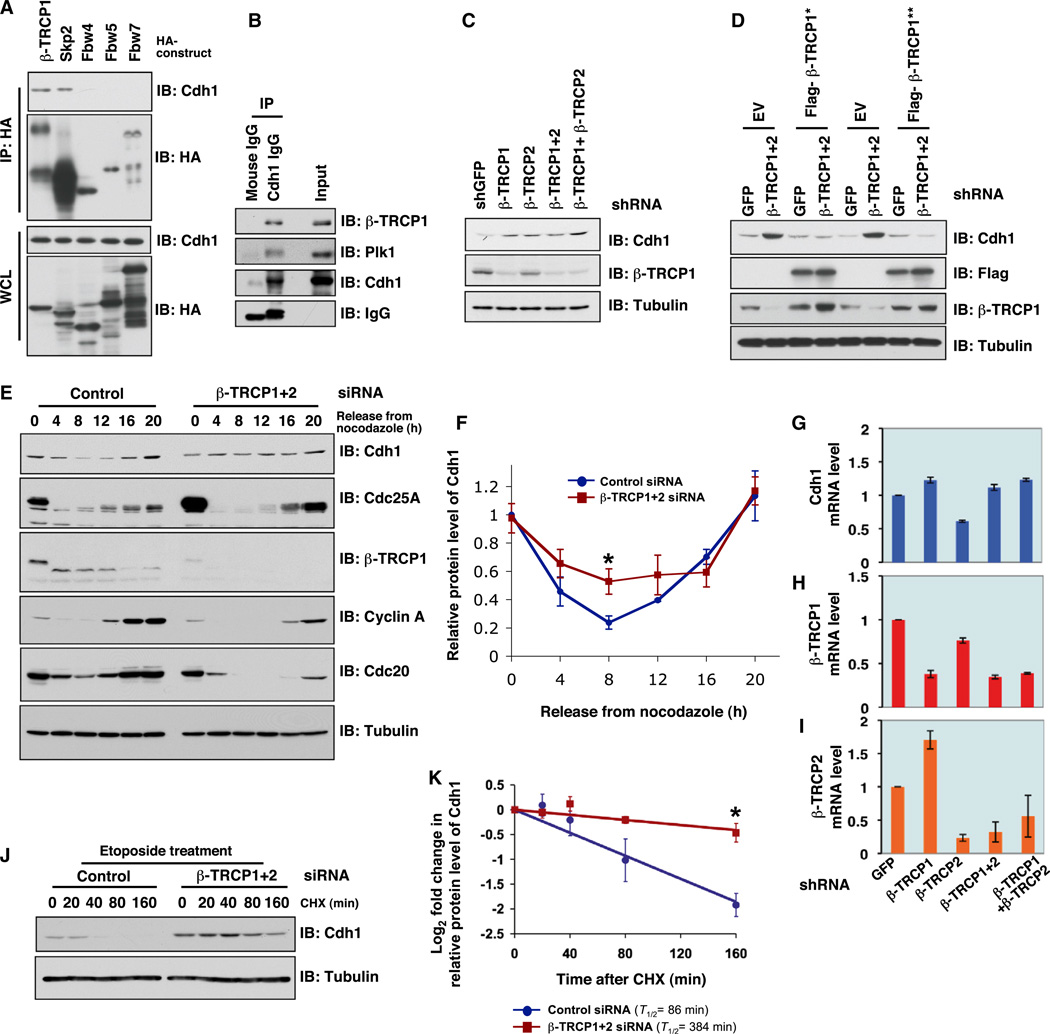
Although Cullin 1 has been reported to control Cdh1 stability (Benmaamar and Pagano, 2005), the identity of the specific F-box protein that binds with Cullin 1 to form a functional SCF type of E3 ligase complex and promotes Cdh1 degradation remains unknown. Because Cdh1 expression fluctuates during the cell cycle, we investigated whether Cdh1 interacts with a panel of F-box proteins with known functions in cell-cycle regulation (Nakayama and Nakayama, 2005). In addition to the known Cdh1 interaction with its substrate Skp2, we only detected a specific interaction between Cdh1 and β-TRCP1, but not with other F-box proteins we examined (Figure 2A). In support of β-TRCP as the specific F-box protein capable of regulating Cdh1 degradation, we detected an interaction between endogenous Cdh1 and β-TRCP1 (Figure 2B).
Figure 2. SCFβ-TRCP Negatively Regulates the Stability of Cdh1.
(A and B) Cdh1 interacts with β-TRCP1 in vivo. (A) Immunoblot (IB) analysis of whole-cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with constructs encoding the indicated HA-tagged F-box proteins. Thirty hours posttransfection, cells were pretreated with the proteasome inhibitor MG132 (10 µM) for 10 hr before harvesting to detect the interaction of endogenous Cdh1 with the indicated HA-tagged F-box proteins. (B) IB analysis of WCL and anti-Cdh1 IP derived from 293T cells. Cells were treated with the proteasome inhibitor MG132 (10 µM) overnight before harvesting to detect the interaction between endogenous Cdh1 and β-TRCP1 proteins.
(C) Depletion of endogenous β-TRCP leads to an increase in endogenous Cdh1 abundance. IB analysis of WCL derived from HeLa cells infected with the indicated lentiviral shRNA constructs. Twenty hours postinfection, cells were selected with 1 µg/ml puromycin for 72 hr to eliminate noninfected cells.
(D) Expression of engineered shRNA-resistant versions of β-TRCP1 in shβ-TRCP-treated cells suppressed Cdh1 upregulation. IB analysis of 293T cells transfected with the indicated shRNA constructs. Where indicated, Flag-β-TRCP1* or Flag-β-TRCP1**, which is engineered to resist shβ-TRCP1 or shβ-TRCP1+2, respectively, was included in the transfection.
(E and F) Depletion of endogenous β-TRCP by siRNA treatment results in elevated Cdh1 expression in late G1/S phases. (E) HeLa cells were transfected with control or β-TRCP1+2 siRNA oligos, arrested with nocodazole and then released back into the cell cycle for immunoblot analysis. Cdc25A was blotted as a positive control of a reported β-TRCP substrate. (F) Quantification of the band intensities in (E). The intensities of Cdh1 bands were normalized to tubulin, then normalized to the t = 0 time point. Data are represented as mean ± SD, n = 3, and *p < 0.05 (Student’s t test).
(G–I) Depletion of endogenous β-TRCP does not significantly affect Cdh1 mRNA levels. Real-time RT-PCR analysis to examine the relative mRNA expression level changes in Cdh1 (G), β-TRCP1 (H), and β-TRCP2 (I) after shRNA treatments as described in (C). Data are represented as mean ± SD, n = 3.
(J and K) Depletion of endogenous β-TRCP extends the half-life of endogenous Cdh1. (J) HeLa cells were transfected with control or β-TRCP1+2 siRNA oligos and then treated with etoposide to induce DNA damage. Afterward, Cdh1 half-life was measured using cycloheximide (CHX). (K) Quantification of the band intensities in (J). The intensities of Cdh1 bands were normalized to tubulin and then normalized to the t = 0 time point. Data are represented as mean ± SD, n = 3, and *p < 0.05 (Student’s t test).
See also Figure S2.
Furthermore, depletion of β-TRCP1 and β-TRCP2 in asynchronized HeLa cells led to a significant increase in steady-state levels of Cdh1 (Figure 2C), which can be rescued by expression of engineered small-hairpin-RNA (shRNA)-resistant β-TRCP1, highlighting the specificity of the shβ-TRCP treatment to result in an elevation of Cdh1 expression (Figure 2D). Compared to the control small-interfering-RNA (siRNA)-treated HeLa cells, degradation of Cdh1 in the late G1-S phase was largely abolished after depletion of β-TRCP (Figures 2E and 2F). Likewise, siβ-TRCP treatment resulted in decreased expression of the Cdh1 substrates cyclin A and Cdc20, suggesting a delayed entry into the S phase (Figures 2E). Moreover, depletion of endogenous β-TRCP1 and β-TRCP2 resulted in an increase in the abundance of both WT-Cdh1 and ΔFizzy-Cdh1, indicating that SCFβ-TRCP possibly governs Cdh1 stability independent of Cdh1 self-ubiquitination (Figure S2A).
