Abstract
LBH589 is a novel pan-HDAC inhibitor which has potent antitumor activity in multiple myeloma and other hematologic malignancies. However, its impact on immune system has not been defined. We here evaluated the effects of LBH589 on human myeloid dendritic cells (DCs) at clinically relevant concentrations. Exposure to LBH589 affected the surface molecule expression on immature and mature DCs, associated with DC maturation (CD83↓), antigen presentation (HLA-ABC↓), and T cell co-stimulation (CD40↓ and CD86↑). LBH589 decreased both protein and polysaccharide antigen uptake capacities by DCs. Importantly, LBH589 impaired DCs function to stimulate antigen-specific immune responses, resulting in the significant reduction of invariant NKT cell (CD1d-restricted) and T cell (MHC-restricted) activation in innate and adaptive immunity. LBH589 also significantly repressed the production of IL-6, IL-10, IL-12p70, IL-23 and TNF-α by TLR3 and TLR4-induced DCs activation, indicating an important role of HDAC activity in immune regulation and inflammation. RelB, a component of NF-κB signaling pathway, was the key component regulated by HDAC inhibition in DCs. Together, our preclinical study demonstrates that LBH589 significantly impairs phenotype and function of DCs, indicating a need for monitoring the immune status in patients receiving HDAC inhibitor therapy. It also provides a rationale to evaluate LBH589 activity for the treatment of inflammation.
Keywords: Histone Deacetylase Inhibitor, LBH589, dendritic cells, T cells, invariant NKT cells, NF-κB
Introduction
Gene expression, cellular differentiation, and survival are regulated by the opposing activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs). Histone acetylation by HATs is associated with activation of transcription through relaxed chromatin structure, whereas deacetylation by HDACs induces a more condensed or inactive chromatin state, leading to gene repression. Although histones are the most thoroughly studied acetylated protein substrates, HDACs are also responsible for modifying the activity of diverse types of nonhistone proteins, including transcription factors and signal transduction mediators (e.g. p53, c-Myb, hsp90, NF-κB, E2F1, sp3, etc). 1-3
It is well recognized that HDAC activity is increased in cancer cells, resulting in altered gene transcription, impaired differentiation, increased cell survival, and dysregulated proliferation.1,4 In the recent years, a group of structurally diverse HDAC inhibitors such as sodium butyrate (small molecular weight carboxylates), Suberoylanilide hydroxamic acid (SAHA) and Trichostatin A (TSA) (Hydroxamic acid), MS-275 (Benzamides), Trapoxin (TPX, Epoxyketones), Apicidin (Cyclic peptides) have been introduced for cancer treatment.1 In addition to the antitumor activities observed, HDAC inhibitors have been reported to possess anti-inflammatory and immunomodulatory properties both in vitro and in vivo. 5-9
LBH589 is a novel pan-HDAC inhibitor belonging to the cinnamic hydroxamic acid class, which displays broad enzyme inhibitory activity in the low nano-molar range3. Preclinical studies have shown that LBH589 has potent antitumor activity. It induces hyperacetylation of core histone proteins, resulting in modulation of protein expression, leading to cell cycle arrest in the G2/M phase and apoptosis. In addition, LBH589 appears to modulate the expression of angiogenesis-related genes, such as hypoxia-inducible factor-1 alpha (HIF-1α) and vascular endothelial growth factor (VEGF), thus impairing endothelial cell chemotaxis and invasion.10-13 LBH589-induced growth inhibition and cytotoxicity has been observed in Cutaneous T cell lymphoma (CTCL), chronic myelogenous leukemia (CML), acute myeloid leukemia (AML), Hodgkin lymphoma, and breast, prostate, colon and pancreatic cancer cell lines.3,14 Currently, LBH589 is undergoing phase I/II clinical trials in multiple myeloma and a series of solid and hematologic malignancies.
Although the role of HDAC inhibition by LBH589 has been widely studied in the malignant cells, its effects on normal human immune function have not yet been defined. Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that play a key role in the initiation and regulation of antigen-specific immune responses via antigen uptake, processing and presentation. DCs also play a crucial in the modulation of innate and adaptive immunity through the production of cytokines (e.g. IL-10, IL-12 and IL-23).15, 16 The effects of HDAC inhibition on DCs-mediated antigen-specific immune responses have been evaluated for some of HDAC inhibitors,9,17 however, it has been restricted to the observation of the MHC-mediated T cell activation. In this study, we have systematically investigated the impact of LBH589 treatment on the function of DCs involved in both classic (MHC-restricted) and non-classic (CD1d-restricted) lymphocyte immune responses. We have also evaluated the effects of LBH589 treatment on the cytokine production by TLR3 and TLR4-induced DCs activation. Our study demonstrates a significant impairment on the phenotype and function of DCs upon LBH589 treatment.
Materials and Methods
Reagent
HDAC inhibitor LBH589 was provided by Novartis Pharmaceutical Inc (Cambridge, MA). It was dissolved in DMSO at 1 mM and kept at −70°C. Drug was diluted in culture medium and used at various concentrations as indicated.
