Abstract
Introduction
In terms of human suffering, tuberculosis has a huge impact on global society, making it arguably the most important infectious disease in history. Despite the devastating impact on society, the tools to fight tuberculosis are very limited. Current standard therapy has been used for over 40 years and threats, such as the HIV epidemic and drug-resistant strains, undermine efforts to control the disease. New drugs are needed to address the challenges faced globally.
Areas covered
Current therapy is briefly reviewed in this paper and then new doses and combinations of existing drugs are presented. New candidate drugs are also discussed, along with the potential benefits and pitfalls of each of the compounds and approaches to therapy.
Expert opinion
Despite the need to develop new drugs, the ability of programs to deliver existing therapies must not be neglected. Directly observed therapy and a standard basic level of care for all patients with tuberculosis, regardless of where they reside, is imperative, and will ensure that new drugs and regimens will have the greatest possible impact. New combination regimens, including PA 824 and TMC207, in combination with existing drugs, are very exciting – not only because of their ability to shorten treatment regimens in pan-susceptible cases, but also because they can be used among drug-resistant strains. Although an effective vaccine will probably be necessary to eliminate tuberculosis, new drugs and combination regimens have the potential to save millions of lives before tuberculosis is finally eliminated.
Keywords: tuberculosis, multidrug resistant tuberculosis, rifampin, rifapentine, TMC207, PA 824, OPC-67683, SQ109, PNU-100480, AZD-5847, moxifloxacin, levofloxacin
1. Introduction
The Plague is a dark and philosophical novel of a small town battling with an untreatable epidemic. It is told from the perspective of a physician who is trying desperately to save the members of the community who are afflicted with the disease [1]. Albert Camus gained worldwide renown for this work and this should not be surprising. He was not only a very talented author, but he also knew what it was like to suffer at the hands of an untreatable disease. When Albert Camus was about to enter college, he was suffering with many of the symptoms of tuberculosis. The inevitable diagnosis soon came and at the age of 17 he was prescribed the best therapy that medical science could offer at the time: rest, red meat, and therapeutic pneumothorax.
In the nearly 100 years since young Albert was sent for treatment of his tuberculosis, tens of millions have died of this disease. This despite the fact that midway through the twentieth century, themodern era of chemotherapy for tuberculosis had begun. It is truly remarkable that the majority of these deaths throughout the twentieth century have occurred after the advent of effective chemotherapy for tuberculosis. Why is that? Why is it that in the decades after the advent of effective chemotherapy, millions had limited access to therapy? Why is it that despite the fact that more people than ever now have access to quality TB care, patients with drug-resistant strains are still offered therapy that is no better, and may be worse, than what was offered to Mr Camus nearly a century earlier?
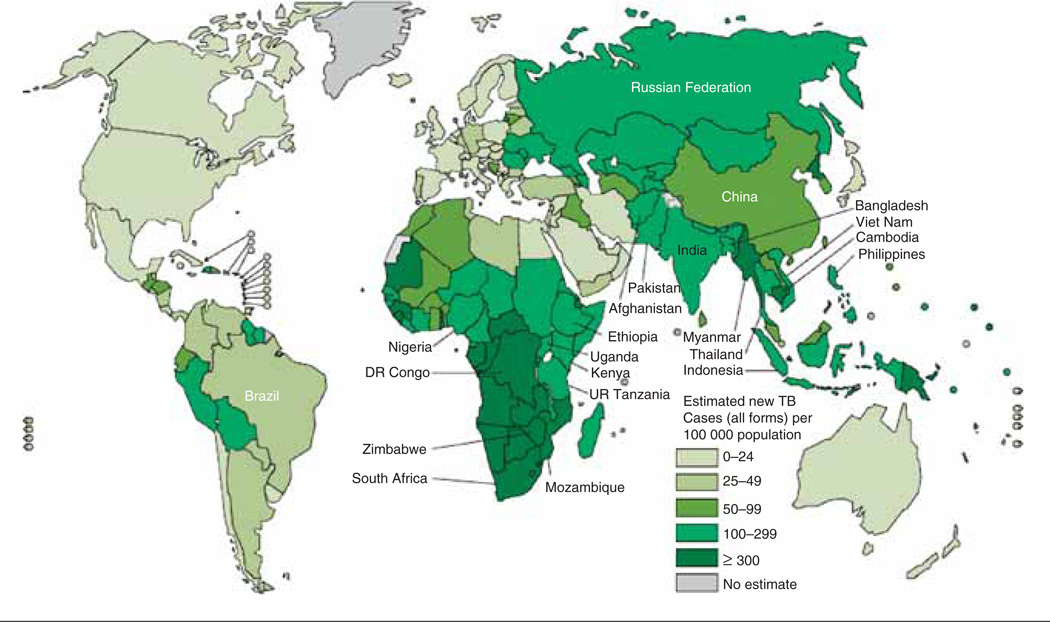
Tuberculosis is a global plague and is arguably the most important infectious disease in history. Over one-third of the world’s population either has evidence of previous tuberculosis exposure or is harboring latent TB infection waiting for the right biological circumstances to arise before developing active disease. With high incidence rates in the most populous regions of the world, there are close to 10 million new cases of active tuberculosis worldwide every year, and this results in just below 2 million deaths annually (Figure 1). Although dramatic advances in the fight against tuberculosis have been made over the last 10 years, these advances have come too late for those who have the least access to high-quality care. Only a part of this high-quality care is related to specific medications. There are a whole host of social, economic, and political factors that determine whether or not somebody will be adequately treated for tuberculosis. How else can you explain millions of deaths occurring annually six decades after the advent of effective prevention, treatment, and cure?
Figure 1. Estimated TB incidence rates, 2010.
Reproduced with permission from the World Health Organization, Global Tuberculosis Control: WHO Report 2011 (http://www.who.int/tb/publications/global_report/en/index.html (accessed 31 October 2011).
Nothing has changed the face of the global tuberculosis epidemic like the emergence and explosive distribution of HIV throughout the world, particularly in areas of high tuberculosis incidence. The HIV epidemic has impacted all aspects of tuberculosis. It has not only changed the epidemiology of tuberculosis; it has also changed the clinical presentation, making it more difficult to diagnose TB in HIV-infected individuals. Patients with tuberculosis have a substantially higher mortality from tuberculosis, especially those with drug-resistant strains. Moreover, treatment of tuberculosis with standard rifampin-based four-drug therapy is complicated in patients with HIV co-infection due to the numerous drug interactions with antiretroviral therapy (ART) such as protease inhibitors [2]. Rifampin is a potent inducer of hepatic CYP3A4, thus rendering protease inhibitors, which are metabolized by these hepatic enzymes, ineffective when co-administered with rifampin [3,4]. Furthermore, health systems in low-resource settings with a high HIV/TB co-infection rate are ill equipped to individualize therapy for both infections, resulting in ineffective therapy, increased toxicity, and poor adherence to therapy [5]. Thus there is a need to develop new drugs for tuberculosis that can be co-administered with protease inhibitors and other ART with increased effectiveness and less toxicity.
In developed countries with adequate infrastructure, drug supplies, and directly observed therapy, cure rates for patients completing all of their therapy can exceed 95% [6,7]. This requires the maintenance of a well-functioning public health program over many decades. However, despite these achievements, stating that ‘a cure for TB’ has been available for over 6 decades is something of an overstatement. Current treatment is cumbersome: it requires multiple medications, it is associated with monitoring, and it is relatively expensive, especially for developing countries. Because of the prolonged duration of therapy, protocols are necessary to maintain adherence. Now, with the advent of drug resistance, plus more frequently encountered complex drug interactions, current therapy is too challenging in many parts of the world. Social factors, and not strictly the biology of the organism, make it difficult to envision current therapy being able to stem the tide of active TB disease over the next 50 years. There is a need for shorter regimens for susceptible tuberculosis disease that are at least not inferior to current therapy and more effective regimens for drug resistant disease that are safe and relatively inexpensive, to allow patients in developing countries to approach the cure rates enjoyed in developed countries. Although no disease in history has ever been eliminated by the use of medications alone, new therapies for TB will have an enormous impact on morbidity and mortality from this disease, one of the greatest killers of the twentieth century.
2. Current TB therapy in 2012
When compared with the treatment of other medical conditions, including other infections, there has been relatively little change in TB therapy over the last 40 years in developed countries other than the widespread use of directly observed therapy. In developing countries, there have been significant changes: the widespread availability of rifampin-based regimens, as well as the wider use of directly observed therapy. The dissemination of TB therapy has been a double-edged sword: in those areas where drug availability exceeded the programs’ ability to monitor therapy and to ensure adherence, drug resistance soon followed. Now, with an estimated 500,000 new cases of multidrug-resistant TB (MDR-TB) worldwide every year, resistance threatens to undermine TB control efforts in many corners of the globe [8]. Indeed, the explosion of drug resistance, particularly in the last 5 years, has provided a strong stimulus for the development of alternatives to current therapy.