Notably, depletion of β-TRCP did not significantly increase Cdh1 mRNA levels, indicating that the observed increase in Cdh1 abundance may be mainly through a posttranslational mechanism(s) (Figure 2G). However, depletion of β-TRCP2, but not β-TRCP1, led to a moderate decrease in Cdh1 mRNA levels, but the biological significance of this change currently remains unclear (Figure 2G). Furthermore, depletion of β-TRCP1 resulted in an approximately 1.7-fold induction of β-TRCP2 mRNA levels (Figure 2I), which might be in part explained by a possible compensation effect. Although β-TRCP1 and β-TRCP2 have been indicated to possess similar enzymatic functions in various in vitro biochemical reactions (Cardozo and Pagano, 2004; Fuchs et al., 2004), there is emerging evidence indicating that their cellular localization or tissue specificity might differ significantly (Lassot et al., 2001; Seo et al., 2009). In addition, β-TRCP1 and β-TRCP2 have been found to respond to different upstream signals (Spiegelman et al., 2001, 2002). Hence, additional studies are required to fully understand the underlying mechanisms and biological outputs for the observed induction of β-TRCP2 upon acute depletion of β-TRCP1. Importantly, in support of a role for β-TRCP in modulating Cdh1 stability, we demonstrated that increased Cdh1 expression in the late G1-S phase following β-TRCP depletion was in part due to prolonged Cdh1 half-life (Figures S2B and S2C). Moreover, Cdh1 half-life was greatly reduced following DNA damage (Liu et al., 2008), whereas depletion of β-TRCP extended the half-life of Cdh1 in this experimental condition (Figures 2J and 2K), confirming the important physiological role of β-TRCP in regulating Cdh1 turnover.
Plk1 and Cdk2/Cyclin A Synergistically Promote the Interaction between Cdh1 and β-TRCP1
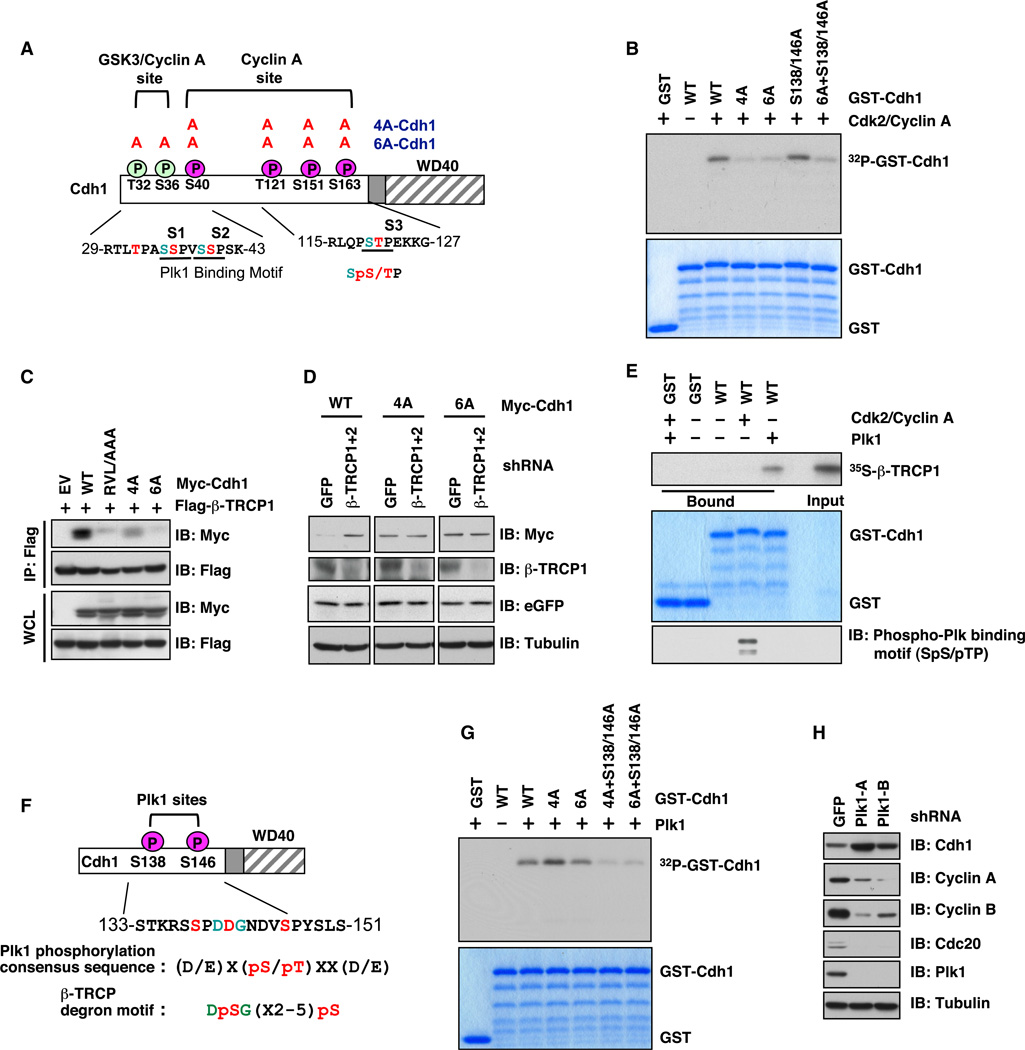
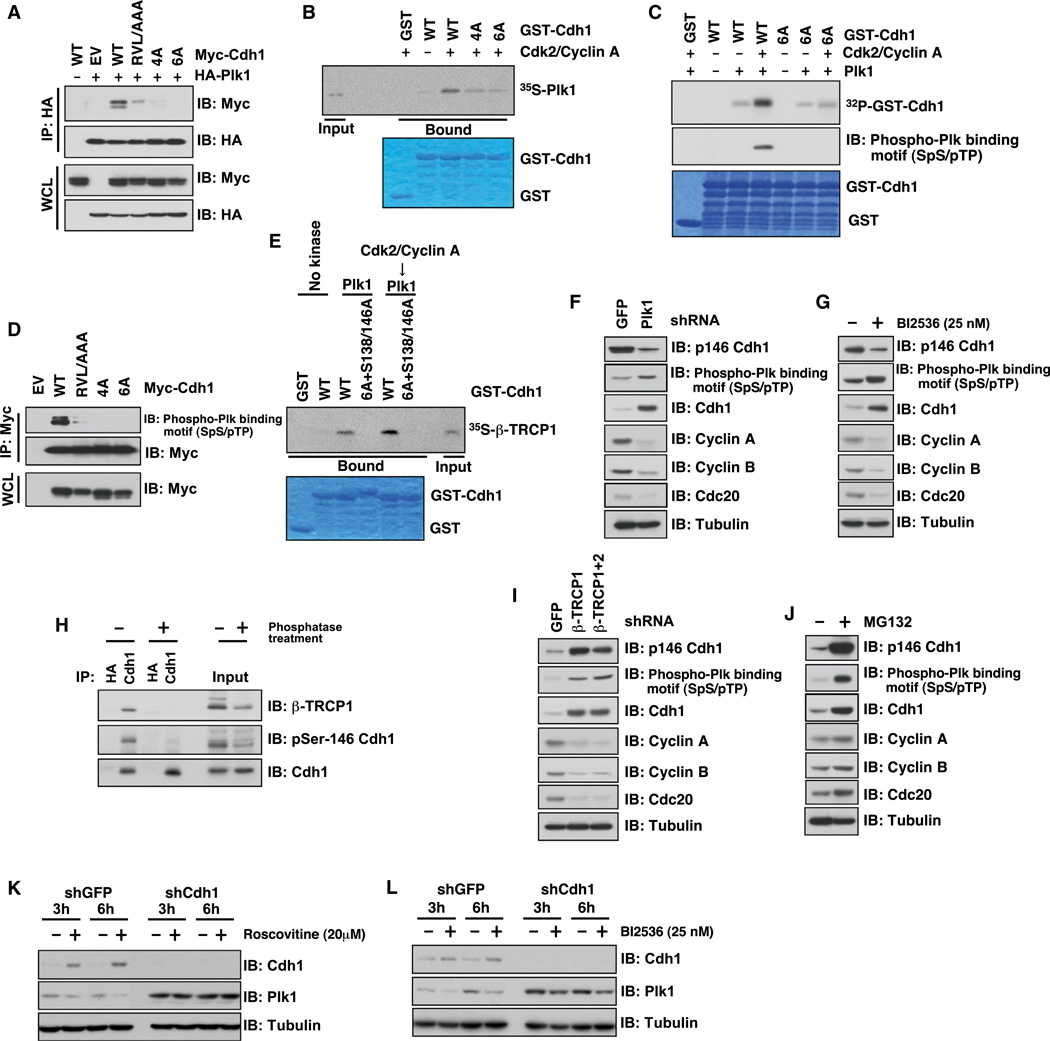
Previous studies from multiple groups showed that most β-TRCP substrates contain a DSGX(2–4)S degron sequence (Cardozo and Pagano, 2004), which must be phosphorylated in order to efficiently interact with β-TRCP.However, we did not find any canonical degron sequence within Cdh1. Consistent with Cdh1 being a Cdk2/cyclinAsubstrate (Lukas et al., 1999; Sørensen et al., 2001), depletion of cyclin A led to an obvious upregulation of endogenous Cdh1 levels in U2OS cells (Figure S3A). Using in vitro kinase assays, we showed that, in addition to the four previously identified Ser/Thr sites (Lukas et al., 1999), there are two possible additional putative Cdk2/cyclin A sites at the N terminus of Cdh1 (Figures 3A and 3B). The in vivo phosphorylation of these sites was further confirmed by mass spectrometry analysis (Dephoure et al., 2008). The conserved cyclin A binding Arg-Val-Leu (RVL) motif at the C terminus of Cdh1 is also known to be critical for its interaction with cyclin A (Sørensen et al., 2001). Interestingly, mutation of the RVL motif or the putative cyclin A phosphorylation sites greatly reduced the interaction between β-TRCP1 and Cdh1 in vivo (Figure 3C). Furthermore, depletion of β-TRCP led to an increase in the abundance of ectopically expressed WT, but not mutant forms of Cdh1 (Figures 3D and S3B). Taken together, these results indicate that phosphorylation of Cdh1 by the Cdk2/cyclin A complex not only is responsible for terminating the E3 ligase activity of Cdh1 (Lukas et al., 1999; Sørensen et al., 2001), but may also serve as a degradation signal for Cdh1.