Generation of DCs
Healthy donor leucopacks were obtained from Dana-Farber Cancer Institute following informed consent approved by the institutional review boards. To generate monocyte-derived immature DCs, CD14+ cells were isolated from peripheral blood mononuclear cells (PBMCs) by magnetic microbeads (Miltenyi Biotec, Auburn, CA) and cultured for 6 days in 10% of heat-inactivated fetal bovine serum (FBS) RPMI1640 medium supplemented with the GM-CSF (1000U/mL) and IL-4 (10ng/mL; both from R&D system, Minneapolis, MN) every 3 days. Mature DCs were induced by LPS (100ng/mL) or poly(I:C) (25μg/mL; both from Sigma-Aldrich, St. Louis, MO) following 6 days of culture. Peripheral blood untouched CD1c (BDCA-1)+ and CD141 (BDCA-3)+ myeloid DCs were directly isolated from PBMCs by human myeloid dendritic cell isolation kit (Miltenyi Biotec) and immediately used for the functional assay.
Isolation of T cells and establish of invariant NKT (iNKT) cell lines
T cells were obtained from PBMCs by negative selection using Pan T cell isolation kit II (Miltenyi Biotec). The establishment of iNKT cell lines was described previously18. In brief, TCRVα24+ cells were enriched from healthy donor PBMCs and stimulated with α-galactosylceramide (α-GalCer) (100ng/mL; Kirin Brewery Co. Ltd., Gunma, Japan)-pulsed mature DCs in the presence of IL-2 (50U/mL; Chiron Corporation, CA). TCRVβ11+ cells were further enriched from the proliferating cells and restimulated with α-GalCer-pulsed mature DCs every week. The frequencies of expanded iNKT cells were monitored by quantifying Vα24+ Vβ11+ cells using flow cytometric analysis.
Antigen uptake assay
The antigen uptake ability by immature DCs was evaluated using Alexa Fluor 488 conjugated- 45-KDa protein A (200μg/mL), 20-KDa protein G (200μg/mL) and FITC conjugated- 40-KD dextran (1mg/mL; all from Invitrogen, Baltimore, MD). In brief, following 24 hours treatment at various concentrations, control or LBH589-treated immature DCs were stained with individual antigen, incubated at 37°C for 45 minutes, washed with RPMI1640, and analyzed by flow cytometry.
DC-mediated T cell immune responses
(1) To determine the effects of LBH589 on immature DCs or directly isolated blood myeloid DCs, cells were pre-treated with or without LBH589 for 24 hours, subsequently collected, extensively washed three times, and then pulsed with tetanus toxoid (0.5μg/mL; Calbiochem, Gibbstown, NJ) overnight in the presence of LPS (100ng/mL). DCs were next harvested, washed, and seeded into 96-well plates to stimulate autologous T cells. (2) To determine the effects of LBH589 on DCs during maturation, DCs following 6 days of culture were treated with or without LBH589 for 24 hours, and simultaneously pulsed with tetanus toxoid (0.5μg/mL) in the presence of LPS. Cells were subsequently harvested, extensively washed three times and added into 96-well plates to culture with autologous T cells. (3) The effect of LBH589 on directly isolated myeloid DCs was also tested by allogeneic immune responses. Cells were pretreated with LBH589 for 24 hours, extensively washed, and loaded into 96-well plates to culture with allogeneic T cells. LBH589 was used at 10 nM for all the experiments. 1×105 T cells were added into each well and cultured with above control or LBH589-treated DCs at ratio of 10:1, in the presence of 40 U/mL of IL-2. In all cases supernatants were collected at 48 hours of culture and subjected to the measurement of IFN-γ production by ELISA.
DC-mediated iNKT cell immune responses
DCs following 6 days of culture were treated with or without LBH589 at 10 nM for 24 hours. During the same time, DCs were pulsing with α-GalCer (100ng/mL) in the presence of LPS (100ng/mL). Cells were subsequently harvested, extensively washed three times, and seeded into 96-well plates. 1×105 iNKT cells were added into each well and cultured with above control or LBH589-treated DCs for 48 hours at ratio of 5:1, in the presence of 40 U/mL of IL-2. Supernatants were collect and subjected to the detection of IFN-γ, IL-2 and IL-4 production by ELISA.
Cytokine production by TLR3 and TLR4-induced DCs activation
DCs following 6 days of culture were placed into 24-well plate at 1×105cells/mL and matured by poly(I:C) (25μg/mL) or LPS (100ng/mL) in the absence or presence of LBH589 at various concentrations. After 24 hours culture, the supernatants were collected for the measurement of IL-6, IL-10, IL-12p70, IL-23 and TNF-α production by ELISA.
