Abstract
Background
Little is known regarding the clinical features, procedural risks, or survival of patients receiving replacement versus new implantable cardioverter-defibrillators (ICDs).
Methods and Results
Entries in the National Cardiovascular Data Registry (NCDR®) ICD Registry™ from 2005 through 2010 were eligible for (N=463,978). Baseline demographic, clinical information, and procedural variables were compared between new (N = 359,993; 77.6%) and replacement (N = 103,985; 22.4%) ICD patients, and entered into a propsensity match model to determine adjusted survival rates. Replacement ICD patients were older (70.7 versus 67.5 years) and more likely to have atrial fibrillation (41.8% vs. 31.4%, P<0.001) and ventricular tachycardia (60.5% vs. 33.9%, P<0.001) compared with new ICD patients. Median battery life was only 4.6 years (25–75% IQR 3.7–5.8) for all replaced devices, 5.8 (25–75% IQR 4.2–7.5) for single-chamber, 5.1 (25–75% IQR 4.1–6.1) for dual-chamber, and 3.9 (25–75% IQR 3.2–4.6) years for biventricular devices. Replacement ICD patients had lower rates of index admission complications (0.9% vs 3.2%, P<0.001) but greater risk for death compared with new ICD patients in unadjusted analysis (HR 1.18, 95%CI 1.16 – 1.20, P<0.0001) and after propensity score matching (HR 1.28, 95% CI 1.25 to 1.30, P < 0.0001).
Conclusions
Patients receiving replacement ICDs are older and are at greater risk for death compared to those receiving initial ICD implants. The battery life of initial ICDs is shorter than previously reported.
Keywords: implantable cardioverter-defibrillators, death, sudden, defibrillation
Background
Approximately 28% of all implantable cardioverter-defibrillator (ICD) implantations are replacements of existing devices, accounting for nearly 30,000 ICD replacements/year in the United States alone.1 Yet little is known regarding the risks and benefits of ICD replacements, as these have been largely excluded from clinical trials and the focus of few observational studies. In the years since initial ICD placement, patients may have acquired additional comorbidities or experienced progression of their underlying heart disease, both of which may affect the impact of ICD therapy on clinical outcomes. The paucity of data describing the characteristics and outcomes of patients receiving ICD replacements is a barrier to risk stratification and prognostication, and explains, in part, the lack of clear indications for replacement in practice guidelines.
Previous risk models for procedural complications have included ICD replacements, but provided only limited comparisons to patients receiving initial implants and no assessment of long-term outcomes.2–4 Guidelines for ICD implantation do not recommend device therapy for patients with life expectancies of less than one year,5 but prospectively identifying these patients is difficult. Scoring systems have modeled survival following ICD implantation, but only focused on initial implantation.6–10 Thus, improved decision-making for this common clinical scenario requires better information regarding outcomes.11
The goals of this study were to describe and compare patients undergoing replacement and new ICD implantation with regard to (a) characteristics at the time of implantation; (b) risk and distribution of index admission complications; and (c) survival. We hypothesized that patients receiving replacement ICDs would be older, would have more accumulated comorbidities, and would have poorer survival following their implantation procedures as compared with patients receiving initial ICD implants.
Methods
Data Source