Figure 3. Plk1 and Cdk2/Cyclin A Synergistically Promote the Interaction between Cdh1 and β-TRCP1.
(A) Schematic diagram of putative Cdk2/cyclin A phosphorylation sites on Cdh1 and Cdh1 mutants used in this study.
(B) Cdk2/cyclin A phosphorylates Cdh1 in vitro. In vitro kinase assays to pinpoint the major Cdk2/cyclin-A-mediated phosphorylation sites in Cdh1. GST-S138A/S146A-Cdh1 was also included in the in vitro kinase assay to illustrate that both Ser138 and Ser146 are major phosphorylation sites mediated by Plk, but not by Cdk2/cyclin A in vitro (see also Figures 2F and 2G).
(C) Phosphorylation of Cdh1 by Cdk2/cyclin A is important for the interaction of Cdh1 with β-TRCP1 in vivo. Flag-tagged β-TRCP1 and various Myc-tagged Cdh1 constructs were expressed in HeLa cells and IPs were performed accordingly. Cells were treated with the proteasome inhibitor MG132 (10 µM) overnight before harvesting to detect the interaction between Flag-β-TRCP1 and Myc-Cdh1 proteins.
(D) Mutation of the Cdk2/cyclin-A-mediated phosphorylation sites in Cdh1 attenuates shβ-TRCP-induced Cdh1 stabilization. IB analysis of HeLa cells transfected with the indicated shRNA constructs together with the indicated Myc-tagged Cdh1 constructs and jellyfish GFP (eGFP) as a transfection control.
(E) Plk1- but not Cdk2/cyclin-A-mediated phosphorylation of Cdh1 promotes the interaction between β-TRCP and Cdh1 in vitro. Autoradiograms show binding of 35S-labeled β-TRCP1 with the indicated GST-Cdh1 proteins (GST protein as a negative control) in the presence of Cdk2/cyclin A and/or Plk1.
(F) Schematic diagram of putative Plk1-mediated phosphorylation sites in Cdh1.
(G) Mapping the Plk1-mediated phosphorylation sites in Cdh1. In vitro kinase assays depicting putative Plk1 phosphorylation sites in Cdh1 (shown in F).
(H) Depletion of endogenous Plk1 stabilizes Cdh1. IB analysis of WCL derived from U2OS cells infected with the indicated lentiviral shRNA constructs. Twenty hours postinfection, cells were selected with 1 µg/ml puromycin for 72 hr to eliminate noninfected cells.
See also Figure S3.
Intriguingly, however, incubation of GST-Cdh1 with purified Cdk2/cyclin A did not promote the interaction between GST-Cdh1 and β-TRCP1 in vitro (Figure 3E), suggesting that Cdk2/cyclin A is not the only regulatory kinase, and possibly additional phosphorylation events mediated by kinases other than Cdk2/cyclin A may be required to trigger the interaction of Cdh1 with β-TRCP (Figure 7A). Upon a closer examination of the Cdh1 protein sequence, it was revealed that Cdh1 contains a suboptimal degron closely resembling the DSGxxS motif, which contains Ser146 (DDGNDVpSP) that may mediate the timely degradation of Cdh1 following UV irradiation (Figure 3F) (Liu et al., 2008). Because both Ser138 and Ser146 appear to be potential Plk1 phosphorylation sites that closely resemble the consensus substrate sequence for Plk1 ([D/E]-X[pS/pT]-X-X-[D/E]) (Dephoure et al., 2008; Nakajima et al., 2003) (Figures 3F and S3C), we performed in vitro kinase assays to confirm that both Ser138 and Ser146 are Plk1 phosphorylation sites (Figure 3G). However, we detected residual Plk1-dependent phosphorylation even in the 6A/S138A/S146A mutant (Figure 3G), indicating that, besides Ser138 and Ser146, there might be additional Plk1 sites present in Cdh1. Nonetheless, these results prompted us to further study a potential role for Plk1 in regulating Cdh1 stability.
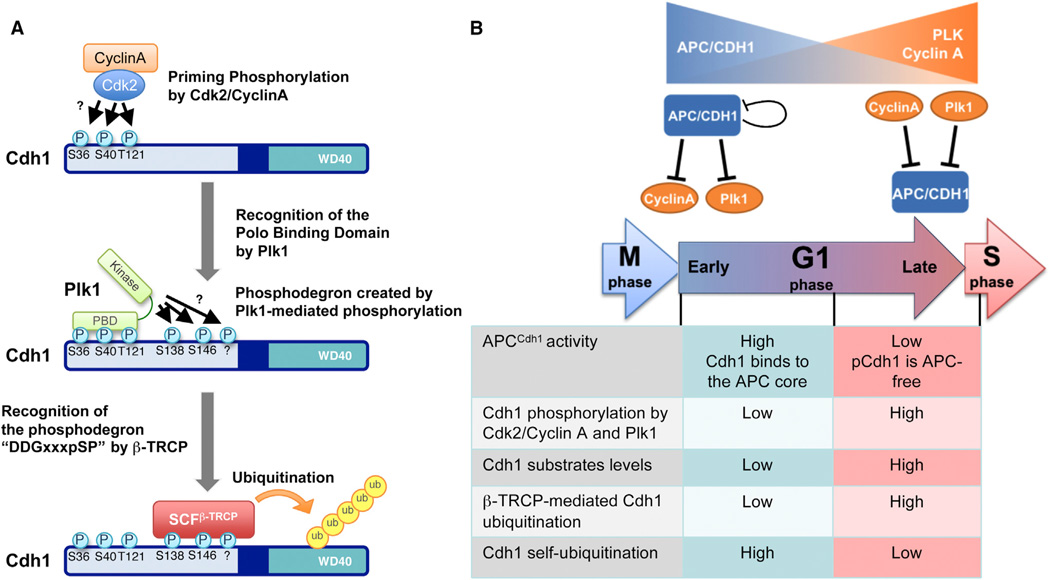
Figure 7. Schemes of the Cell-Cycle-Dependent Cdh1 Degradation by SCFβ-TRCP.