Western blot
DCs following 6 days of culture were seeded into 6-well plates at 1.5×106 cell per well in 3mL DC culture medium, treated with or without LBH589 at various doses (2.5nM, 10nM and 20nM) for 18 hours, or at 10 nM with various time points (12hour, 18 hour and 24hours), in the presence or absence of LPS (100ng/mL) or poly(I:C) (25μg/mL) respectively. Cells were lysed by RIPA-lysis buffer in combination with complete protease inhibitor, 2mM sodium orthovanadatte, 5mM sodium fluoride, 5ug/mL aprotinin, 5ug/mL leupeptin and 1mM phenylmethylsulfonyl fluoride. Proteins were separated by 10% of bis-tris gel electrophoresis, transferred onto polyvinilydene difluoride membranes (Bio-Rad, Hercules, CA) and immunoblotted with anti-NF-κB p65 and RelB (both from Cell Signaling Technology, Beveryly, MA) and anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies.
Cytokine Assays
Released cytokine levels were determined by ELISA. IL-2, IL-4, IL-6, IL-10, IL-12p70, IL-23, IFN-γ, and TNF-α were quantified by the Quantikine immunoassay (R&D Systems) according to the manufacturer's instruction.
Cell viability assay and phenotypic characterization
Cell viability of the DCs with or without LBH589 treatment and of T cells/iNKT cells in response to control or LBH589-pretreated DCs was evaluated by staining with Annexin V and PI (BD Pharmingen, San Diego, CA) and quantifying by flow cytometry. The following antibodies were used for the DCs phenotypic analysis: PE-conjugated anti-CD40, anti-CD80, anti-CD83, anti-CD86, anti-HLA-ABC, and anti-HLA-DR mAbs (BD pharmingen). FITC-conjugated anti-TCRVα24 and PE-conjugated anti-TCRVβ11 mAbs (Immunotech, Marseille, France) were used to monitor the frequencies of iNKT cells. Flow cytometry was performed with a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA).
Results
Effects of HDAC inhibition by LBH589 on DCs survival
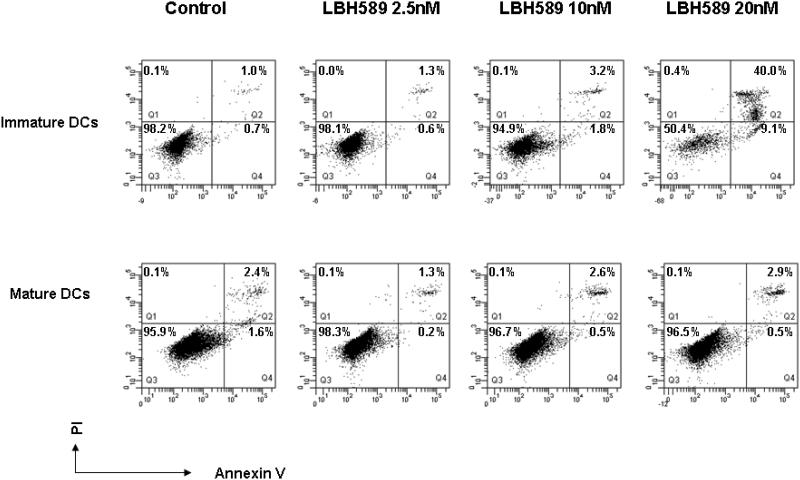
We first evaluated the effect of HDAC inhibition on survival of both immature and mature DCs. Monocyte-derived DCs were cultured with GM-CSF and IL-4 for 6 days and maturation was induced by LPS. Cells were exposed to clinically relevant concentrations of LBH589 (2.5nM to 20nM) for 24 hours and viability was checked by annexin V and PI staining. As seen in Figure 1, although 24 hours treatment with LBH589 at 10nM did not affect the viability of immature DCs (> 90%), a higher concentration at 20 nM induced it. For all subsequent studies with immature DCs we have used up to 10 nM concentration of LBH589 treatment. On the other hand, no significant apoptosis was induced by LBH589 up to 20 nM concentration in mature DC. Similar effects were observed on DCs matured with IL-1β and TNF-α (data not shown).
Figure 1. Effects of HDAC inhibition by LBH589 on DCs survival.
Immature DCs or LPS-induced mature DCs were treated with or without LBH589 for 24 hours at indicated concentrations and subsequently stained with Annexin V and PI. The cell viability was quantified by flow cytometry. LBH589 does not significantly affect the survival of immature DCs up to 10nM of treatment. Mature DCs show more potent survival ability, no apoptosis observed up to 20nM of LBH589 treatment. Data represent one of five independent experiments.