After the addition of ethambutol to the regimen to reduce the risk of drug resistance, no changes have been made to the standard TB regimen for pan-susceptible organisms. The standard recommendation remains initial therapy with four drugs (Table 1) including isoniazid, rifampin, pyrazinamide, and ethambutol for the first 2 months, followed by 4 months of isoniazid and rifampin. This regimen has been shown to be effective when given by directly observed therapy, curing up to 98% of individuals who complete therapy [7]. The most substantial change to standard TB therapy in the United States came in 1998, when rifapentine was approved for TB by the US Food and Drug Administration. It was approved to be given once weekly by directly observed therapy during the continuation phase of treatment. However, when compared with the standard regimen of isoniazid and rifampin given twice weekly, the once-weekly isoniazid and rifapentine regimen was slightly less effective, especially in patients with a high burden of organisms as is seen in cavitary pulmonary disease [9]. Furthermore, a high rate of rifamycin monoresistance was identified in HIV-positive patients treated with once-weekly rifapentine in the continuation phase of therapy [10]. For these reasons, rifapentine has not had a big impact on therapy for active TB disease in the US to date.
Table 1.
Drug regimens for culture-positive pulmonary tuberculosis caused by drug-susceptible organisms.
| Initail Phase | Continual Phase | Range of total doses (minimum§¶ durations) |
Rating‡‡ (evidence)‡ | |||||
|---|---|---|---|---|---|---|---|---|
| Regimen | Drugs | Interval doses## (minimum durations) |
Regimen | Drugs | Interval doses§ (minimum durations) |
HIV− | HIV+ | |
| 1 | INH | Seven days per week for 56 doses (8 wk) or 5 d/wk for 40 doses (8 wk)** | 1a | INH/RIF | Seven days per week for 126 doses (18 wk) or 5 d/wk for 90 doses (18 wk)** | 182–130 (26 wk) | A (I) | A (II) |
| RIF | ||||||||
| PZA | ||||||||
| EMB | ||||||||
| 1b | INH/RIF | Twice weekly for 36 doses (18 wk) | 92–72 (26 wk) | A (I) | A (II)‡‡ | |||
| 1c§§ | INH/RPT | Once weekly for 18 doses (18 wk) | 74–56 (26 wk) | B (I) | E (I) | |||
| 2 | INH | Seven days per week for 14 doses (2 wk), then twice weekly for 12 doses (6 wk) or 5 d/wk for 10 doses (2 wk)** then twice weekly for 12 doses (6 wk) | 2a | INH/RIF | Twice weekly for 36 doses (18 wk) | 62–58 (26 wk) | A (II) | B (II)‡‡ |
| RIF | 2b§§ | INH/RIF | Once weekly for 18 doses (18 wk) | 44–40 (26 wk) | B (I) | E (I) | ||
| PZA | ||||||||
| EMB | ||||||||
| 3 | INH | Three times weekly for 24 doses (8 wk) | 3a | INH/RIF | Three times weekly for 54 doses (18 wk) | 78 (26 wk) | B (I) | B (II) |
| RIF | ||||||||
| PZA | ||||||||
| EMB | ||||||||
| 4 | INH | Seven days per week for 56 doses (8 wk) or 5 d/wk for 40 doses for (8 wk)** | 4a | INH/RIF | Seven days per week for 217 doses (31 wk) or 5 d/wk for 155 doses (31 wk)** | 273–195 (39 wk) | C (I) | C (II) |
| RIF | ||||||||
| EMB | ||||||||
| 4b | INH/RIF | Twice weekly for 62 doses (31 wk) | 118–102 (39 wk) | C (I) | C (II) | |||
EMB = Ethambutol: INH = isoniazid: PZA = pyrazinamide: RIF = rifampin: RPT = rifapentine.
Definitions of evidence ratings: A = preferred, B = acceptable alternative; C = offer when A and B cannot be given: E = should never be given
Definition of evidence ratings: I = randomized clinical trial: ll = data from clinical trials that were not randomized or conducted in other populations: lll = expert opinions.
When DOT is used, drugs may be given 5 days/week and the necessary number of doses adjusted accordingly. Although there are no studies that compare five with seven daily doses, extensive experience indicates that would be an effective practice.
Patients with cavitation on initial chest radiograph and positive cultures at completion of 2 months of therapy should receive a 7-month (31 week; either 217 doses [daily] or 62 doses (twice weekly) continuation phase.
Five-day-a-week administration is always given by DOT. Rating for 5 day/week regimens is A Ill.
Not recommended for HIV-infected patients with CD4+ cell counts < 100 cells/µl.
Options 1c and 2b should be used only in HIV-negative patients who have negative sputum smears at the time of completion of 2 months of therapy and who do not have cavitation on initial chest radiograph (see text). For patients started on this regimen and found to have a possible culture from the 2-month specimen, treatment should be extended an extra 3 months.
Treatment of MDR-TB is a far more complicated matter. Treatment usually entails using at least five drugs to which the organism is confirmed or likely to be susceptible. Table 2 summarizes regimens used in patients with various combinations of drug resistance. In the US and other developed countries, susceptibility is confirmed either by growth-based conventional susceptibility testing or, increasingly more common, by molecular studies looking for specific gene mutations known to cause resistance. In developing countries, very few patients with suspected MDR-TB will have individualized therapy based on confirmed susceptibilities; rather, they will be treated with an empiric regimen based on the patient’s history of previous TB treatment or prevalent resistant patterns in a given jurisdiction. Further complicating the problem of drug resistance is the fact that only half of 1% of cases of MDR-TB worldwide between 2000 and 2009 had access to high-quality medications for MDR therapy [11]. When one considers the fact that so few patients with MDR-TB have access to adequate care, the duration of therapy required to have a chance of curing MDR, and the failure of the DOTS strategy alone to control TB where MDR is common, it is not surprising that the current tools at our disposal will grow increasingly inadequate with time.
Table 2.
Potential regimens for the management of patients with drug-resistant pulmonary tuberculosis.
| Pattern of drug resistance |
Suggested regimen | Duration of treatment (mo) |
Comments |
|---|---|---|---|
| INH (± SM) | RIF, PZA, EMB (an FQN may strengthen the regimen for patients with extensive disease) | 6 | In BMRC trials, 6-mo regimens have yielded ≥ 95% success rates despite resistance to INH if four drugs were used in the initial phase and RIF plus EMB or SM was used throughout.* Additional studies suggested that results were best if PZA was also used throughout the 6 mo (Rating BII),‡ fluoroquinolones were not employed in BMRC studies, but may strengthen the regimen for patients with more extensive disease (Rating BII). INH should be stopped in cases of INH resistance (see text for additional discussion) |
| INH and RIF (± SM) | FQN, PZA, EMB, IA ± alternative agent | 18–24 | In such cases, extended treatment is needed to lessen the risk of relapse. In cases with extensive disease. The use of an additional agent (alternative agents) may be prudent to lessen the risk of failure and additional acquired drug resistance. Resectional surgery may be appropriate (see text) |
| INH and RIF (± SM) and EMB or PZA | FQN, (EMB or PZA if active). two alternative agents | 24 | Use of first-line agents to which there is susceptibility.Add two or more alternative agents in case of extensive disease. Surgery should be considered (see text) |
| RIF | INH, EMB, FQN, supplemented with PZA for the first 2 months (an IA may be included for the first 2–3 months for patients with extensive disease) | 12–18 | Daily and three times weekly regimens of INH, PZA and SM given for 9 mo were effective in a BMRC trial§ (Rating BI). However extended use of an injectable agent may not be feasible. It is not known if EMB would be as effective as SM in these regimens. An all oral-regiman for 12–18 mo should be effective (Rating BIII). But for more extensive disease and/or to shorten duration (e.g., to 12 months), an injectable agent may be added in the initial 2 mo of therapy (Rating BIII). |
BMRC = British Medical Research Council: EMB = ethambutol; FQN = fluoroquinolone : IA = Injectable agent: INH = isoniazid; PZA = pyrazinamide; RIF = rifampin; SM = streptomycin.
FQN = Fluoroquinolone; most experience involves ofloxacin, levofloxacin, or ciprofloxacin.
IA = Injectable agents = Ethionamide, cycloserine, (streptomycin, amikacin, or kanamycin) or the polypeptide capreomycin.
Alternative agents = Ethionamide, cycloserine, p-aminosalicylic acid, clarithromycin, amoxicillin-clavunate, linezolid.
Source; Mitchison DA, Nunn AJ. Influence of initial drug resistance to short-course chemotherapy of pulmonary tuberculosis Am Rev Respir Dis 1986; 133; 423–430.
Source; Hong Kong Chest Service, British Medical Research Council. Five-year follow-up chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis 1986;133;423–430.
Source Hong Kong Chest Service, British Medical Research Council; controlled trial of 6-month regimens of daily and intermittent streptomycin plus isoniazed plus pyrazinamide for pulmonary tuberculosis in Hong Kong. Am Res Dis 19977; 115; 727–735.