(A) A proposed model showing how priming phosphorylation of Cdh1 by the Cdk2/cyclin A kinase complex allows Plk1 to bind to Cdh1 and phosphorylate Cdh1 at Ser138 and Ser146. Phosphorylation of Cdh1 at Ser138 and Ser146 then triggers its interaction with, and subsequent ubiquitination by, SCFβ-TRCP.
(B) Schematic illustration of the crosstalk between Cdh1 and its substrates cyclin A/Plk1 to influence the self-ubiquitination and SCFβ-TRCP-mediated ubiquitination of Cdh1 in different cell-cycle phases. In early G1 phase, APC/Cdh1 actively degrades its substrates Plk1 and cyclin A. As a result, the abundance of both cyclin A and Plk1 are kept low and Cdh1 mainly undergoes self-ubiquitination. On the other hand, when the cell cycle approaches the late G1 phase, the accumulated Plk1 and cyclin A lead to increased phosphorylation of Cdh1, which leads to dissociation of Cdh1 from the APC core complex to terminate its E3 ligase activity and self-ubiquitination but, on the other hand, triggers its interaction with, and subsequent ubiquitination and degradation by, the SCFβ-TRCP E3 ligase complex.
See also Figure S7.
SCFβ-TRCP Promotes the Ubiquitination and Degradation of Cdh1 in a Plk1-Dependent Manner
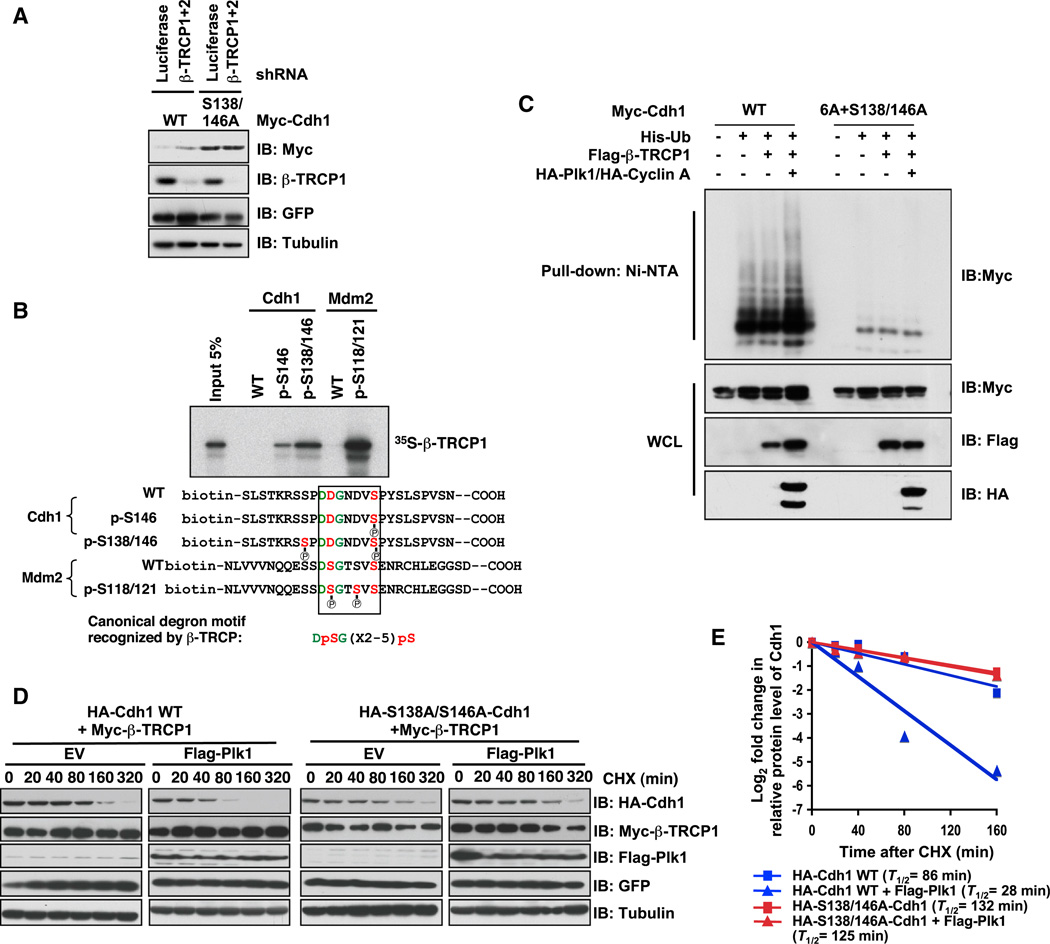
In agreement with a role for Plk1 in Cdh1 stability control, we detected an interaction between endogenous Cdh1 and Plk1 (Figure 2B) and further found that depletion of endogenous Plk1, by various shRNA constructs, led to increased levels of Cdh1 (Figures 3H and S3D), which correlates with an extended Cdh1 half-life (Figures S3E and S3F). Moreover, alanine replacement of the Ser138 and Ser146 residues in Cdh1 abolished the elevation of Cdh1 induced by depletion of β-TRCP, arguing for a critical role for Plk1 in β-TRCP-mediated Cdh1 degradation (Figure 4A). More importantly, using in vitro peptide pull-down assays, we found that biotinylated Cdh1 peptides interacted with 35S-labeled β-TRCP1 only when Ser146 and/or Ser138 were phosphorylated (Figure 4B). In addition, Plk1-mediated phosphorylation of GST-Cdh1 resulted in a significant increase in β-TRCP-mediated binding with bacterially purified recombinant GST-Cdh1 in vitro (Figure S4A). Importantly, in keeping with the notion that prior phosphorylation of substrates is critical for their recognition by β-TRCP (Cardozo and Pagano, 2004; Fuchs et al., 2004), these identified Cdk2/cyclin A and Plk1 phosphorylation sites were crucial for β-TRCP-dependent ubiquitination of Cdh1 both in vivo and in vitro (Figures 4C and S4B). Consistent with the critical role of Ser146 as part of the DSGxxS degron sequence (Figures 4B and S3C) mediating the degradation of Cdh1 by β-TRCP, coexpression of β-TRCP and Plk1 significantly decreased the half-life of WT, but not the S138A/S146A version of Cdh1 (Figures 4D and 4E). Thus, these results coherently support a critical role for Plk1 in governing β-TRCP-mediated Cdh1 ubiquitination. However, the potential presence of additional Plk1 sites besides S138 and S146 in Cdh1 (Figure 3G) indicates that similar to many well-characterized β-TRCP substrates, including Gli3 (Wang and Li, 2006) and Mdm2 (Inuzuka et al., 2010), there might be multiple suboptimal degrons mediating the ubiquitination of Cdh1 by SCFβ-TRCP.
Figure 4. SCFβ-TRCP Promotes the Ubiquitination and Degradation of Cdh1 in a Plk1-Dependent Manner.
(A) Mutation of Plk1-mediated phosphorylation sites in Cdh1 attenuates shβ-TRCP-induced Cdh1 stabilization. Immunoblot (IB) analysis of HeLa cells transfected with the indicated shRNA constructs together with the WT or the S138A/S146A mutant form of Cdh1.
(B) Plk1-mediated phosphorylation of Cdh1 at Ser138 and Ser146 is important for interaction between Cdh1 and β-TRCP1 in vitro. Autoradiograms showing recovery of 35S-labeled β-TRCP1 bound to indicated biotinylated peptides.
(C) In vivo ubiquitination assays illustrate that SCFβ-TRCP promotes Cdh1 ubiquitination in a cyclin A/Plk1-dependent manner.
(D and E) Mutation of Plk1-mediated phosphorylation sites in Cdh1 increases the half-life of Cdh1. (D) HeLa cells were transfected with the indicated HA-Cdh1 constructs together with the Myc-β-TRCP1 and Flag-Plk1 plasmids. Twenty hours posttransfection, cells were split into 60 mm dishes and, after another 20 hr, treated with 20 µg/ml cycloheximide (CHX). At the indicated time points, whole-cell lysates were prepared for immunoblot analysis. (E) Quantification of the band intensities in (D). Cdh1 band intensity was normalized to tubulin and then normalized to the t = 0 controls.