HDAC inhibition by LBH589 modulates phenotypes of immature and mature DCs
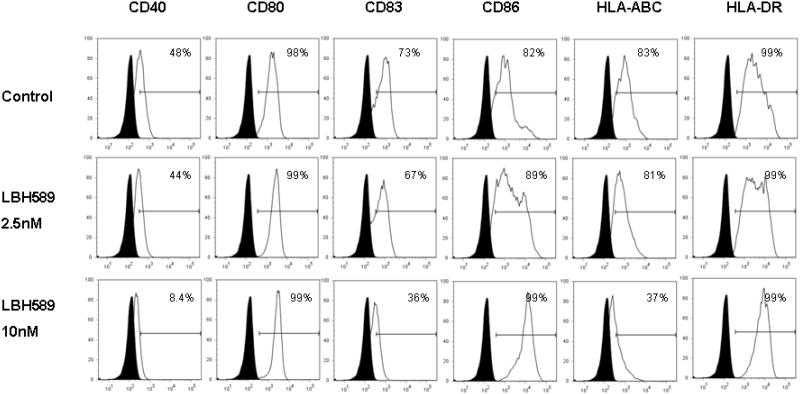
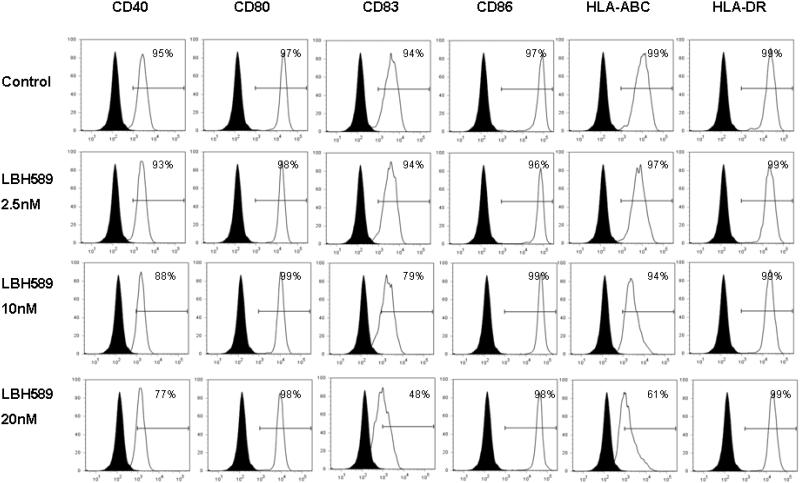
We next investigated the effects of HDAC inhibition on the expression of a series of surface molecules that are critical for DC function. Immature and mature DCs were cultured with or without LBH589 for 24 hours at various concentrations. Compared to the control, LBH589 treatment at 10 nM led to a significantly downregulation of CD40, CD83 and HLA-ABC expression on immature DCs. In contrast, we also observed the upregulation of CD86 expressions upon LBH589 treatment (Figure 2A). Similar to those observed on the immature DCs, LBH589 treatment (10 nM and 20 nM) resulted in the decreased expression of CD40, CD83 and HLA-ABC on mature DCs, compared to control. No obvious alteration was shown on the expression of CD86 (Figure 2B). CD80 and HLA-DR expressions were not significantly affected by LBH589 in both immature and mature DCs.
Figure 2. Effects of HDAC inhibition by LBH589 on the phenotype of immature and mature DCs.
Immature DCs or LPS-induced mature DCs were treated with or without LBH589 for 24 hours at indicated concentrations. The control or drug-treated DCs were then stained with PE-conjugated anti-CD40, anti-CD80, anti-CD83, anti-CD86, anti-HLA-ABC and anti-HLA-DR mAbs respectively for the phenotypic analysis at immature (Fig.2 A) and mature stages (Fig.2 B). Data represent the percentage of cells staining positive for each surface molecules (blank) compared to the isotype control (filled). Data represent one of three independent experiments.
HDAC inhibition by LBH589 decreases antigen uptake by immature DCs
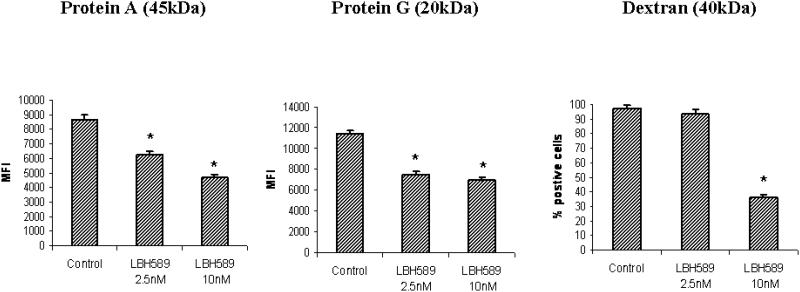
We next tested whether HDAC inhibition affected the antigen uptake capacity by immature DCs, using various size and forms of antigens. We observed LBH589 treatment (2.5 nM and 10 nM) significantly reduced uptake of both protein antigens (45-KDa protein A and 20-KDa protein G) by DCs, compared to control. Our data also demonstrate that HDAC inhibition repressed the ability of polysaccharide antigen uptake, evidenced by significant inhibition of Dextran (40-KDa) uptake by DCs upon LBH589 treatment (10nM) (Figure 3).
Figure 3. HDAC inhibition by LBH589 decreases antigen uptake capacity by DCs.
Following 24 hours of LBH589 treatment at indicated concentrations, the control or LBH589-treated immature DCs were stained with Alexa Fluor 488 conjugated- 45-KDa protein A, 20-KDa protein G or FITC conjugated- 40-KD dextran. The antigen uptake capacity by immature DCs was analyzed by flow cytometry. LBH589 treatment inhibits the uptake capacity of both protein and polysaccharides antigens by immature DCs (* p<0.05). Results are shown as the mean ± SD of triplicate assays. Data represent one of three independent experiments.