3. Barriers to a solution
There are a number of barriers to developing new TB drugs. The most important of these surround the cost of these medications and their administration in a well-functioning program. Perhaps the most important of all of these challenges has been the high cost of developing new drugs, which is estimated to be between $115 and $240 million for each new drug [12]. Since tuberculosis is largely a disease of the developing world, or in poorer populations in developed nations, there has traditionally been little economic incentive to invest in new drugs for tuberculosis. Most TB programs in the developing world struggle to provide therapy where a single patient can be cured for about $20. This leaves little room for recovery of costs after the substantial investment necessary for research and development of new medications. Because of the economic disincentive to the pharmaceutical industry, governments, nongovernmental agencies, and private industry have established innovative collaborations to increase the investment in TB drug research and development. Included among these collaborations are the Centers for Disease Control and Prevention TB trials consortium (TBTC), the Global Alliance for TB Drug Development, and the Critical Path to TB Drug Regimens. These collaborations have led to an unprecedented optimism that new and very powerful drugs and drug combinations will hit the market soon.
Another challenge is time. It often takes at least 8 years from the time that a potential drug candidate is identified to the time it reaches market. With tuberculosis, this time may be longer, because the current standard to assess the efficacy of anti-TB regimens in Phase III clinical trials is the relapse rate after 2 years of effective therapy. Also tuberculosis, like cancer, is treated with multiple drugs in various combinations and will require evaluating efficacy for entire regimens. Also, contributing to the cost is a relative scarcity of clinical trial sites in high-incidence countries that have the capacity to undertake Phase II and III clinical trials. Developing these sites, and the inefficiency from inexperienced sites, contributes significantly to the cost of developing new drug regimens, and affects the ability to perform randomized clinical trials.
4. Potential solutions
4.1 Vaccine development
The global eradication of smallpox is the best example of what can be achieved when vigorous efforts are directed to developing and disseminating an effective, safe, and inexpensive vaccine. The translation of discoveries in the laboratory into an operational public health context leading to the elimination of smallpox is frequently held up as the gold standard by which all other global disease eradication programs are measured. In fact, many investigators and policymakers feel that the path to achieve the World Health Organization’s (WHO) goal of reducing TB rates globally to < 1 case per million population by 2050 will be reached not by new drugs, but by an effective vaccine. There is good reason to hold such a view. A brief look at childhood diseases, particularly in developed countries, gives ample evidence for such optimism. But closer inspection tempers the enthusiasm behind any view that a vaccine alone will be sufficient.
Over the last 10 – 20 years, there has been a considerable increase in funding for TB basic science research, with much of this focused on developing a better understanding of the immunogenicity of TB. This investment is bearing fruit. At the end of 2010, there were 11 candidate vaccines in clinical trials all over the world. But because of the complex immunology of TB, it is uncertain whether any of these candidate vaccines will reach clinical use. Over 2 billion people have evidence of latent TB infection, but only 10% of these individuals will develop active TB disease. The quandary of latency is a formidable barrier to developing an effective vaccine. Since 90% of those infected with latent TB do not develop disease and presumably have lifelong immunity to reactivation of latent disease, the goal for a new vaccine would be to induce this lifelong immunity in the 10% of those with evidence of latent infection who currently develop active TB at some point during their lives [13]. Even if establishing immunity is possible, maintaining that immunity will be very difficult because of the many insults that can impair a sustained immune response to prevent reactivation. Infections with other mycobacteria, co-infection with HIV, and helminth infestation can modify the immune system’s ability to maintain cell-mediated immunity over many years [14].
4.2 Modified dosing of rifampin
Rifampin is considered the most important drug in standard TB therapy. Its mechanism of action occurs by binding to the beta subunit of RNA polymerase. This in turn inhibits RNA transcription, shutting down protein synthesis in Mycobacterium tuberculosis. [15]. Rifampin is felt to be the most important drug in standard TB therapy because it has the most potent sterilizing activity [16]. Sterilizing activity has been defined as the process of killing organisms between the second day and the completion of the second month of therapy; it is measured by the bacteriological response over the first 2 months of treatment [17]. However, this definition is more of a surrogate marker for sterilizing activity. Essentially, sterilizing activity is the ability of drugs to prevent post-treatment relapses. It is this sterilizing activity that is most important to shorten therapy [18]. Unlike isoniazid, where there appears to be a plateau in activity with increasing dosages, rifampin has no detectable plateau. This has led various investigators to question: what is the ideal dose of rifampin?
Early studies in mice showed that there was an increased sterilizing activity with increasing dose in rifampin, and a human early bactericidal activity (EBA) study showed that the 2-day EBA of rifampin is doubled if the dose is doubled [19,20]. Numerous subsequent studies in humans showed that there was increased culture conversion at 2 months with an increased dose of rifampin [21,22]. More recently, Goutelle et al. used Monte Carlo simulation to model concentration of rifampin in plasma and airway epithelium lining fluid, and used the model to assess the sterilizing activity of rifampin. They concluded that 1200 mg of rifampin was associated with better results than conventional dosing with 600 mg of rifampin [23]. A systematic review of randomized controlled trials that evaluate a range of rifampin doses was recently published [24]. The study reviewed 14 trials with a total of 4256 participants. Several trials were identified as increasing the likelihood of culture conversion among patients receiving ≥ 900 mg of rifampin. Side effects were uncommon; however, an increased incidence of flu-like syndrome was seen when doses > 900 mg were given intermittently. The authors concluded that there was sufficient evidence to justify Phase II and III clinical trials evaluating high-dose rifampin. Several clinical trials are now enrolling patients to determine whether high-dose rifampin can shorten treatment durations. A series of three clinical trials to be carried out in Africa have been designed and are currently enrolling patients. These trials will include pharmacokinetic and the EBA assessments, leading to larger, multicenter Phase IIb studies using the MTD [25].
4.3 Other rifamycins
Although rifapentine has not gained traction as a component of standard TB therapy in combination with isoniazid and other first-line TB drugs, the potential of rifapentine to shorten therapy and contribute to TB control remains great. Rifapentine has a longer half-life than rifampin, and its Cmax of 10 – 30 µg/ml is slightly higher than rifampin’s [26]. Also like rifampin, increased doses > 600 mg may have the potential to shorten TB treatment. Murine studies have shown that when combined with moxifloxacin, increased sterilizing activity is noted. The RIFAQUIN study, a Phase III study that began enrolling patients in 2008, is designed to evaluate whether a rifapentine- and moxifloxacin-containing regimen can reduce the duration of therapy. Where rifapentine will most rapidly have an impact in TB control efforts is with latent TB infection. The PREVENT TB study, completed in 2011, enrolled > 8000 patients in a noninferiority protocol and compared rifapentine 900 mg versus isoniazid 900 mg once weekly in directly observed therapy for 12 weeks versus 9 months of isoniazid 300 mg daily self-administered therapy. This study showed that the rifapentine-containing regimen was not inferior to 9 months of isoniazid [27].
Rifabutin is currently used mainly as a substitute for rifampin in standard TB regimens for pan-susceptible disease in patients who have significant drug interactions, such as those individuals who are on protease inhibitors. Although it has a longer half-life than either rifampin or rifapentine, it has dose-related toxicities such as uveitis, polyarthralgia, and hepatitis [28]. Therefore it is unlikely that any expanded role for rifabutin will be sought beyond its roles as a selective substitute for rifampin.
5. New drugs
5.1 TMC207 (bedaquiline)
TMC207 is a new diarylquinoline formerly referred to as R207910 or the J compound. It is an exciting new drug developed for TB that was discovered by high-throughput screening of a library of 10,000 compounds [29]. Its mechanism of action is novel. Its biological target is the F0 subunit of the ATP synthase encoded by atpE, and because of this novel mechanism of action there is no cross-resistance with existing TB drugs [30,31]. It is metabolized by CYP450 to an active M2 metabolite, but does so differently in mice compared with humans. The expected 50% reduction of serum concentrations by rifampin, along with changes in the concentrations of the long-lasting M2 metabolite, make the combined use of TMC207 and rifampin problematic [32,33].
Important insights have been gleaned from studies in the mouse model. Ibrahim showed that a 2-month regimen combining TMC207 with pyrazinamide (PZA) led to culture conversion [34]. Also, it appears that TMC207 is more bactericidal than conventional therapy. When TMC207 was given at 25 mg/kg, it was found to have a greater bactericidal effect than a regimen of isoniazid, rifampin, and PZA [30]. This increased bactericidal activity has led to hopes that a combination of TMC207 with PZA and rifampin, isoniazid, or moxifloxacin will lead to curative regimens that can be substantially less than 6 months [35]. To take this one step further, in another study conducted in the mouse model, TMC207 combined with PZA and rifapentine sterilized markedly better than a regimen of isoniazid, rifampin, and PZA, with relapse rates of 0 versus 100% after 2 months of therapy [36]. Human trials have also been promising, but with some mixed results. An EBA study showed that TMC207 given daily at 400 mg has significantly less EBA than isoniazid or rifampin [32]. However, it must be kept in mind that TMC207 has time-dependent bactericidal effects, and that if the study is carried out longer or used in combination with PZA more rapid killing of organisms would be observed. It is also important to consider that EBA studies may not be predictive of sterilizing ability. Results of EBA studies for TMC207 and other agents discussed in this paper need to be interpreted with this limitation in mind. PZA, for example, differs from isoniazid in that it has a slow and steady EBA response; yet it is a very important sterilizing drug.