See also Figure S4.
Phosphorylation of Cdh1 by Cdk2/Cyclin A Creates Plk1 Binding Sites for the Subsequent Phosphorylation of Cdh1 by Plk1
The substrate specificity of Plk1 was previously determined to be mediated largely by the two tandem Polo-Box domains (PBD) at the C terminus of Plk1, which preferentially bind to the SpS/pTP motif that is also called PBD binding motif (Elia et al., 2003a, 2003b). Cdh1 was reported to be phosphorylated by Cdk2/cyclin A at the S40, S151, S163, and T121 sites (Lukas et al., 1999), among which both the S40 and T121 sites match the stringent PBD binding motif (Figure 3A). Thus, we next explored whether phosphorylation of Cdh1 by Cdk2/cyclin A creates possible Plk1 binding sites for subsequent phosphorylation of Cdh1 at Ser146 by Plk1. In support of this notion, mutating Cdh1 at either the cyclin A interaction site (RVL/AAA) or the putative Cdk2/cyclin A sites impaired the interaction between Cdh1 and Plk1 (Figures 5A and 5B). Using in vitro kinase assays, we demonstrated that the impaired interaction might be partly due to reduced phosphorylation of the PBD binding motifs in Cdh1 (Figure 5C, middle panel). In keeping with this result, pretreatment of GST-Cdh1 with purified Cdk2/cyclin A, but not Plk1 could promote a significant increase in phosphorylation of the PBD binding motifs in vitro (Figure S5A, middle panel). As such, pretreatment of GST-Cdh1 with cyclin A/Cdk2 induced the interaction of Plk1 with Cdh1 in vitro (Figure 5B) as well as Plk1-dependent phosphorylation of Cdh1 in vitro (Figure 5C, top panel) and in vivo (Figure 5D). Consistent with these observations, sequential kinase treatment of GST-Cdh1 with Cdk2/cyclin A and Plk1 augmented the interaction between Cdh1 and β-TRCP1 in vitro (Figure 5E). Importantly, Plk1-dependent phosphorylation of Cdh1 (Figures 5C and 5D), as well as the interaction between Plk1 and Cdh1 (Figure 5B), and the interaction between Cdh1 and β-TRCP1 (Figure 5E), were all abolished by Ala replacements of the characterized cyclin A/Cdk2 sites of Cdh1. These data indicate that Cdk2/cyclin-A-mediated Cdh1 phosphorylation is a critical step for Plk1 to interact with, and phosphorylate Cdh1, which subsequently induces β-TRCP binding to Cdh1 (Figure 7A).
Figure 5. Phosphorylation of Cdh1 by Cdk2/Cyclin A Creates Plk1 Binding Sites for Subsequent Phosphorylation of Cdh1 at Ser146 by Plk1.
(A and B) Cdk2/cyclin-A-mediated phosphorylation of Cdh1 is important for the interaction between Plk1 and Cdh1. (A) Immunoblot (IB) analysis of whole-cell lysates (WCL) and immunoprecipitates (IP) derived from 293T cells transfected with HA-Plk1 and the indicated Cdh1 constructs. Thirty hours posttransfection, cells were pretreated with the proteasome inhibitor MG132 (10 µM) for 10 hr before harvesting to detect the interaction between Myc-Cdh1 and HA-Plk1 proteins.
(B) Autoradiograms showing the recovery of 35S-labeled Plk1 to the indicated GST-Cdh1 proteins (GST protein as a negative control) incubated with Cdk2/cyclin A before the in vitro pull-down assays.
(C) Cdk2/cyclin-A-mediated phosphorylation of Cdh1 primes for the subsequent phosphorylation of Cdh1 by Plk1 in vitro. Purified Plk1 and/or Cdk2/cyclin A proteins were incubated with 3 µg of the indicated GST-Cdh1 proteins in the presence of γ-32P-ATP. The kinase reaction products were resolved by SDS-PAGE, and phosphorylation was detected by autoradiography (top panel). The phosphorylation in the PBD motif(s) was detected by IB analysis (middle panel).
(D) Phosphorylation of Cdh1 by Cdk2/cyclin A creates Plk1 binding motifs in Cdh1. IB analysis of WCL and IP derived from 293T cells transfected with the indicated Myc-Cdh1 constructs. Thirty hours posttransfection, cells were pretreated with the proteasome inhibitor MG132 (10 µM) for 10 hr before harvesting to stabilize the phosphorylated Myc-Cdh1 protein.
(E) Phosphorylation of Cdh1 by Plk1 enhances the binding between Cdh1 and β-TRCP in vitro. Autoradiograms showing recovery of 35S-labeled β-TRCP1 to the indicated GST-Cdh1 proteins (GST protein as a negative control) incubated with Plk1 before the in vitro pull-down assays. Where indicated, the GST-Cdh1 proteins were treated with Cdk2/cyclin A prior to the in vitro Plk1 kinase assay.
(F and G) Inhibition of Plk1 reduces the phosphorylation of Cdh1 at Ser146 in vivo. (F) IB analysis of WCL derived from HeLa cells infected with the indicated lentiviral shRNA constructs. Twenty hours postinfection, cells were selected with 1 µg/ml puromycin for 72 hr to eliminate noninfected cells. (G) IB analysis of WCL derived from HeLa cells treated with the Plk inhibitor BI2536 (25 nM) for 6 hr before harvesting.
(H) The in vivo interaction between Cdh1 and β-TRCP is phosphorylation dependent. IB of WCL and anti-Cdh1 IP derived from HeLa cells (anti-HA was used as a negative control). Cells were treated with the proteasome inhibitor MG132 overnight before harvesting for immunoblot analysis. Where indicated, the whole-cell lysates were treated with the l-phosphatase before immunoprecipitation.
(I and J) Inhibition of Cdh1 degradation results in elevated phosphorylation on Ser146 and Plk binding motifs in Cdh1. (I) IB analysis of WCL derived from HeLa cells infected with the indicated lentiviral shRNA constructs. Twenty hours postinfection, cells were selected with 1 µg/ml puromycin for 72 hr to eliminate noninfected cells. (J) IB analysis of WCL derived from HeLa cells treated with the 26S proteasome inhibitor MG132 (10 µM) for 12 hr before harvesting.
(K) Inhibition of Cdk2/cyclin A increases Cdh1 levels. IB analysis of WCL derived from shCdh1- (or shGFP as a negative control) infected HeLa cells that are treated with the Cdk2 inhibitor roscovitine (20 µM) for the indicated time periods before harvesting.
(L) Inhibition of Plk1 increases Cdh1 levels. IB analysis of WCL derived from shCdh1- (or shGFP as a negative control) infected HeLa cells that are treated with the Plk inhibitor BI2536 (25 nM) for the indicated time periods before harvesting.
See also Figure S5.
In order to obtain more insights into how Plk1-mediated phosphorylation of Ser146 affects Cdh1 stability, we developed a specific antibody against pSer146-Cdh1 (Figure S5B). Importantly, inactivating endogenous Plk1 by either shRNA (Figure 5F) or the pharmacological Plk1 inhibitor, BI2536 (Steegmaier et al., 2007) (Figure 5G), led to a significant reduction in phosphorylation of Cdh1 at Ser146, arguing that Ser146 is a physiological Plk1 site in vivo. In further support of a critical role of Ser146 in β-TRCP1-mediated degradation of Cdh1, we found that endogenous β-TRCP1 preferentially interacts with endogenous Cdh1 that is phosphorylated at Ser146 (Figure 5H), and depletion of β-TRCP (Figure 5I) or inhibiting the 26S proteasome by MG132 (Figure 5J) resulted in elevated phosphorylation of Ser146-Cdh1 in HeLa cells.