HDAC inhibition by LBH589 impairs DC-mediated T cell activation
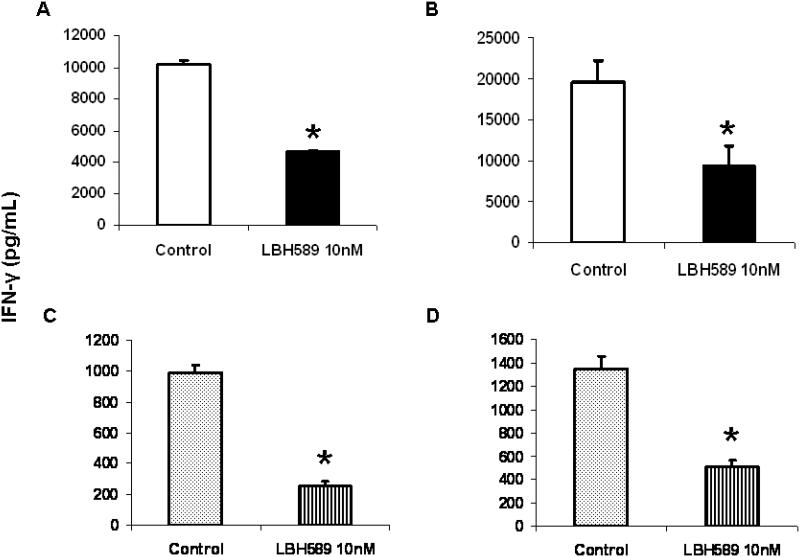
To investigate whether HDAC inhibition affected DCs capacity to activate antigen-specific T cell response, the IFN-γ production by T cells in response to control or LBH589 (10 nM) treated- DCs was measured by ELISA. As seen in figure 4A, upon stimulation with tetanus toxoid-pulsing DCs which were pretreated with LBH589 at immature stage, the IFN-γ production was significantly reduced at 48 hours of culture, indicating a repressed DC function to T cell activation. Exposure of DCs to LBH589 during LPS-induced maturation resulted in the similar effect, as seen in figure 4B. Importantly, we confirmed that LBH589 pretreatment of freshly isolated blood myeloid DCs led to significant inhibition of antigen-specific (Figure 4C) and allogenic (Figure 4D) T cell immune responses. To demonstrate that the impaired IFN-γ production by T cells was not due to the effect of residual LBH589, we performed the T cell apoptosis assay after 48 hours of incubation in above conditions. No significant difference in T cell viability was observed between cultures with control and LBH589-treated DCs following wash out (supplemental data).
Figure 4. HDAC inhibition by LBH589 impairs DC-mediated T cell activation.
(A) LBH589 effect on immature DCs. Cells were pretreated with or without LBH589 for 24 hours, subsequently pulsed with tetanus toxoid (0.5μg/mL) overnight in the presence of LPS, and then used to stimulate autologous T cells. (B) LBH589 effect on DCs during maturation. DCs following 6 days of culture were pulsed with tetanus toxoid (0.5μg/mL) in the presence of LPS, treated with or without LBH589 for 24 hours, and then used to stimulate autologous T cells. (C) LBH589 effect on blood myeloid DCs-mediated antigen-specific T cell responses. We follow the same description above in (A). (D) LBH589 effect on blood myeloid DCs-mediated allogeneic lymphocyte responses. Cells were pretreated with LBH589 for 24 hours and used to stimulate allogeneic T cells. LBH589 was used at 10 nM for all experiments. 1×105 T cells were added into each well and cultured with above control or LBH589-treated DCs at ratio of 10:1. In all cases Supernatants were collected at 48 hours of incubation and IFN-γ production was measured by ELISA. Compared to control, LBH589 treatment to DCs significantly impairs the IFN-γ production by T cells (* p<0.05). Results are shown as the mean ± SD of triplicate assays. Data represent one of three independent experiments.