A Phase II trial to investigate the use of TMC207 in patients with MDR-TB began in 2007. The first stage of the study showed more rapid culture conversion and good tolerance when TMC207 was added to a standard MDR-TB regimen versus placebo added to the same regimen [37].
In summary, TMC207 is an exciting new drug with a novel mechanism of action that not only holds promise for MDR patients, but also has potential to shorten the regimen for those with susceptible disease. There are a few concerns that will need to be further elucidated. First, because of the better results seen with TMC207 when used in combination with PZA, it is unclear how effective TMC207 will be in patients with PZA resistance, a potentially common additional form of resistance in areas of high MDR-TB incidence. Secondly, in the Phase II study mentioned above, QTc prolongation and gastrointestinal upset were seen among patients. What the clinical significance will be is uncertain at this time. Nonetheless, it is expected that TMC207 will continued to move along in clinical trials.
5.2 Nitroimidazole derivatives
5.2.1 PA 824
PA 824 is a nitroimidazole derivative that was developed from a series of nitroimidazofurans originally investigated as radio-sensitizers for cancer chemotherapy [38]. The lead compound was found to be mutagenic, but it was found to have significant bactericidal activity against replicating tuberculosis organisms in vitro [39]. PA 824 is a pro-drug with a novel mechanism of action [40]. The active metabolite(s) probably attack M. tuberculosis, but not other mycobacteria, by inhibiting ketomycolic acid synthesis and protein synthesis, as well as possibly generating intracellular NO [35,41,42].
In vitro studies have shown that the MICs of PA 824 against susceptible and resistant strains of TB are very low, in the range of 0.015 – 0.53 µg/ml, and that activity was concentration dependent [40,43]. In the mouse model, PA 824 has bactericidal activity similar to that of isoniazid and sterilizing activity comparable to rifampin [44]. Although the addition of PA 824 to regimens that contain rifampin showed no significant improvement in sterilizing effect [45], the combination of PA 824 to moxifloxacin, and PZA cured mice more rapidly [43] than the standard therapy regimen using isoniazid, rifampin, and PZA [46]. These data suggest that there is a role for PA 824 to shorten regimens for TB, but in only those regimens that do not contain isoniazid – or, more importantly, rifampin.
In a Phase I extended EBA study conducted in South Africa, sputum smear-positive patients were investigated using escalating doses of PA 824 over 14 days. Although less bactericidal activity was noted in the first 2 days compared with standard four-drug therapy with isoniazid, rifampin, PZA, and ethambutol, significant and continued bactericidal activity was noted throughout the 14 days of the trial [47]. No serious adverse effects have been identified from the human studies conducted to date [48]. PA 824 is now in Phase II trials.
PA 824, with its narrow spectrum and novel mechanism of action, is a promising new drug candidate for TB therapy among susceptible and resistant strains. However, some challenges lie ahead. PA 824 is highly protein bound (94%), which could affect drug delivery into certain types of tissues [49]. Also, despite the fact that no adverse effects have been noted in small Phase I studies, recent animal studies suggest the possibility that ocular and male reproductive toxicity may be a potential toxicity in humans [50].
5.2.2 OPC-67683 (delamanid)
OPC-67683 is a compound that is closely related to PA 824 and is also chemically related to metronidazole. It lacks the mutagenicity of the compounds originally discovered in this class and has a very high potency both in vitro and in vivo against drug-susceptible and -resistant strains of M. tuberculosis [40,51]. In fact, although the mechanism of action of OPC-67683 is similar to that of PA 824, inhibition of cell wall biosynthesis, it is ~ 20 times more potent than PA 824 and has a remarkably low MIC of 0.006 – 0.024 µg/ml in vitro [51,52]. OPC-67683 requires intracellular activation by M. tuberculosis and there is cross-resistance with PA 824 [53]. Mutations that occur within the gene that encodes for ddn, the enzyme responsible for activation, causes resistance to both OPC-67683 and PA 824 [54]. It is unclear whether mutations in other genes that confer resistance to PA 824 will also confer resistance to OPC-67683. Despite the likely cross-resistance with PA 824, OPC-67683 does not show cross-resistance with other TB medications. Other important characteristics include a relatively long half-life of ~ 8 h, no significant activation of liver enzymes, and a very good post-antibiotic effect [51,52].
In a mouse model, 2 months of OPC-67683 in combination with rifampin and pyrazinamide followed by 2 months of OPC-67683 and rifampin resulted in culture negativity faster than a standard first-line regimen of isoniazid, rifampin, pyrazinamide, and ethambutol [51]. Phase I studies have shown good tolerance among healthy volunteers in doses up to 400 mg with no adverse effects being observed [38]. The initial EBA studies have shown low bactericidal activity during the first 4 days. However, a recently completed study among patients with smear-positive disease in South Africa revealed better results when the study extended over 14 days. Forty-eight patients with smear-positive tuberculosis (63% male; 54.7 ± 9.9 kg; 30.7 ± 10.8 years) were randomly assigned to receive OPC-67683 at doses of 100, 200, 300 or 400 mg daily for 14 days. Colony-forming units (cfu) of M. tuberculosis were counted on agar plates from overnight sputum collections to calculate EBA, defined as fall in log10 cfu/ml sputum per day. The EBA of OPC-67683 was monophasic and not significantly different between dosages; however, more patients receiving 200 mg (70%) and 300 mg (80%) experienced a response of ≥ 0.9 log10 cfu/ml sputum decline over 14 days than those receiving 100 mg (45%) and 400 mg (27%). The authors concluded that OPC-67683 at all dosages was safe, well tolerated and demonstrated significant exposure-dependent EBA over 14 days. Phase II studies continue to enroll patients, including MDR-TB, to further assess efficacy and safety in human subjects [47].
In summary, the novel mechanism of action, high potency, and lack of induction of metabolism by CYP enzymes has raised the hopes and expectations of this agent to be of use not only in MDR-TB but also in patients receiving ART for HIV co-infection. The initial EBA studies have given way to somewhat more promising data, encouraging hopes for this drug’s usefulness in the long term.
5.3 SQ109
SQ109 is an ethylenediamine that was originally identified by screening a library of > 60,000 compounds related to ethambutol [55]. Although derived from ethambutol, it is different enough molecularly to be considered as more than a second-generation ethambutol. It exerts its mechanism of action on the cell wall, but its exact target is unknown [55–57]. Since the incidence of resistance to SQ109 in vitro is low, it is thought that multiple mutations are necessary, suggesting the possibility of more than one target of SQ109 [53]. It has an MIC of 0.11 – 0.64 µg/ml against resistant and susceptible strains of M. tuberculosis, including ethambutol-resistant strains [57,58]. It appears to be bactericidal within macrophages [59,60] and has an exceptionally long half-life of just over 60 h.
In animal studies, there is evidence of extensive tissue distribution and concentration [55,57], probably due to the enhanced binding to tissue rather than plasma proteins [58]. In daily doses given alone, SQ109 is similar in activity to ethambutol; but when used in combination with isoniazid, rifampin, and pyrazinamide rather than ethambutol, SQ109 leads to increased bactericidal activity. Phase I studies have shown that the drug has been well tolerated by healthy volunteers in single doses up to 300 mg [35].
Perhaps the most intriguing addition to TB therapy may be its remarkable synergistic effect on other TB drugs. In vitro, there is synergy between SQ109 and rifampin and isoniazid, d even below the MIC concentrations of SQ109; but no additive effect was noted when combined with ethambutol and pyrazinamide [38]. In another study conducted in the mouse model, after 2 months of therapy of SQ109 substituted for ethambutol in a regimen of rifampin and isoniazid with or without pyrazinamide, the M. tuberculosis load was 1.5 log10 lower than observed with a standard regimen of isoniazid, rifampin, pyrazinamide, and ethambutol [61]. This synergistic effect is not limited to the first-line drugs. Reddy et al., in a study funded by the maker of SQ109, found that when SQ109 and TMC207 were combined in vitro with each other and with rifampin, the combination of SQ109 with TMC207 improved the TMC207 MIC four- to eightfold, improved the bactericidal activity of either drug alone, and enhanced the drug post-antibiotic effect by 4 h [62]. There is significant optimism for SQ109, given its synergistic properties and mechanism of action. If evidence surfaces that SQ109 is an efflux inhibitor, this will be significant, since there is growing evidence that efflux pump inhibitors have the potential to improve the efficiency of tuberculosis therapy [63]. Its entry into Phase II studies should further define its future role in TB therapy. It has recently been licensed by its manufacturer for clinical use in Russia [64].
