Abstract
Vaccination with cytokine-producing tumor cells generates potent immune responses against tumors outside the central nervous system (CNS). The CNS, however, is a barrier to allograft and xenograft rejection, and established tumors within the CNS have failed to respond to other forms of systemic immunotherapy. To determine what barriers the "immunologically privileged" CNS would pose to cytokine-assisted tumor vaccines and what cytokines would be most efficacious against tumors within the CNS, we irradiated B16 murine melanoma cells producing murine interleukin 2 (IL-2), IL-3, IL-4, IL-6, gamma-interferon, or granulocyte-macrophage colony stimulating factor (GM-CSF) and used these cells as subcutaneous vaccines against tumors within the brain. Under conditions where untransfected B16 cells had no effect, cells producing IL-3, IL-6, or GM-CSF increased the survival of mice challenged with viable B16 cells in the brain. Vaccination with B16 cells producing IL-4 or gamma-interferon had no effect, and vaccination with B16 cells producing IL-2 decreased survival time. GM-CSF-producing vaccines were also able to increase survival in mice with pre-established tumors. The response elicited by GM-CSF-producing vaccines was found to be specific to tumor type and to be abrogated by depletion of CD8+ cells. Unlike the immunity generated against subcutaneous tumors by GM-CSF, however, the effector responses generated against tumors in the CNS were not dependent on CD4+ cells. These data suggest that cytokine-producing tumor cells are very potent stimulators of immunity against tumors within the CNS, but effector responses in the CNS may be different from those obtained against subcutaneous tumors.
Full text
PDF

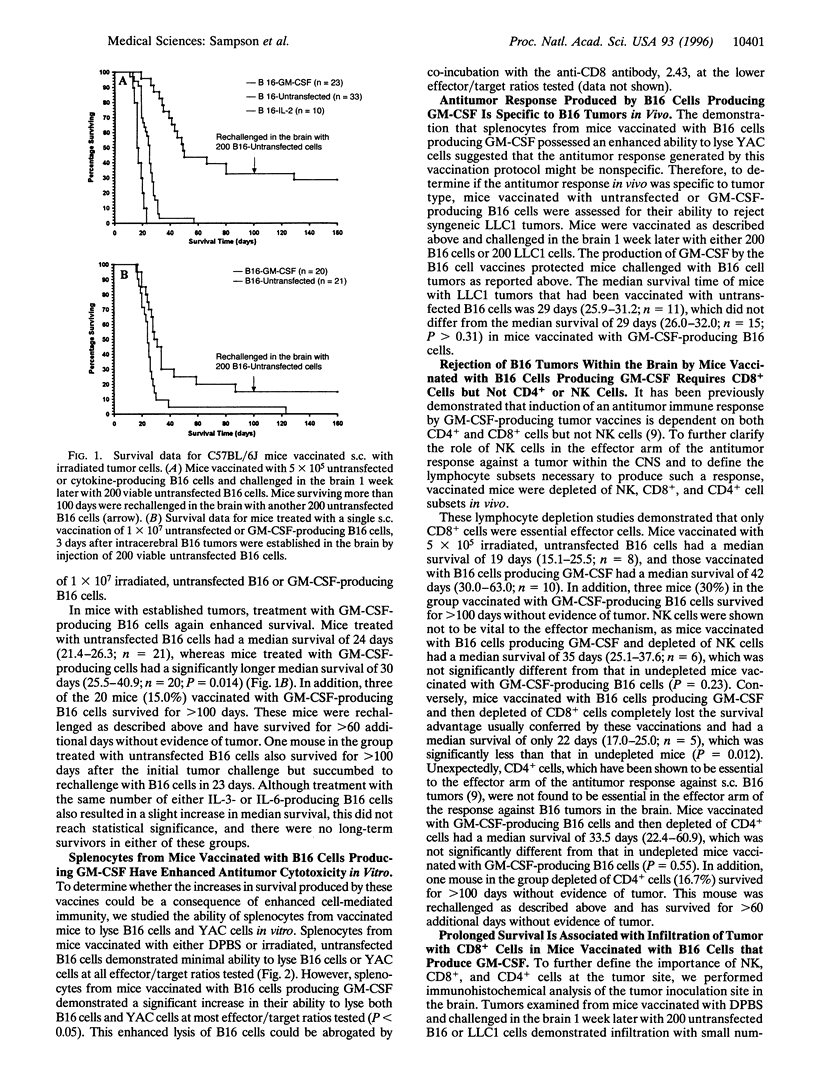
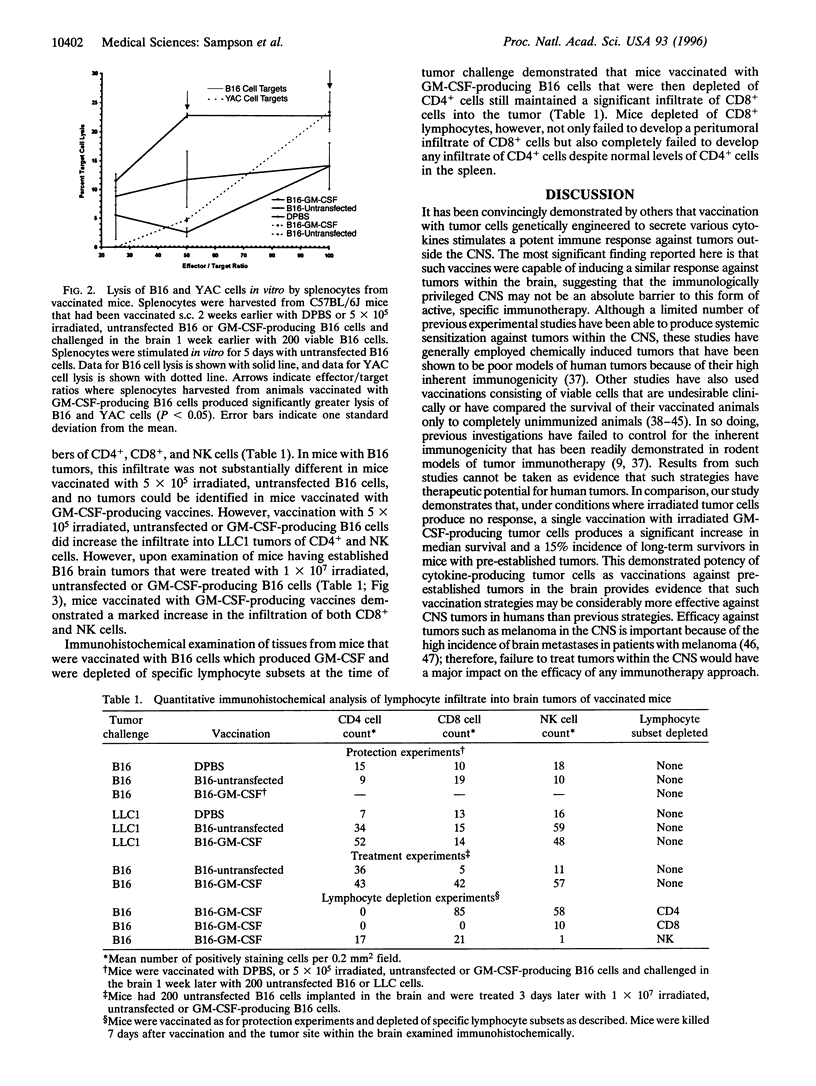


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright L., Madigan J. C., Gaston M. R., Houchens D. P. Therapy in an intracerebral murine glioma model, using Bacillus Calmette-Guérin, neuraminidase-treated tumor cells, and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Cancer Res. 1975 Mar;35(3):658–665. [PubMed] [Google Scholar]
- Amer M. H., Al-Sarraf M., Baker L. H., Vaitkevicius V. K. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978 Aug;42(2):660–668. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Amer M. H., Al-Sarraf M., Vaitkevicius V. K. Clinical presentation, natural history and prognostic factors in advanced malignant melanoma. Surg Gynecol Obstet. 1979 Nov;149(5):687–692. [PubMed] [Google Scholar]
- Bernard C. C., Carnegie P. R. Experimental autoimmune encephalomyelitis in mice: immunologic response to mouse spinal cord and myelin basic proteins. J Immunol. 1975 May;114(5):1537–1540. [PubMed] [Google Scholar]