HDAC inhibition by LBH589 impairs DCs-mediated iNKT cell activation
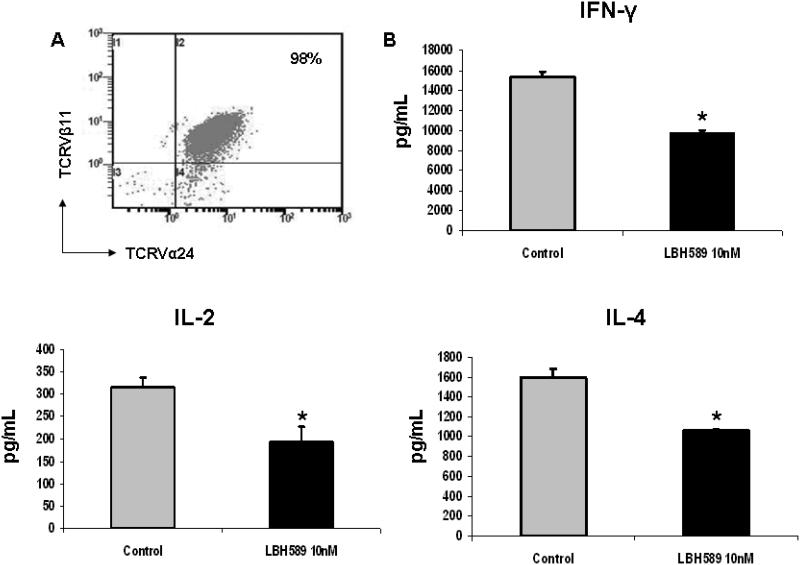
In addition to presenting peptide antigens, DCs also present glycolipids to activate iNKT cells. We therefore addressed whether HADC inhibition affected DCs function to activate CD1d-restricted iNKT cells, by measuring the cytokines production by iNKT cells upon stimulation with α-GalCer-pulsing control or LBH589 (10 nM) treated DCs. The frequency of Vα24+Vβ11+ cells from established primary iNKT cell lines was 98%, confirmed by flow cytometric analysis (Figure 5A). As seen in figure 5B, compared to control, significant reduction of both Th1-type cytokines (IFN-γ and IL-2) and Th2-type cytokine (IL-4) produced by iNKT cells was observed in the culture of LBH589-treated DCs, demonstrating an impaired DC function to iNKT cell activation. No significant difference in iNKT cell viability was observed between stimulation with control or LBH589-treated DCs (supplemental data).
Figure 5. HDAC inhibition by LBH589 impairs DC-mediated iNKT cell activation.
(A) The frequency of Vα24+β11+ cells in the established primary iNKT cell lines was analyzed by flow cytometry. Result represents the frequency of three iNKT cell lines. (B) DCs following 6 days of culture were treated with or without LBH589 (10 nM) for 24 hours, and simultaneously pulsed with α-GalCer (100ng/mL) in the presence of LPS. Control or LBH589-treated DCs were next seeded into 96-well plates to stimulate iNKT cells (1×105 cells/well) at the ratio of iNKT cells and DCs of 5:1. Supernatants were collect at 48 hours of incubation and subjected to the ELISA. The production of IFN-γ, IL-2 and IL-4 by iNKT cells is significantly repressed upon LBH589 treatment (* p<0.05). Results are shown as the mean ± SD of triplicate assays. Data represent one of three independent experiments.
HDAC inhibition by LBH589 represses cytokine production by DCs
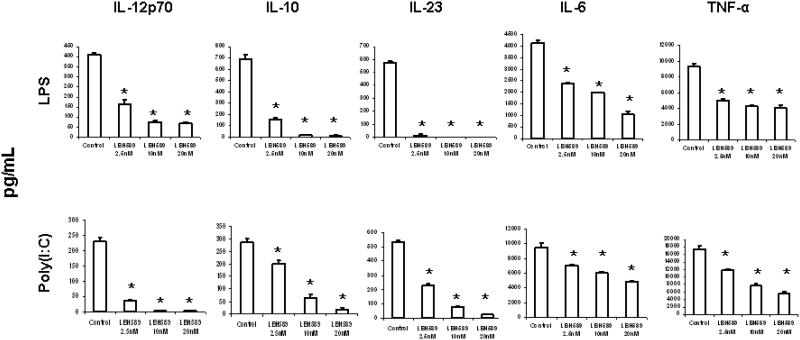
DCs play a crucial role in the innate and adaptive immune responses through cytokine regulation. Thus, we evaluated whether HDAC inhibition could modulate the cytokine production by DCs. DCs were treated with or without LBH589 (2.5nM, 10nM and 20nM) for 24 hours during LPS- or poly(I:C) induced maturation and the cytokine production was measured by ELISA. As seen in Figure 6, compared to controls, LBH589 treatment significantly inhibited a series of cytokine production by DCs, including IL-6, IL-10, IL-12p70, IL-23 and TNF-α. The significant reduction appeared at the lowest LBH589 concentration (2.5 nM), suggesting an essential role for HDAC activity in the cytokine production by DCs.
Figure 6. HDAC inhibition by LBH589 represses cytokine production by DCs.
DCs following 6 days of culture 6 were treated with or without LBH589 at indicated concentrations (2.5nM, 10nM and 20nM) during LPS (100ng/mL) or poly(I:C) (25μg/mL)- induced maturation. Supernatants were collected at 24 hours of incubation and subjected to the measurement of IL-6, IL-10, IL-12p70, IL-23 and TNF-α production by ELISA. LBH589 treatment leads to significant reduction of all cytokine production from 2.5 nM to 20 nM (* p<0.05). Results are shown as the mean ± SD of triplicate assays. Data represent one of three independent experiments.