5.4 Oxazolidinones
The oxazolidinones block protein synthesis in M. tuberculosis by interrupting the formation of the ribosomal initiation complex [35]. The only drug in this class in clinical use today is linezolid, a drug that is used off label to treat MDR-TB and extensively drug-resistant (XDR)-TB. It has substantial in vitro activity against M. tuberculosis, with MIC90 values of 0.5 – 1.0 µg/ml [65]. Linezolid probably contributes to sputum culture conversion in many cases of drug-resistant TB, and there is ample anecdotal and case-report evidence that this is the case [66–68]. However, the relative contribution of linezolid to sputum conversion in patients being treated for resistant TB is unknown. Furthermore, linezolid is associated with substantial adverse effects that are more common in long-term use, as would be necessary to treat TB. Myelosuppression, peripheral neuropathy, and optic neuropathy have all been reported in case series of patients treated with linezolid [52,66,69,70]. Although adjusting the dose frequency may reduce the incidence of myelosuppression, that may not be the case for the incidence of neuropathy [71].
Despite the limitations of linezolid, the oxazolidinones are still a promising new addition to the TB treatment armamentarium. Two new compounds that emerged as TB drug candidates are PNU-100480 and AZD-5847, both of which are in Phase I studies (ClinicalTrials.gov identifier NCT00990990 and NCT 01037725). PNU-100480 was first described over 15 years ago. When compared with linezolid in the mouse model, it had significantly greater bactericidal activity [72]. More recently, investigators compared the combination of PNU-100480, moxifloxacin, and pyrazinamide, which does not contain either rifampin or isoniazid, and found that it was more active than rifampin, isoniazid, and pyrazinamide [73]. Wallis et al. studied 19 healthy volunteers receiving two escalating single oral doses of PNU-100480 and eight subjects received four daily doses of 300 mg of linezolid. They found that single doses of PNU-100480 up to 1000 mg were well tolerated and demonstrated superior antituberculous activity compared with linezolid at 300 mg at steady state [74]. PNU-100480 also has favorable in vitro activity against M. tuberculosis when compared with linezolid. In a study comparing the susceptibility of clinical tuberculosis isolates to PNU-100480 and linezolid using the MGIT™ 960 culturing system (BD Diagnostic Systems, Sparks, MD), the mean MIC for PNU-100480 was 3.2 times lower than that for linezolid [75].
AZD-5847 is the other oxazolidinone that has entered Phase I. An ascending-dose study of the pharmacokinetics, safety, and tolerance has been completed. This drug is being developed by private industry and no other information is available at this time. The oxazolidinones have excellent bioavailability and good activity against M. tuberculosis isolates, but the issues related to tolerance during the longer duration of therapy necessary for TB will need to be addressed definitively. Although frequently thought of as a drug to use in cases of drug-resistant TB, these new drug candidates may have a role in shortening TB regimens.
5.5 Pyrrole derivative LL-3858
Several pyrrole derivatives were developed in the search for compounds with activity against mycobacteria. LL-3858 is a pyrrole derivative being developed by Lupin Ltd. Its mechanism of action is unknown, but an early study reported an MIC range of 0.06 – 0.5 µg/ml independent of resistance to isoniazid and rifampin [76]. In that same study in the mouse model, investigators found that addition of LL-3858 to a regimen of isoniazid, rifampin, and pyrazinamide enhanced the sterilizing activity of the regimen [76]. Other than the report from Arora et al., no other publications have been produced and no further information is available publicly about its current status.
6. Alternate use of existing agents
6.1 Fluoroquinolones
The fluoroquinolones are broad-spectrum antibiotics that are widely used for a host of bacterial infections other than tuberculosis. They exert their activity inhibiting bacterial DNA gyrase, an enzyme critical to maintaining DNA supercoils during chromosomal replication [77,78]. Because of their excellent bioavailability, side-effect profile, and activity against M. tuberculosis the fluoroquinolones have become indispensible components of TB care, especially in patients with MDR-TB or who are intolerant of one or more oral first-line TB drugs. Second only to the rifamycins in terms of potency, there has been intense interest in recent years to evaluate this class of drugs for their potential to shorten current therapy.
Of the five most commonly used fluoroquinolones, the most active against M. tuberculosis are moxifloxacin and gatifloxacin, followed by levofloxacin, with ofloxacin and ciprofloxacin being the least active. As a class, these drugs are bactericidal and studies are underway to determine whether fluoroquinolones can be used to shorten treatment duration [78,79]. They are distributed widely throughout the body, including the intracellular space, which probably contributes to their efficacy [80]. Optimal dosing would require larger doses; but, with the exception of levofloxacin, large increases in conventional dosing may not be possible [50].
Studies in the mouse model have demonstrated that substituting moxifloxacin for isoniazid leads to more rapid sterilization and a stable cure [81,82]. These and other studies provided the basis for EBA studies that have further bolstered the contention that fluoroquinolones have the potential to substantially decrease treatment duration. In one study, investigators showed that 400 mg of moxifloxacin was similar to isoniazid 300 mg in early bactericidal activity, with a log10 decrease in cfu of 0.209 versus 0.273 [83]. Another study showed the relative parity in EBA across the three most active fluoroquinolones, dosed at gatifloxacin 400 mg, moxifloxacin 400 mg, and levofloxacin 1000 mg [84].
Several Phase II trials designed to determine whether the fluoroquinolones can shorten treatment duration have been completed. The first of these was the TB Trials Consortium Study 27, which showed that substitution of moxifloxacin for ethambutol in a standard four-drug regimen resulted in a higher rate of sputum conversion at 4 and 6 weeks, but not at 8 weeks [85]. A study with a similar design conducted in Brazil found that a higher rate of sputum conversion persisted through Week 8 of therapy [86]. A study by the OFLOTUB group evaluated the substitution of ethambutol with moxifloxacin 400 mg, ofloxacin 800 mg, or gatifloxacin 400 mg. The use of ofloxacin was not associated with any improvement in sputum colony counts, but the use of moxifloxacin and gatifloxacin was associated with faster reductions in sputum colony counts [85]. The consistently encouraging aspect of these studies is that the quinolones have been safe and well tolerated in the wide range of populations studied. Slightly discouraging, however, is that the results have been somewhat mixed, or modest at best, in terms of shortening treatment.
Perhaps the most intriguing potential use of the fluoroquinolones is their use in completely novel treatment combinations. In a recent study using the mouse model, investigators conducted an experiment evaluating novel combinations composed of TMC207, pyrazinamide, PA 824, moxifloxacin and rifapentine. TMC207 plus pyrazinamide plus either rifapentine or moxifloxacin were most effective, curing 100 and 67% of mice, respectively, in 2 months of treatment. Four months of the standard regimen did not cure any mice, whereas the combination of TMC207, PA 824 and moxifloxacin cured 50% [87]. Extending the reach of quinolones in the treatment of MDR-TB is also an intriguing area of inquiry. Investigators working in Bangladesh conducted an observational study over 10 years and evaluated the outcomes of 206 patients with laboratory-confirmed MDR-TB. Patients were assigned to one of six standardized treatment regimens. The most effective regimen substantially reduced the time of treatment from 18 – 24 months to a minimum of 9 months, with gatifloxacin as the cornerstone of therapy. A regimen of gatifloxacin, clofazamine, ethambutol, and pyrazinamide supplemented by prothionamide, kanamycin, and high-dose isoniazid during a 4-month intensive phase resulted in a relapse-free cure of 87.9% (95% CI, 82.7 – 91.6) [88].
The REMoxTB study is a multicenter international Phase III trial investigating two 4-month regimens: 2 months of rifampin, isoniazid, pyrazinamide, moxifloxacin followed by 2 months of rifampin, isoniazid, moxifloxacin, or 2 months of ethambutol, moxifloxacin, rifampin, and pyrazinamide followed by 2 months of rifampin and moxifloxacin (ClinicalTrials.gov identifier NCT00864383). Also open is a separate Phase II randomized, open-label trial of a rifapentine plus moxifloxacin-based regimen during the intensive phase of therapy (ClinicalTrials.gov identifier NCT00728507). The RIFAQUIN trial is being conducted by The International Consortium for Trials of Chemotherapeutic Agents in Tuberculosis. This study is a randomized controlled international multicenter clinical trial to evaluate high-dose rifapentine combined with moxifloxacin to treat pulmonary tuberculosis [53].