In light of these findings, and given that both cyclin A and Plk1 are themselves Cdh1 substrates, it appears that there exists an elegant negative feedback loop within the cell cycle that ensures timely inactivation of APC/Cdh1 prior to S phase onset (Figure 7B). Interestingly, in support of a negative role for both Cdk2/cyclin A and Plk1 in Cdh1 stability control, both the Cdk2 inhibitor roscovitine (Figure 5K) and the Plk1 inhibitor BI2536 (Figure 5L) led to an increase in Cdh1 abundance, and a subsequent decrease in the Cdh1 substrate Plk1. Furthermore, roscovitine or BI2536-induced decrease of Plk1 was impaired in Cdh1-deficient cells (Figures 5K and 5L). Notably, as Cdh1-deficient cells displayed elevated expression of both the Plk1 and cyclin A oncoproteins, compared with shGFP-treated cells, shCdh1-treated cells were much more sensitive to the treatment of roscovitine and/or BI2536 (Figure S5C), a phenotype recently described as ‘‘oncogene addiction’’ (Sharma and Settleman, 2007). Our results indicate that further preclinical studies are warranted to explore the synergistic usage of Cdk2 and Plk1 inhibitors in anticancer treatment, especially for Cdh1-deficient patients.
SCFβ-TRCP Negatively Regulates Cdh1 Stability to Ensure Proper Cell-Cycle Progression
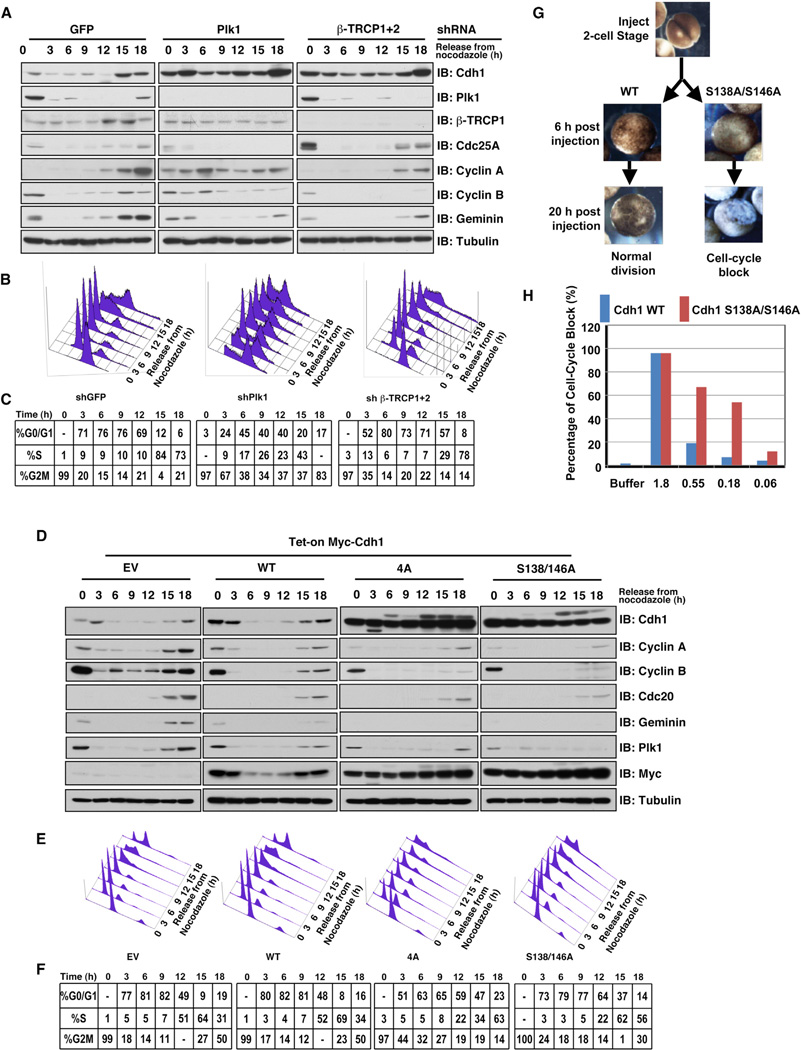
Next, we intended to determine the biological significance of β-TRCP1-mediated ubiquitination and subsequent degradation of Cdh1. To this end, concomitant with β-TRCP1 or Plk1 depletion, there was an increase in Cdh1 abundance that correlated with elevated abundance of pSer146-Cdh1 (Figure S6A), and a reduction in both cyclin B and Geminin expression levels in the late G1 phase, which resulted in delayed S phase entry as judged by fluorescence-activated cell sorting (FACS) analysis (Figures 6A–6C and S6B). Consistent with a previous report (Oshimori et al., 2006), depletion of Plk1 also resulted in an impaired mitotic exit (Figures 6B, 6C, and S6B). In further support of our hypothesis that Cdh1 dysregulation causes β-TRCP-depletion-mediated cell-cycle defects, compared to EV or WT-Cdh1-expressing cells, tetracycline (Tet)-induced expression of nondegradable Cdh1 mutants (4A-Cdh1 and S138A/S146A-Cdh1) phenocopies the depletion of β-TRCP, leading to a retarded S phase entry (Figures 6D–6F). Notably, further studies revealed that, compared to WT-Cdh1, the half-lives of both 4A-Cdh1 and S138A/S146A-Cdh1 were extended in the G1 phase (Figures S6C–S6F), arguing for a critical role for these phosphorylation sites in triggering the timely degradation of Cdh1 by β-TRCP in the G1 phase.
Figure 6. SCFβ-TRCP Negatively Regulates Cdh1 Stability to Ensure Proper Cell-Cycle Progression.
(A–C) Depletion of endogenous Plk1 or endogenous β-TRCP stabilizes Cdh1 and delays the S phase entry. HeLa cells were infected with the indicated shRNA constructs followed by selection with 1 µg/ml puromycin for 72 hr to eliminate noninfected cells. Afterward, the resulting cell lines were arrested with nocodazole and then released back into the cell cycle for immunoblot (IB) analysis (A). FACS was performed in (B) to monitor the changes of cell-cycle distribution (C).
(D–F) Expression of nondegradable Cdh1 leads to a retarded S phase entry. HeLa cells were infected with pTRIPZ lentiviral vectors that allow the ectopic expression of the indicated Cdh1 cDNA under the control of doxycycline. The infected cells were selected with 1 µg/ml puromycin for 72 hr to eliminate noninfected cells. Afterward, the resulting cell lines were arrested with nocodazole for 18 hr and then released back into the cell cycle for IB analysis (D). To exclude a possible indirect cell-cycle effect derived from aberrant overexpression of Cdh1, 100 ng/ml doxycycline was added to various cell lines 3 hr before adding nocodazole to induce the expression of WT-Cdh1 at levels comparable to endogenous Cdh1, whereas 4A and S138/S146A-Cdh1 were expressed approximately 3- to 4-fold over endogenous Cdh1 in part due to prolonged half-lives (see also Figures S6C–S6F). The blots were probed and exposed identically to compare the inducible expression of the WT and mutant Cdh1 at the same experimental condition. FACS was performed in (E) to monitor the changes of cell-cycle distribution (F).
(G and H) Expression of nondegradable Cdh1 mutants enhances the cell-cycle blockage phenotype of Xenopus embryos. Fertilized Xenopus embryos were injected at 2-cell stage with variable amounts of mRNA encoding WT or S138A/S146A-Cdh1. Pictures were taken at the indicated time points after injection, showing normal division or cell-cycle blockage phenotype as an example (G). The percentage of cell-cycle blockage phenotype is quantified in (H). The experiments were performed twice, with a total of 30–40 embryos injected for each category.
See also Figure S6.
On the other hand, Tet-inducible expression of Plk1 resulted in the increased degradation of Cdh1 and a subsequent increase in Cdh1 substrates (Figure S6G). Importantly, our findings could also be reproduced in silico using a mathematical (ordinary differential equation [ODE]) model described previously (Conradie et al., 2010). Specifically, when Cdh1 degradation or phosphorylation rate is set to zero, the cell-cycle duration is increased, whereas cyclin A and Cdc20 expression levels are decreased (Figure S6H). Notably, the simulated results (Figure S6H) match the critical findings that have been observed in our experimental settings (Figures 6D–6F). Furthermore, consistent with the notion that many key cell-cycle regulators play a critical role in differentiation (Fasano et al., 2007; Hong et al., 2009; Janzen et al., 2006), the nondegradable S138A/S146A mutant of Cdh1 displayed elevated ability in inhibiting Xenopus oocyte development (Figures 6G and 6H). Therefore, these results further illustrate the biological significance of the SCFβ-TRCP -Cdh1 axis in cell-cycle regulation (Figures 7 and S7L). For more details, see also Extended Results.