- Bertram J. S., Janik P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980 Nov;11(1):63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Pitts O. M., Wikstrand C. J. Induction of lethal experimental allergic encephalomyelitis in nonhuman primates and guinea pigs with human glioblastoma multiforme tissue. J Neurosurg. 1981 Jul;55(1):32–42. doi: 10.3171/jns.1981.55.1.0032. [DOI] [PubMed] [Google Scholar]
- Cassel W. A., Weidenheim K. M., Campbell W. G., Jr, Murray D. R. Malignant melanoma. Inflammatory mononuclear cell infiltrates in cerebral metastases during concurrent therapy with viral oncolysate. Cancer. 1986 Apr 1;57(7):1302–1312. doi: 10.1002/1097-0142(19860401)57:7<1302::aid-cncr2820570709>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cikes M., Friberg S., Jr, Klein G. Progressive loss of H-2 antigens with concomitant increase of cell-surface antigen(s) determined by Moloney leukemia virus in cultured murine lymphomas. J Natl Cancer Inst. 1973 Feb;50(2):347–362. doi: 10.1093/jnci/50.2.347. [DOI] [PubMed] [Google Scholar]
- Connor J., Bannerji R., Saito S., Heston W., Fair W., Gilboa E. Regression of bladder tumors in mice treated with interleukin 2 gene-modified tumor cells. J Exp Med. 1993 Apr 1;177(4):1127–1134. doi: 10.1084/jem.177.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger R. H., Axler D. A., Koestner A., Liss L. Tumor-specific transplantation immunity to intracerebral challenge with cells from a methylnitrosourea- induced brain tumor. J Med. 1975;6(3-4):249–259. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dranoff G., Crawford A. D., Sadelain M., Ream B., Rashid A., Bronson R. T., Dickersin G. R., Bachurski C. J., Mark E. L., Whitsett J. A. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994 Apr 29;264(5159):713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Gansbacher B., Bannerji R., Daniels B., Zier K., Cronin K., Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity. Cancer Res. 1990 Dec 15;50(24):7820–7825. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Grooms G. A., Eilber F. R., Morton D. L. Failure of adjuvant immunotherapy to prevent central nervous system metastases in malignant melanoma patients. J Surg Oncol. 1977;9(2):147–153. doi: 10.1002/jso.2930090208. [DOI] [PubMed] [Google Scholar]
- Hewitt H. B., Blake E. R., Walder A. S. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976 Mar;33(3):241–259. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurford R. K., Jr, Dranoff G., Mulligan R. C., Tepper R. I. Gene therapy of metastatic cancer by in vivo retroviral gene targeting. Nat Genet. 1995 Aug;10(4):430–435. doi: 10.1038/ng0895-430. [DOI] [PubMed] [Google Scholar]