Despite the momentum and excellent safety, tolerance, and bioavailability of the fluoroquinolones, there are significant concerns regarding the widespread use of these agents as first-line therapy. The foremost of these concerns is the development of resistance. Resistance of M. tuberculosis to the fluoroquinolones is attributed to mutations in the gyrA and gyrB genes that encode for the gyrase enzyme [89,90,91], and exposure to fluoroquinolones among tuberculosis patients has been associated with the development of resistance in relatively short periods of time. One study showed that 13% of patients receiving fluoroquinolones for ≥ 10 days prior to their diagnosis of tuberculosis had TB strains that were resistant to the fluoroquinolones [92]. Even patients for whom documentation of previous exposure to fluoroquinolones is difficult have high rates of fluoroquinolones resistance, particularly among MDR-TB patients. A recent study from India showed that 15% of isolates from patients with susceptibility to first-line TB drugs were resistant to the fluoroquinolones, as well as almost 40% of isolates with MDR-TB [93]. These studies document the risk of resistance to the fluoroquinolones with current practice. However, the development of new agents such as PA 824 and TMC207, as well as the use of fluoroquinolones with the rifamycins as first-line therapy, will lead to new combination regimens. Drusano and colleagues sound a cautionary signal in a recent publication regarding pharmacokinetic mismatching of drugs when used in novel combinations. To maximize adherence, many programs and some clinical trials administer therapy 5 days per week rather than 7 days per week. Using an in vitro pharmacokinetic model, these investigators showed that when drugs with different half-lives such as moxifloxacin and rifampin were used in combination with a 5-day/week schedule, breakthrough resistance was observed – concluding that the mismatched half-life led to functional monotherapy during the sixth and seventh days of the week and hence the development of resistance [94].
Although as a class the fluoroquinolones are safe and relatively well tolerated, it must be kept in mind that not all of the fluoroquinolones are similar in their side-effect profile. Gatifloxacin has been associated with an increased risk of dysglycemia among elderly patients, which eventually led to its being removed from the US market [95]. Moxifloxacin has also been recently associated with significant adverse events. In 2008, Bayer submitted a ‘Dear Doctor’ letter in the US to warn physicians about severe hepatic and dermatologic adverse events which, though rare, required monitoring. Lastly, moxifloxacin increases the QTc interval to a small and probably insignificant degree, but it is uncertain what this effect may be when moxifloxacin is used in combination with new drugs such as TMC207.
7. Expert opinion
It has frequently been said that tuberculosis is a social disease with medical implications. Literally, tens of millions of people have died in the decades since effective chemotherapy for tuberculosis has been developed. Currently available treatments resulting cure rates of 95 – 98%, but these rates are achieved only in the context of the right social, economic, and political conditions to ensure that a prolonged complicated regimen is administered appropriately. In areas of the world were these conditions exist, namely economically advanced countries, tuberculosis rates have been declining despite the emergence of drug resistance as well as HIV infection within known populations. So what we do? How do we level the playing field to slow the inexorable climb of morbidity and mortality in high-incidence areas, mostly within the developing world?
For many, the answer to that question has been to develop new drugs. If treatment regimens were 4 or even 2 months in duration, rather than the current ≥ 6 months, there would be fewer opportunities to fail. Certainly, new drugs with fewer side effects and drug interactions would be a huge advantage over what is currently available, and would simplify therapy for many countries grappling with the TB epidemic. However, although no one would argue that new drugs are desperately needed in the battle against tuberculosis, it must be remembered that tuberculosis, in many ways, is first a social disease before a medical disease.
The cornerstone of tuberculosis care worldwide has been the use of directly observed therapy (DOT), which has played a critical role in reducing the incidence of drug resistance. Many national programs, primarily in the developed countries, credit the use of DOT with stemming the tide of their resurgence of tuberculosis in the mid 1980s. In many parts of the world, what is called ‘directly observed’ therapy is anything but. Because of limited resources and other social and political barriers, true observation of all doses taken by the patient is not the standard of care. Patients continue to receive medications delivered once a week or given by a family member, but not truly as directly observed therapy as has been studied and is the global standard of care. Although shortened regimens – as may be possible with new agents being developed – are certainly welcomed, if these medications are not properly administered and protected, drug resistance will develop just as easily as it has previously with conventional first-line therapy. Now more than ever – especially in light of the possibility of new TB drugs, and as TB combinations begin rolling out in high-incidence, low-resource areas – we need to ensure that DOT is truly directly observed. High-incidence countries that have low resources are many times capable, far beyond current expectations. It is time to end the subtle bigotry of reduced expectations and ensure that patients worldwide receive the same basic standard of care, including DOT.
That said, there certainly is a great deal of excitement regarding the future of tuberculosis therapy; and with good reason. For the first time since the advent of modern chemotherapy for tuberculosis, there are a whole host of new medications available that will revolutionize therapy. Also, it is clear that, especially in most countries besieged by the HIV epidemic, current treatments and resources are not sufficient to stem the tide of the TB epidemic. Further, the emergence of MDR- and XDR-TB in high-incidence areas virtually mandates new drugs to reduce the burden of disease. In the short term, the approaches that use modification of the dosing of rifampin as well as more widespread use of rifapentine will in a very practical way offer the possibility of reducing treatment duration for uncomplicated, non–HIV-infected patients. Of course, this will only be effective if one knows the strain being treated is susceptible to rifampin. Fortunately, despite the emergence of drug resistance throughout the world, the overwhelming majority of new cases of tuberculosis remain susceptible to rifampin.
New combination regimens – including PA 824 and TMC207 in combination with existing drugs – are very exciting, not only for their ability to shorten treatment regimens and pan-susceptible cases, but also because they can be used amongst drug-resistant strains. PA 824 is particularly exciting because of its lack of liver enzyme induction and the potential for its concurrent use with ART. The results of ongoing clinical trials should shed light on what the immediate impact of these medications may be within the next 3 – 5 years.
More widespread use of the fluoroquinolones is also encouraging; however, it does have significant drawbacks. The fluoroquinolones are the cornerstone of therapy for MDR disease, and this approach has been widely implemented and used in the most difficult cases. However, more widespread use of the fluoroquinolones in the treatment of pan-susceptible disease will probably result in further resistance to the quinolones among new cases, whether or not they have been previously treated. The drug interactions with new agents will need to be watched very carefully as new regimens developed in combination with moxifloxacin and other fluoroquinolones begin to be utilized more widely.
It must be remembered that although new medications and new combination regimens will revolutionize therapy and reduce suffering for millions of people, the elimination of tuberculosis will be possible only with the development of an effective vaccine. Reduction of poverty and economic development is correlated with declining tuberculosis rates, and in fact that is what is being seen in many parts of the world today. However, it will take more than that. An effective and safe vaccine truly holds the most promise of achieving that most elusive of goals, the elimination of tuberculosis. As we ponder the potential for such elimination, we must bear in mind that increased access to highly effective treatment regimens in the context of strong TB programs incorporating DOT should be the goal – not just new and exciting drugs. If we fail to ensure that therapy is administered appropriately, we will ultimately have little more at our disposal than what was offered to young Albert Camus nearly a century ago.
Article highlights.
Tuberculosis is a global plague and is arguably the most important infectious disease in history.
Over one-third of the world’s population either has evidence of previous TB exposure or is harboring latent TB infection waiting for the right biological circumstances to arise before developing active disease.
When compared to the treatment of other medical conditions including other infections, there has been relatively little change in TB therapy over the last 40 years in developed countries other than the widespread use of directly observed therapy.
There are substantial data from animal studies and some human studies to suggest that modifying dosing of current standard TB drugs, especially rifampin, may provide an opportunity to shorten existing regimens without losing effectiveness.
New combination regimens including PA 824 and TMC207 in combination with existing drugs are very exciting not only because of their ability to shorten treatment regimens in pan-susceptible cases, but because of their ability to be used among drug-resistant strains as well.
PA 824 is particularly exciting because of its lack of liver enzyme induction and the potential for its concurrent use with antiretroviral therapy.
Widespread use of the fluoroquinolones is encouraging; however, more widespread use of the fluoroquinolones in the treatment of pan-susceptible disease will probably result in further resistance to the quinolones among new cases whether or not they have been previously treated.
Now more than ever, especially in light of the possibility of new TB drugs and combinations rolling out in high-incidence, low-resource areas, we need to ensure that directly observed therapy (DOT) really is directly observed.
Acknowledgements
The authors would like to thank JD Hosford, MPH for her exceptionally helpful assistance in preparing this manuscript.
Declaration of interest
This work supported in part by the NIH/NCRR Clinical and Translational Science Award to the University of Florida (UL1 RR029890). C Peloquin has received financial support from CDC, NIH and Jacobus Pharmaceuticals, and is a consultant for Otsuka. M Lauzardo has received financial support from CDC.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Camus A. Modern Library. New York, NY: 1948. The Plague, Trans. Stuart Gilbert. [Google Scholar]
- 2.Manosuthi W, Sungkanuparph S, Tantanathip P, et al. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R Study. Clin Infect Dis. 2009;48(12):1752–1759. doi: 10.1086/599114. [DOI] [PubMed] [Google Scholar]
- 3.La Porte CJL, Colbers EPH, Bertz R, et al. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004;48:1553–1560. doi: 10.1128/AAC.48.5.1553-1560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl 1):S63–S75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 5. Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375(9729):1906–1919. doi: 10.1016/S0140-6736(10)60409-6. •• This excellent review of the impact of HIV on the TB epidemic was written by a group of clinicians and investigators with unsurpassed experience.