HDAC inhibition by LBH589 impairs NF-κB signaling pathway in DCs
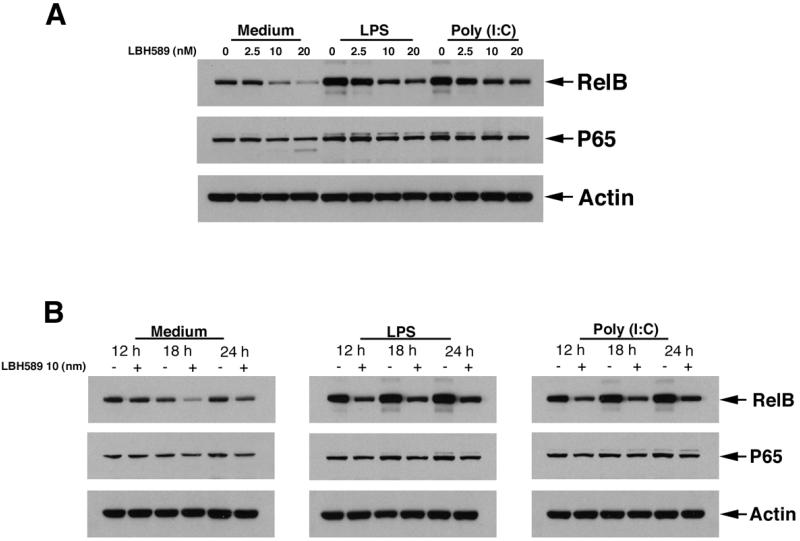
We next evaluated the mechanism responsible for LBH589-induced cytokine reduction by DCs. We observed that HDAC inhibition by LBH589 significantly reduced the expression of RelB, a NF-κB signaling component, in a dose-dependent manner (2.5nM, 10nM and 20nM) following 18 hour of treatment in both immature DCs and DCs during LPS or poly(I:C)-induced maturation. We also observed the reduction of RelB expression from 12 hours to 24 hours of LBH589 treatment at 10 nM. We did not observe a significant alteration in the expression of p65, another NF-κB component, in above conditions (Figure 7).
Figure 7. HDAC inhibition by LBH589 impairs NF-κB signaling pathway in DCs.
DCs following 6 days of culture were seeded into 6-well plates with or without LBH589 at indicated doses (2.5nM, 10nM and 20nM) for 18 hours (A), or at 10nM with indicated time points (12hour, 18 hour and 24hours) (B), in the presence or absence of LPS (100 ng/mL) or poly(I:C) (25μg/mL). Cells were then harvested and lysed, proteins separated by 10% of bis-tris gel electrophoresis and immunoblotted with anti-RelB, anti-NF-κB p65 and anti-actin antibodies. LBH589 treatment significantly reduces the expression of RelB in DCs.
Discussion
In the present study, we report that HDAC inhibition by LBH589 significantly modulates the phenotype and function of myeloid DCs. HDAC inhibition led to both up- and down- regulation of important surface molecules on DCs which were responsible for antigen-specific immune responses. CD40, CD80 and CD86 are the co-stimulatory molecules mediating T cell activation. Upon LBH589 treatment, CD40 expression was significantly reduced at both immature and mature states, while the CD86 expression was up-regulated on immature DCs. These discrepant results are consistent with the other report evaluating effects of LAQ824, another HDAC inhibitor, on DC 17, whereas SAHA induced downregulation of both CD40 and CD86 expression on DC 9. We also monitored the alteration of antigen presenting molecules. The expression of HLA-ABC was significantly inhibited. In combination to the reduction of CD83 on both immature and mature DCs, our results suggest the HDAC activity may play an important role in DC maturation as well as in generation of T cell responses.
Importantly, in the present study, we demonstrate that HDAC activity play a crucial role in DC-mediated antigen-specific immune responses, via both classic (MHC-mediated) and non-classic (CD1d-mediated) antigen presenting pathways. With MHC-restricted manner, DCs present peptides to activate T cells, the key mediators in the adaptive immunity. Our study has shown that LBH589 treatment of immature DCs or during LPS-induced maturation repressed their ability to activate T cells. LBH589 pretreatment of directly isolated myeloid DCs also showed similar effect. The published reports of similar effect of other HDAC inhibitors (SAHA, MS-275) on DC-mediated T cell immune responses suggests that these actions are related with the effect of the drugs on the type of target (HDAC) rather than a separate direct drug effects. iNKT cells constitute an innate lymphocyte lineage that has an important role in regulating immune responses. iNKT cells recognize glycolipid ligands presented by CD1d and are characterized by their capacity to rapidly produce large amounts of immunoregulatory cytokines to activate downstream immune effectors.19-21 We here report that LBH589 also repressed DC-mediated antigen-specific iNKT cell immune responses. To our knowledge, it is the first report to reveal the role HDAC activity in CD1d-mediated antigen presentation.
Together, our study revealed HDAC inhibition on DCs significantly affected both innate and adaptive immune responses, mediated by the major components of iNKT cells and T cells respectively. These may be caused by reduced expression of surface molecules associated with T/iNKT cell activation and by inhibited antigen uptake ability, which were demonstrated in this study; Or, due to repressed antigen processing and/or presentation capacities upon HDAC inhibition. Further evaluation of the molecular mechanisms of action of LBH589 on MHC class I, MHC class II and CD1d pathways will benefit our understanding of HDAC activity in DC-mediated antigen processing and presentation. Moreover, due to the crucial role of HDAC activity in DCs function, an attention should be caught for avoiding the combination of HDAC inhibitor with antigen-specific vaccination strategies.