DISCUSSION
We and others previously showed that APC/Cdh1 degrades Skp2 in the early G1 phase (Bashir et al., 2004; Wei et al., 2004), indicating that early cell-cycle events set the tempo and ensure the timely achievement of later events. However, relatively little is known about the upstream pathways that control Cdh1’s stability and E3 ligase activity. Here, we report that reciprocally SCFβ-TRCP could suppress APC by promoting Cdh1 for ubiquitination and subsequent degradation. This work demonstrates that cell-cycle progression behaves less as a linear pathway and more like an interwoven network, or coupled oscillatory modules (Lu and Cross, 2010).
By elucidating the dual repressive mechanism between SCF and APC, we will likely gain a better understanding of how the orchestration of E3 ligase activities controls the timely degradation of key cell-cycle regulators to govern proper cell-cycle progression. More importantly, we identified the Cdh1 substrates cyclin A and Plk1 as the modifying enzymes that govern Cdh1 stability. In early G1 phase where APC/Cdh1 is mostly active, APC/Cdh1 plays a dominant role, promoting the degradation of its substrates including cyclin A and Plk1. When the cell cycle progresses to mid-late G1 phases, the accumulation of cyclin A and Plk1 to a critical threshold (Hsu et al., 2002) leads to β-TRCP-mediated ubiquitination and degradation of Cdh1 by sequential phosphorylation of Cdh1 to create the β-TRCP-recognizable phospho-degron(s). Because Cdh1 is one of the master regulators of cell-cycle progression, the timely degradation of Cdh1 plays a critical role in determining the length of the G1 phase (Robbins and Cross, 2010; Wäsch and Cross, 2002). To this end, this bimodual regulation highlights the critical role of β-TRCP in mediating APC/Cdh1’s ability to control the timing of G1-S transition. Given that overexpression of both cyclin A and Plk1 is frequently observed in human carcinomas (Liu et al., 2008), these studies could provide a possible molecular mechanism for dysregulated cell cycle in pathological conditions such as cancer and indicate that combined treatments using Cdk2 and Plk1 inhibitors (for example, roscovitine and BI2536, respectively) may have a synergistic effect in suppressing tumor cell growth.
Previous studies have demonstrated that Cdh1 undergoes self-ubiquitination in a D-box-dependent manner (Listovsky et al., 2004). As Cdk2/cyclin-A- or Cdk1/cyclin-B-mediated phosphorylation of Cdh1 terminates its E3 ligase activity (Peters, 2002), it is plausible that these phosphorylation events also inhibit the self-ubiquitination of Cdh1. As complete degradation of cyclin A and cyclin B is required for mitotic exit (Harper et al., 2002; Peters, 2002), in the early G1 phase, both cyclin A and cyclin B expression levels are low such that APC/Cdh1 E3 ligase is mostly active, where the self-ubiquitination of Cdh1 should primarily occur. On the other hand, with the progressive accumulation of Cdh1 substrates including cyclin A and Plk1 in the mid-late G1 phases, the degradation mechanism of Cdh1 is shifted from self-ubiquitination to β-TRCP-mediated ubiquitination. Therefore, a nonphosphorylatable Cdh1 mutant (such as 4A or 6A-Cdh1) is defective in β-TRCP-mediated ubiquitination, but instead may undergo constant self-ubiquitination.
Importantly, aberrant regulation of Cdh1 abundance has been reported to result in severe cell-cycle defects. Depletion of Cdh1 in primary human fibroblasts led to premature senescence (Gao et al., 2009a), whereas overexpressing Cdh1 leads to G1 cell-cycle arrest coupled with endoreplication (Sørensen et al., 2000). Given that Cdh1 expression levels are critical for proper cell-cycle progression, our studies reveal that its stability is subjected to tight regulation by both β-TRCP and self-ubiquitination. Notably, the existence of multiple degradation mechanisms has also been characterized for many other critical cell-cycle regulators including p53 and p21 (Brooks and Gu, 2011; Lu and Hunter, 2010). For more details, see also Extended Discussion.
EXPERIMENTAL PROCEDURES
Immunoblots and Immunoprecipitation
Cells were lysed in EBC buffer (50 mM Tris [pH 7.5], 120 mM NaCl, 0.5% NP-40) supplemented with protease inhibitors (Complete Mini, Roche) and phosphatase inhibitors (phosphatase inhibitor cocktail set I and II, Calbiochem). The protein concentrations of the lysates were measured using the Bio-Rad protein assay reagent on a Beckman Coulter DU-800 spectrophotometer. The lysates were then resolved by SDS-PAGE and immunoblotted with indicated antibodies. For immunoprecipitation, 800 µg lysates were incubated with the appropriate antibody (1–2 µg) for 3–4 hr at 4°C followed by 1 hr incubation with Protein A Sepharose beads (GE Healthcare). Immunocomplexes were washed five times with NETN buffer (20 mM Tris [pH 8.0], 100 mM NaCl, 1 mM EDTA, and 0.5% NP-40) before being resolved by SDS-PAGE and immunoblotted with indicated antibodies as described previously (Gao et al., 2011; Wan et al., 2011).
siRNA
As described previously, siRNA oligos were transfected into subconfluent cells with oligofectamine or Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions (Wei et al., 2004). Human siRNA oligos against Cdh1 have been described previously (Wei et al., 2004). A human siRNA oligo that can deplete both β-TRCP1 and β-TRCP2 (sense, 5′-AAGUGGAAUUUGUGGAACAUC-3′) has been validated previously (Jin et al., 2003). A human siRNA oligo against Cullin 1 (sense, 5′-GGUCGCUUCAUAAACAACATT-3′ has been described and validated previously (Benmaamar and Pagano, 2005; Gao et al., 2010). E2F-1 scramble and Luciferase GL2 siRNA oligonucleotides were purchased from Dharmacon and served as negative controls.
Cell Culture and Synchronization
Cell culture, including synchronization and transfection, has been described previously (Wei et al., 2004). For synchronization, HeLa cells were arrested in G2/M phase in 330 nM nocodazole for 18 hr and then washed with PBS three times and replated into nocodazole-free fresh medium. The 293T cells engineered to express a Cullin 1-deletion (Cul1-N252) in a doxycycline-inducible manner were kind gifts from Dr. Michele Pagano (Benmaamar and Pagano, 2005). Lentiviral shRNA virus packaging and subsequent infection of various cell lines were performed according to the protocol described previously (Boehm et al., 2005). For cell viability assays, cells were plated at 10,000 per well in 96-well plates, and incubated with appropriate medium containing roscovitine (Cell Signaling), alone or in combination with Plk1 inhibitor BI2536 (Selleckchem) for the indicated time period. Assays were performed with CellTiter-Glo Luminescent Cell Viability Assay Kit according to the manufacturer’s instructions (Promega). Cycloheximide (CHX) experiments were performed as described previously (Gao et al., 2009b).
Xenopus Embryos and RNA Injection
The animals and procedures used in this study were in accordance with the guidelines and approval of the Harvard Medical School Institutional Animal Care and Use Committees. An Animal Welfare Assurance of Compliance is on file with the Office of Laboratory Animal Welfare (OLAW) (#A3431-01). Xenopus embryos culture and RNA injection were described previously (Kroll et al., 1998). In brief, Xenopus embryos were obtained from previously unovulated X. laevis frogs (NASCO) by in vitro fertilization, dejellied, and cultured at 16°C–18°C in 0.1 × Marc’s Modified Ringer’s solution (Peng, 1991) containing 50 µg/ml gentamicin. Embryos were staged according to Nieuwkoop and Faber (1967). Embryos were injected with RNAs in 0.2 × Marc’s Modified Ringer’s solution containing 5% Ficoll and 50 µg/ml gentamicin and cultured in the same media at 16°C–18°C. For more details, see also Extended Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS
We thank Qing Zhang, Adriana Tron, and Shavali Shaik for critical reading of the manuscript; Michele Pagano, Jiri Lukas, and William Hahn for providing reagents; Lewis Cantley and Alex Toker for helpful suggestions; and members of the Wei and Kirschner labs for useful discussions. W.W. is an ACS research scholar and an LLS research scholar. H.F. is supported by the JSPS Fellowship. Y.L. is a Damon Runyon cancer research fellow and a Lallage Feazel Wall Fellow. This work was supported in part by NIH grants (GM089763 and GM094777 to W.W., AG041218 to H.I., and GM39023 to M.W.K.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Results, Extended Discussion, Extended Experimental Procedures, and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.07.031.