- 6.Combs DL, O’Brien RJ, Geiter LJ. USPHS tuberculosis short-course chemotherapy trial 21: effectiveness, toxicity, and acceptability. Ann Intern Med. 1990;112(6):397–406. doi: 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 7.Cohn DL, Catlin BJ, Peterson KL, et al. A 62-Dose 6-Month therapy for pulmonary and extrapulmonary tuberculosis. Ann Intern Med. 1990;112(6):407–415. doi: 10.7326/0003-4819-76-3-112-6-407. [DOI] [PubMed] [Google Scholar]
- 8.Wells C. Global Impact of multidrug-resistant pulmonary tuberculosis among HIV-infected and other immunocompromised hosts: epidemiology, diagnosis, and strategies for management. Curr Infect Dis Rep. 2010;12(3):192–197. doi: 10.1007/s11908-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 9.Tam CM, Chan SL, Lam CW, et al. Rifapentine and isoniazid in the continuation phase of treating pulmonary tuberculosis. Initial report. Am J Respir Crit Care. 1998;157(6):1726. doi: 10.1164/ajrccm.157.6.9707037. [DOI] [PubMed] [Google Scholar]
- 10. Vernon A, Burman W, Benator D, et al. Acquired rifamycin monoresistance in patients with HIV-related tuberculosis treated with once-weekly rifapentine and isoniazid. Lancet. 1999;353(9167):1843–1847. doi: 10.1016/s0140-6736(98)11467-8. • A landmark paper that points out the risks and potential pitfalls of highly intermittent therapy.
- 11.Keshavjee S, Farmer PE. Time to put boots on the ground: making universal access to MDR-TB treatment a reality [Editorial] Int J Tuberc Lung Dis. 2010;14(10):1222–1225. [PubMed] [Google Scholar]
- 12.Gardner CA, Acharya T, Pablos-Mendez A. The Global alliance for tuberculosis drug development–accomplishments and future directions. Clin Chest Med. 2005;26(2):341–347. doi: 10.1016/j.ccm.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 13. Kaufmann SHE. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2010;33(4):567–577. doi: 10.1016/j.immuni.2010.09.015. • An excellent review of the strategies employed in developing vaccines for tuberculosis.
- 14.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3(8):e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telenti A, Imboden P, Marchesi F, et al. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341(8846):647–651. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson JM, Mitchison DA. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am Rev Respir Dis. 1981;123(4 Pt 1):367. doi: 10.1164/arrd.1981.123.4.367. [DOI] [PubMed] [Google Scholar]
- 17.Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first.14 days. Am J Respir Crit Care. 2003;167(10):1348. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 18.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units 1946 – 1986, with relevant subsequent publications. Int J Tuberc Lung Dis. 1999;3(10s2):S231–S279. [PubMed] [Google Scholar]
- 19.Verbist L, Gyselen A. Antituberculous activity of rifampin in vitro and in vivo and the concentrations attained in human blood. Am Rev Respir Dis. 1968;98(6):923. doi: 10.1164/arrd.1968.98.6.923. [DOI] [PubMed] [Google Scholar]
- 20.Diacon AH, Patientia RF, Venter A, et al. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob agents Chemother. 2007;51(8):2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decroix G, Kreis B, Sors C, et al. Comparison between regimes of rifampicin-isoniazid administered daily and administered twice a week (initial results of a comparative study conducted in 4 medical services of the Parisian region) Rev Tuberc Pneumol. 1969;33(6):751. [PubMed] [Google Scholar]
- 22.Kreis B, Pretet S, Birenbaum J, et al. Two three-month treatment regimens for pulmonary tuberculosis. Bull Int Union Tuberc Lung Dis. 1976;51(1):71. [PubMed] [Google Scholar]
- 23.Goutelle S, Bourguignon L, Maire PH, et al. Population modeling and monte carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob Agents Chemother. 2009;53(7):2974. doi: 10.1128/AAC.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steingart KR, Jotblad S, Robsky K, et al. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review [Review article] Int J Tuberc Lung Dis. 2011;15(3):305–316. [PubMed] [Google Scholar]
- 25.Boeree J. Global clinical trials for the treatment of TB with thioridazine. Recent Patent Antiinfect Drug Discov. 2011;6(2):99–103. doi: 10.2174/157489111796064533. [DOI] [PubMed] [Google Scholar]
- 26.Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet. 2001;40(5):327–341. doi: 10.2165/00003088-200140050-00002. [DOI] [PubMed] [Google Scholar]
- 27. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for treatment of latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. •• A recent but very influential paper that will change the way physicians and public health programs treat latent tuberculosis.
- 28.Skinner MH, Blaschke TF. Clinical pharmacokinetics of rifabutin. Clin Pharmacokinet. 1995;28(2):115–125. doi: 10.2165/00003088-199528020-00003. [DOI] [PubMed] [Google Scholar]
- 29.Sacchettini JC, Rubin EJ, Freundlich JS. Drugs versus bugs: in pursuit of the persistent predator mycobacterium tuberculosis. Nat Rev Microbiol. 2008;6(1):41–52. doi: 10.1038/nrmicro1816. [DOI] [PubMed] [Google Scholar]
- 30.Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of mycobacterium tuberculosis. Science. 2005;307(5707):223. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 31.Koul A, Dendouga N, Vergauwen K, et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat Chem Biol. 2007;3(6):323–324. doi: 10.1038/nchembio884. [DOI] [PubMed] [Google Scholar]
- 32.Lounis N, Gevers T, Van Den Berg J, Andries K. Impact of the interaction of R207910 with rifampin on the treatment of tuberculosis studied in the mouse model. Antimicrob Agents Chemother. 2008;52(10):3568. doi: 10.1128/AAC.00566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez OD, Palomo JB, Ortega MS, Ribas FM. Prudent use of antibiotics and suggestions for improvement from community and hospital pharmacy. Enferm Infecc Microbiol Clin. 2010;28(Suppl 4):36–39. doi: 10.1016/S0213-005X(10)70041-0. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim M, Andries K, Lounis N, et al. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother. 2007;51(3):1011. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nuermberger EL, Spigelman MK, Yew WW. Current development and future prospects in chemotherapy of tuberculosis. Respirology. 2010;15(5):764–778. doi: 10.1111/j.1440-1843.2010.01775.x. • An excellent review of new drugs for tuberculosis written by a leader in the field of new drug development.
- 36.Nuermberger E. Novel TMC207-containing regimens have sterilizing activity in murine tuberculosis. Presented at ICAAC; 17 – 20 September 2011; Chicago, IL. [Google Scholar]
- 37.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 38.Shi R, Sugawara I. Development of new anti-tuberculosis drug candidates. Tohoku J Exp Med. 2010;221(2):97–106. doi: 10.1620/tjem.221.97. [DOI] [PubMed] [Google Scholar]
- 39.Ashtekar DR, Costa-Perira R, Nagrajan K, et al. In vitro and in vivo activities of the nitroimidazole CGI 17341 against mycobacterium tuberculosis. Antimicrob Agents Chemother. 1993;37(2):183. doi: 10.1128/aac.37.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stover CK, Warrener P, VanDevanter DR, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405(6789):962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Manjunatha U, Boshoff HIM, et al. PA-824 kills nonreplicating mycobacterium tuberculosis by intracellular NO release. Science. 2008;322(5906):1392. doi: 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maroz A, Shinde SS, Franzblau SG, et al. Release of nitrite from the antitubercular nitroimidazole drug PA-824 and analogues upon one-electron reduction in protic, non-aqueous solvent. Org Biomol Chem. 2009;8(2):413–418. doi: 10.1039/b915877d. [DOI] [PubMed] [Google Scholar]
- 43.Lenaerts AJ, Gruppo V, Marietta KS, et al. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob Agents Chemother. 2005;49(6):2294. doi: 10.1128/AAC.49.6.2294-2301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyagi S, Nuermberger E, Yoshimatsu T, et al. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother. 2005;49(6):2289. doi: 10.1128/AAC.49.6.2289-2293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuermberger E, Rosenthal I, Tyagi S, et al. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother. 2006;50(8):2621. doi: 10.1128/AAC.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nuermberger E, Tyagi S, Tasneen R, et al. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin and pyrazinamide in the murine model of tuberculosis. Antimicrob Agents Chemother. 2008;524:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15(7):949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 48.Ginsberg AM, Laurenzi MW, Rouse DJ, et al. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob Agents Chemother. 2009;53(9):3720. doi: 10.1128/AAC.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Y, Coates ARM, Mitchison DA. Comparison of the sterilising activities of the nitroimidazopyran PA-824 and moxifloxacin against persisting Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2008;12(1):69–73. [PubMed] [Google Scholar]
- 50.Mitnick CD, McGee B, Peloquin CA. Tuberculosis pharmacotherapy: strategies to optimize patient care. Expert Opin Pharmacother. 2009;10:381–401. doi: 10.1517/14656560802694564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3(11):e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yew WW, Lange C, Leung CC. Treatment of tuberculosis: update 2010. Eur Respir J. 2011;37(2):441. doi: 10.1183/09031936.00033010. • Good review of current and future treatment options for tuberculosis.