- Kida Y., Cravioto H., Hochwald G. M., Hochgeschwender U., Ransohoff J. Immunity to transplantable nitrosourea-induced neurogenic tumors. II. Immunoprophylaxis of tumors of the brain. J Neuropathol Exp Neurol. 1983 Mar;42(2):122–135. doi: 10.1097/00005072-198303000-00002. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J., Hershfield M. S. Determinants of deoxyadenosine toxicity in hybrids between human T- and B- lymphoblasts as a model for the development of drug resistance in T-cell acute lymphoblastic leukemia. Cancer Res. 1985 Apr;45(4):1579–1586. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ley V., Langlade-Demoyen P., Kourilsky P., Larsson-Sciard E. L. Interleukin 2-dependent activation of tumor-specific cytotoxic T lymphocytes in vivo. Eur J Immunol. 1991 Mar;21(3):851–854. doi: 10.1002/eji.1830210350. [DOI] [PubMed] [Google Scholar]
- Mitchell M. S. Relapse in the central nervous system in melanoma patients successfully treated with biomodulators. J Clin Oncol. 1989 Nov;7(11):1701–1709. doi: 10.1200/JCO.1989.7.11.1701. [DOI] [PubMed] [Google Scholar]
- Mullen C. A., Coale M. M., Levy A. T., Stetler-Stevenson W. G., Liotta L. A., Brandt S., Blaese R. M. Fibrosarcoma cells transduced with the IL-6 gene exhibited reduced tumorigenicity, increased immunogenicity, and decreased metastatic potential. Cancer Res. 1992 Nov 1;52(21):6020–6024. [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Porgador A., Bannerji R., Watanabe Y., Feldman M., Gilboa E., Eisenbach L. Antimetastatic vaccination of tumor-bearing mice with two types of IFN-gamma gene-inserted tumor cells. J Immunol. 1993 Feb 15;150(4):1458–1470. [PubMed] [Google Scholar]
- Porgador A., Gansbacher B., Bannerji R., Tzehoval E., Gilboa E., Feldman M., Eisenbach L. Anti-metastatic vaccination of tumor-bearing mice with IL-2-gene-inserted tumor cells. Int J Cancer. 1993 Feb 1;53(3):471–477. doi: 10.1002/ijc.2910530320. [DOI] [PubMed] [Google Scholar]
- Porgador A., Tzehoval E., Katz A., Vadai E., Revel M., Feldman M., Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992 Jul 1;52(13):3679–3686. [PubMed] [Google Scholar]
- Porgador A., Tzehoval E., Vadai E., Feldman M., Eisenbach L. Immunotherapy via gene therapy: comparison of the effects of tumor cells transduced with the interleukin-2, interleukin-6, or interferon-gamma genes. J Immunother Emphasis Tumor Immunol. 1993 Oct;14(3):191–201. [PubMed] [Google Scholar]
- Pulaski B. A., McAdam A. J., Hutter E. K., Biggar S., Lord E. M., Frelinger J. G. Interleukin 3 enhances development of tumor-reactive cytotoxic cells by a CD4-dependent mechanism. Cancer Res. 1993 May 1;53(9):2112–2117. [PubMed] [Google Scholar]
- SCHEINBERG L. C., SUZUKI K., DAVIDOFF L. M., BEILIN R. L. Immunization against intracerebral transplantation of a glioma in mice. Nature. 1962 Mar 24;193:1194–1195. doi: 10.1038/1931194a0. [DOI] [PubMed] [Google Scholar]