When exposed to pathogens, particularly the ligands for Toll-like receptors (TLRs), DCs produce an array of pro-inflammatory cytokines, which create the environment in which T cells are expansion and differentiation. 22 Cytokine specifically involved in the induction of given T helper cell subset have been demonstrated that IL-12 for Th1, and IL-23 for Th17 cells.16,22,23 We compared these cytokine productions by LPS or poly(I:C) activated- DCs with or without LBH589 treatment. A significant reduction was seen for both IL-12 and IL-23 production at 2.5 nM drug concentration. In addition, we showed significant repression of the IL-6, IL-10 and TNF-α production by LBH589-treated DCs. Together, our data demonstrate that HDAC activity plays a crucial role in immune system via modulating DCs-produced cytokines, which are important components in regulating T cell polarization, NK cell activation and cytotoxic activity, as well as inflammation.
To address the mechanism mediating the LBH589-induced reduction in cytokine production by DCs, we evaluated the alteration in proteins of MAPK, NF-κB and IRF-5 signaling pathways which participate in TLR3 or TLR4 activation to produce pro-inflammatory cytokines. 24-26 Our study revealed that RelB of the NF-κB signaling pathway is the key component regulated by HDAC. No obvious alteration in MAPK (p38, JNK) and IRF5 signaling pathways was observed either by protein expression or phosphorylation of studies (data not shown). We have also evaluated the effect of LBH589 on the expression of IDO, a repressor of DCs activation. However, our data showed that LBH589 significantly down-regulated IDO expression after 12 to 24 hours of treatment in both LPS and poly(IC) induced- DC activation (no IDO expression seen in the immature DCs), which is opposite to our understanding of repressive function of IDO in DCs (data not shown). Further studies to elucidate this finding may bring us a new insight into understanding the HDAC activity in DCs.
In summary, our study demonstrates that HDAC inhibition by LBH589 significantly impairs the biology of myeloid DCs. Our pre-clinical data indicates a need to monitor immune functions in patients receiving the HDAC inhibitor therapy. In contrast, the significantly reduced pro-inflammatory cytokine production may suggest a need to investigate the use of this agent in treatment of inflammatory disease as well as autoimmune disorders.
Supplementary Material
Acknowledgement
We thank Novartis Pharmaceutical Inc (Cambridge, MA) for kindly providing LBH589. We also thank Kirin Brewery Co. Ltd. (Gunma, Japan) for kindly providing α-GalCer. This work is supported in part by National Institutes of Health grants P050-100707 and PO1-78378 (N.C.M and K.C.A), and a Merit Review Award from the Research Service Veterans Health Care (N.C.M).
Footnotes
Conflict-of –interest disclosure: The authors declare no competing financial interests.
References
- 1.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 2.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 3.Atadja P. Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett. 2009;280:233–241. doi: 10.1016/j.canlet.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 5.Leoni F, Zaliani A, Bertolini G, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JH, Oh SW, Kang MS, Kwon HJ, Oh GT, Kim DY. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin Exp Allergy. 2005;35:89–96. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 7.Camelo S, Iglesias AH, Hwang D, et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164:10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Bode KA, Schroder K, Hume DA, et al. Histone deacetylase inhibitors decrease Toll-like receptor-mediated activation of proinflammatory gene expression by impairing transcription factor recruitment. Immunology. 2007;122:596–606. doi: 10.1111/j.1365-2567.2007.02678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Inves. 2008;118:2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scuto A, Kirschbaum M, Kowolik C, et al. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood. 2008;111:5093–5100. doi: 10.1182/blood-2007-10-117762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha TL, Chuang MJ, Wu ST, et al. Dual degradation of aurora A and B kinases by the histone deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of renal cancer cells. Clin Cancer Res. 2009;15:840–850. doi: 10.1158/1078-0432.CCR-08-1918. [DOI] [PubMed] [Google Scholar]
- 12.Qian DZ, Kato Y, Shabbeer S, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 13.Verheul HM, Salumbides B, Van Erp K, et al. Combination strategy targeting the hypoxia inducible factor-1 alpha with mammalian target of rapamycin and histone deacetylase inhibitors. Clin Cancer Res. 2008;14:3589–3597. doi: 10.1158/1078-0432.CCR-07-4306. [DOI] [PubMed] [Google Scholar]
- 14.Glaser KB. HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Brogdon JL, Xu Y, Szabo SJ, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 18.Song W, van der Vliet HJ, Tai YT, et al. Generation of antitumor invariant natural killer T cell lines in multiple myeloma and promotion of their functions via lenalidomide: a strategy for immunotherapy. Clin Cancer Res. 2008;14:6955–6962. doi: 10.1158/1078-0432.CCR-07-5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Vliet HJ, Molling JW, Nishi N, et al. Polarization of Valpha24+ Vbeta11+ natural killer T cells of healthy volunteers and cancer patients using alpha-galactosylceramide-loaded and environmentally instructed dendritic cells. Cancer Res. 2003;63:4101–4106. [PubMed] [Google Scholar]
- 21.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–131. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 24.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.