- 53.Van Den Boogaard J, Kibiki GS, Kisanga ER, et al. New drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother. 2009;53(3):849. doi: 10.1128/AAC.00749-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurdle JG, Lee RB, Budha NR, et al. A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents. J Antimicrob Chemother. 2008 Nov;62(5):1037–1045. doi: 10.1093/jac/dkn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Protopopova M, Hanrahan C, Nikonenko B, et al. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1, 2-ethylenediamines. J Antimicrob Chemother. 2005;56(5):968. doi: 10.1093/jac/dki319. [DOI] [PubMed] [Google Scholar]
- 56.Spigelman M, Gillespie S. Tuberculosis drug development pipeline: progress and hope. Lancet. 2006;367(9514):945–947. doi: 10.1016/S0140-6736(06)68388-8. [DOI] [PubMed] [Google Scholar]
- 57.Chen P, Gearhart J, Protopopova M, et al. Synergistic interactions of SQ109, a new ethylene diamine, with front-line antitubercular drugs in vitro. J Antimicrob Chemother. 2006;58(2):332. doi: 10.1093/jac/dkl227. [DOI] [PubMed] [Google Scholar]
- 58.Jia L, Tomaszewski JE, Hanrahan C, et al. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine based antitubercular drug. Br J Pharmacol. 2005;144(1):80–87. doi: 10.1038/sj.bjp.0705984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia L, Coward L, Gorman GS, et al. Pharmacoproteomic effects of isoniazid, ethambutol, and N-geranyl-N -(2-adamantyl) ethane-1, 2-diamine (SQ109) on Mycobacterium tuberculosis H37Rv. J Pharmacol Exp Ther. 2005;315(2):905. doi: 10.1124/jpet.105.087817. [DOI] [PubMed] [Google Scholar]
- 60.Jia L, Tomaszewski JE, Noker PE, et al. Simultaneous estimation of pharmacokinetic properties in mice of three anti-tubercular ethambutol analogs obtained from combinatorial lead optimization. J Pharm Biomed Anal. 2005;37(4):793–799. doi: 10.1016/j.jpba.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 61.Nikonenko BV, Protopopova M, Samala R, et al. Drug therapy of experimental TB: improved outcome by combining SQ109, new diamine antibiotic, with existing TB drugs. Antimicrob Agents Chemother. 2007;51:1563–1565. doi: 10.1128/AAC.01326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reddy VM, Einck L, Andries K, Nacy CA. In vitro interactions between new antitubercular drug candidates SQ109 and TMC207. Antimicrob Agents Chemother. 2010;54(7):2840. doi: 10.1128/AAC.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Louw GE. Rifampicin reduces susceptibility to Ofloxacin in Rifampicin-resistant mycobacterium tuberculosis through efflux. Am J Respir Crit Care Med. 2011;184:269–276. doi: 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sequella Licenses Rights to Commercialize SQ109 for Tuberculosis in Russia to Maxwell Biotech Venture Fund Subsidiary. [Accessed January 2012]; Available from http://online.wsj.com/article/PR-CO-20110425-900011.html. [Google Scholar]
- 65.Rodriguez JC, Ruiz M, Lopez M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2002;20(6):464–467. doi: 10.1016/s0924-8579(02)00239-x. [DOI] [PubMed] [Google Scholar]
- 66.Fortun J, Martin-Davila P, Navas E, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005;56(1):180. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]
- 67.Ntziora F, Falagas ME. Linezolid for the treatment of patients with atypical mycobacterial infection: a systematic review [Review Article] Int J Tuberc Lung Dis. 2007;11(6):606–611. [PubMed] [Google Scholar]
- 68.Von der Lippe B, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB): a report of ten cases. J Infect. 2006;52(2):92–96. doi: 10.1016/j.jinf.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 69.French G. Safety and tolerability of linezolid. J Antimicrob Chemother. 2003;51(Suppl 2):ii45. doi: 10.1093/jac/dkg253. [DOI] [PubMed] [Google Scholar]
- 70.Nam H-S, Koh W-J, Kwon OJ, et al. Daily half-dose linezolid for the treatment of intractable multidrug-resistant tuberculosis. Int J Antimicrob Agents. 2009;33(1):92–93. doi: 10.1016/j.ijantimicag.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Park IN, Hong S-B, Oh Y-M. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother. 2006;58(3):701–704. doi: 10.1093/jac/dkl298. [DOI] [PubMed] [Google Scholar]
- 72.Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999;43(5):1189. doi: 10.1128/aac.43.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams KN, Stover CK, Zhu T, et al. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother. 2009;53(4):1314. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallis RS, Jakubiec WM, Kumar V, et al. Pharmacokinetics and whole-blood bactericidal activity against Mycobacterium tuberculosis of single doses of PNU-100480 in healthy volunteers. J Infect Dis. 2010;202(5):745. doi: 10.1086/655471. [DOI] [PubMed] [Google Scholar]
- 75.Alffenaar JWC, van der Laan T, Simons S, et al. Susceptibility of clinical mycobacterium tuberculosis isolates to a potentially less toxic derivate of linezolid, PNU-100480. Antimicrob Agents Chemother. 2011;55(3):1287. doi: 10.1128/AAC.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arora S. Eradication of mycobacterium tuberculosis infection in 2 months with LL-3858: a preclinical study. Int J Tuberc Lung Dis. 2004;8(11) Suppl 1:S29. [Google Scholar]
- 77.Shindikar AV, Viswanathan CL. Novel fluoroquinolones: design, synthesis, and in vivo activity in mice against Mycobacterium tuberculosis H37Rv. Bioorg Med Chem. 2005;15(7):1803–1806. doi: 10.1016/j.bmcl.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 78. Garcia-Rodriguez JA, Garcia AC. In-vitro activities of quinolones against mycobacteria. J Antimicrob Chemother. 1993;32(6):797. doi: 10.1093/jac/32.6.797. • Early paper showing the benefit of quinolones against mycobacteria.
- 79.Rastogi N, Goh KS. In vitro activity of the new difluorinated quinolone sparfloxacin (AT-4140) against Mycobacterium tuberculosis compared with activities of ofloxacin and ciprofloxacin. Antimicrob Agents Chemother. 1991;35(9):1933. doi: 10.1128/aac.35.9.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paramasivan CN, Sulochana S, Kubendiran G, et al. Bactericidal action of gatifloxacin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005;49(2):627–631. doi: 10.1128/AAC.49.2.627-631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004;169(3):421. doi: 10.1164/rccm.200310-1380OC. [DOI] [PubMed] [Google Scholar]
- 82.Nuermberger EL, Yoshimatsu T, Tyagi S, et al. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am J Respir Crit Care Med. 2004;170(10):1131–1134. doi: 10.1164/rccm.200407-885OC. [DOI] [PubMed] [Google Scholar]
- 83.Pletz MWR, De Roux A, Roth A, et al. Early bactericidal activity of moxifloxacin in treatment of pulmonary tuberculosis: a Prospective, Randomized Study. Antimicrob Agents Chemother. 2004;48(3):780–782. doi: 10.1128/AAC.48.3.780-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson JL, Hadad DJ, Boom WH, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;6(10):605–612. [PubMed] [Google Scholar]
- 85.Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first two months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174(3):331–338. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- 86.Conde MB, Efron A, Loredo C, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373(9670):1183–1189. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rustomjee R, Lienhardt C, Kanyok T, et al. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12(2):128–138. [PubMed] [Google Scholar]
- 88.Tasneen R, Li SY, Peloquin CA, et al. Sterilizing activity of novel TMC207-and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Van Deun A, Maug AKJ, Salim MAH, et al. Short, highly effective and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. • An important paper with a potential big impact on future treatment strategies in high-incidence, low-resource settings.
- 90.Aubry A, Veziris N, Cambau E, et al. Novel gyrase mutations in quinolone-resistant and hypersusceptible clinical isolates of mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob Agents Chemother. 2006;50(1):104. doi: 10.1128/AAC.50.1.104-112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veziris N, Martin C, Brossier F, et al. Treatment failure in a case of extensively drug-resistant tuberculosis associated with selection of a GyrB mutant causing fluoroquinolone resistance. Eur J Clin Microbiol Infect Dis. 2007;26(6):423–425. doi: 10.1007/s10096-007-0298-0. [DOI] [PubMed] [Google Scholar]
- 92.Devasia RA, Blackman A, Gebretsadik T, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis: the effect of duration and timing of fluoroquinolone exposure. Am J Respir Crit Care Med. 2009;180(4):365. doi: 10.1164/rccm.200901-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verma U, Sharma R, Gupta P, et al. New uses for old drugs: novel therapeutic options. Indian J Pharmacol. 2005;37(5):279. [Google Scholar]
- 94.Drusano GL, Sgambati N, Eichas A, et al. Effect of administration of moxifloxacin plus rifampin against mycobacterium tuberculosis for 7 of 7 days versus 5 of 7 days in an in vitro pharmacodynamic system. mBio. 2011;2(4):e00108–e00111. doi: 10.1128/mBio.00108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354(13):1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]