- SCHEINBERG L. C., SUZUKI K., EDELMAN F., DAVIDOFF L. M. STUDIES IN IMMUNIZATION AGAINST A TRANSPLANTABLE CEREBRAL MOUSE GLIOMA. J Neurosurg. 1963 Apr;20:312–317. doi: 10.3171/jns.1963.20.4.0312. [DOI] [PubMed] [Google Scholar]
- Saito S., Bannerji R., Gansbacher B., Rosenthal F. M., Romanenko P., Heston W. D., Fair W. R., Gilboa E. Immunotherapy of bladder cancer with cytokine gene-modified tumor vaccines. Cancer Res. 1994 Jul 1;54(13):3516–3520. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Schackert H. K., Itaya T., Schackert G., Fearon E., Vogelstein B., Frost P. Systemic immunity against a murine colon tumor (CT-26) produced by immunization with syngeneic cells expressing a transfected viral gene product. Int J Cancer. 1989 May 15;43(5):823–827. doi: 10.1002/ijc.2910430514. [DOI] [PubMed] [Google Scholar]
- Siesjö P., Visse E., Lindvall M., Salford L., Sjögren H. O. Immunization with mutagen-treated (tum-) cells causes rejection of nonimmunogenic rat glioma isografts. Cancer Immunol Immunother. 1993 Jul;37(1):67–74. doi: 10.1007/BF01516944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staib L., Harel W., Mitchell M. S. Protection against experimental cerebral metastases of murine melanoma B16 by active immunization. Cancer Res. 1993 Mar 1;53(5):1113–1121. [PubMed] [Google Scholar]
- Tjuvajev J., Gansbacher B., Desai R., Beattie B., Kaplitt M., Matei C., Koutcher J., Gilboa E., Blasberg R. RG-2 glioma growth attenuation and severe brain edema caused by local production of interleukin-2 and interferon-gamma. Cancer Res. 1995 May 1;55(9):1902–1910. [PubMed] [Google Scholar]
- Vieweg J., Rosenthal F. M., Bannerji R., Heston W. D., Fair W. R., Gansbacher B., Gilboa E. Immunotherapy of prostate cancer in the Dunning rat model: use of cytokine gene modified tumor vaccines. Cancer Res. 1994 Apr 1;54(7):1760–1765. [PubMed] [Google Scholar]
- Walker W. S. Origins of macrophage diversity: functional and phenotypic analysis of cloned populations of mouse splenic macrophages. Cell Immunol. 1987 Jul;107(2):417–432. doi: 10.1016/0008-8749(87)90249-8. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kuribayashi K., Miyatake S., Nishihara K., Nakayama E., Taniyama T., Sakata T. Exogenous expression of mouse interferon gamma cDNA in mouse neuroblastoma C1300 cells results in reduced tumorigenicity by augmented anti-tumor immunity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9456–9460. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrand C. J., Bigner D. D. Surface antigens of human glioma cells shared with normal adult and fetal brain. Cancer Res. 1979 Aug;39(8):3235–3243. [PubMed] [Google Scholar]