REFERENCES
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- Benmaamar R, Pagano M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle. 2005;4:1230–1232. doi: 10.4161/cc.4.9.2048. [DOI] [PubMed] [Google Scholar]
- Boehm JS, Hession MT, Bulmer SE, Hahn WC. Transformation of human and murine fibroblasts without viral oncoproteins. Mol. Cell. Biol. 2005;25:6464–6474. doi: 10.1128/MCB.25.15.6464-6474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Gu W. p53 regulation by ubiquitin. FEBS Lett. 2011;585:2803–2809. doi: 10.1016/j.febslet.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Conradie R, Bruggeman FJ, Ciliberto A, Csikász-Nagy A, Novaák B, Westerhoff HV, Snoep JL. Restriction point control of the mammalian cell cycle via the cyclin E/Cdk2:p27 complex. FEBS J. 2010;277:357–367. doi: 10.1111/j.1742-4658.2009.07473.x. [DOI] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science. 2003a;299:1228–1231. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell. 2003b;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Fuchs SY, Spiegelman VS, Kumar KG. The many faces of beta-TrCP E3 ubiquitin ligases: reflections in the magic mirror of cancer. Oncogene. 2004;23:2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Korenjak M, Tseng A, Wu T, Wan L, Kirschner M, Dyson N, Wei W. Cdh1 regulates cell cycle through modulating the claspin/Chk1 and the Rb/E2F1 pathways. Mol. Biol. Cell. 2009a;20:3305–3316. doi: 10.1091/mbc.E09-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat. Cell Biol. 2009b;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Wan L, Inuzuka H, Berg AH, Tseng A, Zhai B, Shaik S, Bennett E, Tron AE, Gasser JA, et al. Rictor forms a complex with Cullin-1 to promote SGK1 ubiquitination and destruction. Mol. Cell. 2010;39:797–808. doi: 10.1016/j.molcel.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tan MK, Fukushima H, Locasale JW, Liu P, Wan L, Zhai B, Chin YR, Shaik S, et al. mTOR drives its own activation via SCF(bTrCP)-dependent degradation of the mTOR inhibitor DEPTOR. Mol. Cell. 2011;44:290–303. doi: 10.1016/j.molcel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: it’s not just for mitosis any more. Genes Dev. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, Reimann JD, Sørensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1) Nat. Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- Inuzuka H, Tseng A, Gao D, Zhai B, Zhang Q, Shaik S, Wan L, Ang XL, Mock C, Yin H, et al. Phosphorylation by casein kinase I promotes the turnover of the Mdm2 oncoprotein via the SCF(beta-TRCP) ubiquitin ligase. Cancer Cell. 2010;18:147–159. doi: 10.1016/j.ccr.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Jin J, Shirogane T, Xu L, Nalepa G, Qin J, Elledge SJ, Harper JW. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 2003;17:3062–3074. doi: 10.1101/gad.1157503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck JM, Summers MK, Tedesco D, Ekholm-Reed S, Chuang LC, Jackson PK, Reed SI. Cyclin E overexpression impairs progression through mitosis by inhibiting APC(Cdh1) J. Cell Biol. 2007;178:371–385. doi: 10.1083/jcb.200703202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll KL, Salic AN, Evans LM, Kirschner MW. Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development. 1998;125:3247–3258. doi: 10.1242/dev.125.16.3247. [DOI] [PubMed] [Google Scholar]
- Lassot I, Ségéral E, Berlioz-Torrent C, Durand H, Groussin L, Hai T, Benarous R, Margottin-Goguet F. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCF(betaTrCP) ubiquitin ligase. Mol. Cell. Biol. 2001;21:2192–2202. doi: 10.1128/MCB.21.6.2192-2202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AW, Inuzuka H, Fukushima H, Wan L, Liu P, Gao D, Sun Y, Wei W. Regulation of APC(Cdh1) E3 ligase activity by the Fbw7/cyclin E signaling axis contributes to the tumor suppressor function of Fbw7. Cell Res. 2013;23:947–961. doi: 10.1038/cr.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listovsky T, Oren YS, Yudkovsky Y, Mahbubani HM, Weiss AM, Lebendiker M, Brandeis M. Mammalian Cdh1/Fzr mediates its own degradation. EMBO J. 2004;23:1619–1626. doi: 10.1038/sj.emboj.7600149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Li W, Fujita T, Yang Q, Wan Y. Proteolysis of CDH1 enhances susceptibility to UV radiation-induced apoptosis. Carcinogenesis. 2008;29:263–272. doi: 10.1093/carcin/bgm251. [DOI] [PubMed] [Google Scholar]
- Lu Y, Cross FR. Periodic cyclin-Cdk activity entrains an autonomous Cdc14 release oscillator. Cell. 2010;141:268–279. doi: 10.1016/j.cell.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle. 2010;9:2342–2352. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Sørensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, Bartek J, Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- Miller JJ, Summers MK, Hansen DV, Nachury MV, Lehman NL, Loktev A, Jackson PK. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20:2410–2420. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin. Cell Dev. Biol. 2005;16:323–333. doi: 10.1016/j.semcdb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Hatakeyama S, Nakayama K. Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem. Biophys. Res. Commun. 2001;282:853–860. doi: 10.1006/bbrc.2001.4627. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin): a Systematical and Chronological Survey of the Development from Fertilized Egg Till the End of Metamorphosis (Amsterdam: North Holland) 1967 [Google Scholar]
- Oshimori N, Ohsugi M, Yamamoto T. The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 2006;8:1095–1101. doi: 10.1038/ncb1474. [DOI] [PubMed] [Google Scholar]
- Peng HB. Xenopus laevis: Practical uses in cell and molecular biology. Solutions and protocols. Methods Cell Biol. 1991;36:657–662. [PubMed] [Google Scholar]
- Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Cross FR. Requirements and reasons for effective inhibition of the anaphase promoting complex activator CDH1. Mol. Biol. Cell. 2010;21:914–925. doi: 10.1091/mbc.E09-10-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E, Kim H, Kim R, Yun S, Kim M, Han JK, Costantini F, Jho EH. Multiple isoforms of beta-TrCP display differential activities in the regulation of Wnt signaling. Cell. Signal. 2009;21:43–51. doi: 10.1016/j.cellsig.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–3231. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- Sørensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J. Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol. Cell. Biol. 2000;20:7613–7623. doi: 10.1128/mcb.20.20.7613-7623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J. A conserved cyclin-binding domain determines functional interplay between anaphase-promoting complex-Cdh1 and cyclin A-Cdk2 during cell cycle progression. Mol. Cell. Biol. 2001;21:3692–3703. doi: 10.1128/MCB.21.11.3692-3703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman VS, Stavropoulos P, Latres E, Pagano M, Ronai Z, Slaga TJ, Fuchs SY. Induction of beta-transducin repeat-containing protein by JNK signaling and its role in the activation of NF-kappaB. J. Biol. Chem. 2001;276:27152–27158. doi: 10.1074/jbc.M100031200. [DOI] [PubMed] [Google Scholar]
- Spiegelman VS, Tang W, Katoh M, Slaga TJ, Fuchs SY. Inhibition of HOS expression and activities by Wnt pathway. Oncogene. 2002;21:856–860. doi: 10.1038/sj.onc.1205132. [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, Gürtler U, Garin-Chesa P, Lieb S, Quant J, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Wan L, Zou W, Gao D, Inuzuka H, Fukushima H, Berg AH, Drapp R, Shaik S, Hu D, Lester C, et al. Cdh1 regulates osteoblast function through an APC/C-independent modulation of Smurf1. Mol. Cell. 2011;44:721–733. doi: 10.1016/j.molcel.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li Y. Evidence for the direct involvement of betaTrCP in Gli3 protein processing. Proc. Natl. Acad. Sci. USA. 2006;103:33–38. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäsch R, Cross FR. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature. 2002;418:556–562. doi: 10.1038/nature00856. [DOI] [PubMed] [Google Scholar]
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.