This study analyzed data from the National Cardiovascular Data Registry (NCDR®) ICD Registry™. This registry was created in 2005 after the Centers for Medicare and Medicaid Services (CMS) national coverage decision for primary prevention ICD implantation. The initial goal was to create a prospective, observational database that would include all Medicare beneficiaries receiving ICDs for primary prevention of sudden cardiac death. Although hospitals are not required to submit data for non-Medicare patients, >75% of the 1489 hospitals participating in the registry have entered data on all ICD implantations regardless of indication or insurance, and it is estimated that 90% of all ICD implants in the United States are captured by this dataset, with more than 10,000 cases entered monthly.1 All data entry was performed using the ICD Registry™ Data Collection From v1.08.12 Participating sites receive formal training on data collection and entry by the NCDR®. After submission, data are evaluated for quality and returned to sites if incomplete. Data from the ICD Registry™ have been used to address key clinical research questions in prior studies.13, 14
Study Population
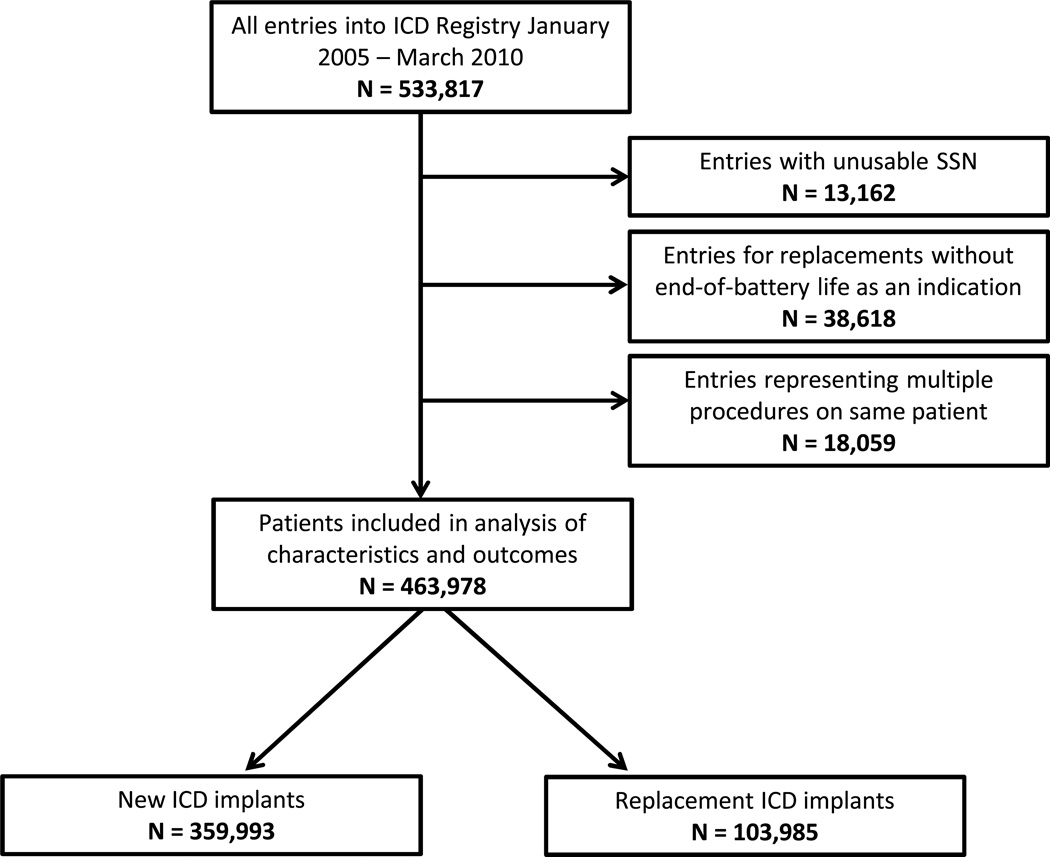
All patients receiving replacement or new ICDs between January 1, 2005 and March 30, 2010 were eligible for inclusion (Figure 1). Patients missing social security numbers, and those entered twice into the database (for initial and then replacement implantation) were excluded. As the primary focus of this study was comparing initial implantation with routine replacement, subjects who did not have “end of expected battery life” as one of the reasons for replacement were also excluded.
Figure 1.
All patients entered into the ICD Registry from January 2005 – March 2010 were eligible. Those without usable social security numbers and those with multiple entries into the database were also excluded. For those patients receiving replacement ICDs, those whose reason(s) for replacement did not include end of expected battery life were also excluded.
Variables
The ICD Registry™ collects over 130 standardized data elements describing demographic, clinical information and procedural information for each patient receiving an ICD implant. Patient files are linked to the Social Security Death Index to determine patient vital status, which was available up to 10/1/2011. For this report, variables were selected a priori from the ICD Registry™ that were felt to be necessary to describe and examine the characteristics and outcomes of patients receiving replacement ICD devices based on American College of Cardiology / American Heart Association guidelines for device based therapy5 as well as published literature regarding ICD outcomes (Table 1).6, 8
Table 1.
Baseline Characteristics of Replacement and New ICD Recipients
| Characteristic | Total n = 463978 |
Replacement ICD n = 103985 |
New ICD n = 359993 |
Absolute Standardized Difference (%) |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 68.2 ± 12.9 | 70.7 ± 12.4 | 67.5 ± 12.9 | 21.0 |
| Male | 340606 (73.4%) | 78644 (75.6%) | 261962 (72.8%) | 8.4 |
| Caucasian | 384396 (82.8%) | 91068 (87.6%) | 293328 (81.5%) | 15.3 |
| Hispanic | 22856 (4.9%) | 3786 (3.6%) | 19070 (5.3%) | 7.5 |
| Clinical History | ||||
| Any Ischemic Heart Disease | 302234 (65.1%) | 70542 (67.8%) | 231692 (64.4%) | 7.9 |
| Prior myocardial infarction | 246136 (53.0%) | 57929 (55.7%) | 188207 (52.3%) | 7.7 |
| Previous CABG | 161335 (34.8%) | 41324 (39.8%) | 120011 (33.3%) | 14.4 |
| Prior percutaneous coronary intervention | 150330 (32.4%) | 32636 (31.4%) | 117694 (32.7%) | 1.1 |
| Congestive Heart Failure | 355661 (76.7%) | 75804 (72.9%) | 279857 (77.8%) | 1.7 |
| Non-Ischemic Dilated Cardiomyopathy | 0.7 | |||
| No | 317748 (68.5%) | 75841 (73.0%) | 241907 (67.2%) | |
| Yes Within the past 3 months | 24983 (5.4%) | 955 (0.9%) | 24028 (6.7%) | |
| Yes 3 to 9 months | 20140 (4.3%) | 643 (0.6%) | 19497 (5.4%) | |
| Yes Greater than 9 months | 100877 (21.8%) | 26484 (25.5%) | 74393 (20.7%) | |
| NYHA Class | 16.0 | |||
| Class I | 65574 (14.2%) | 21679 (20.9%) | 43895 (12.2%) | |
| Class II | 171070 (36.9%) | 43376 (41.8%) | 127694 (35.5%) | |
| Class III | 209362 (45.2%) | 36505 (35.2%) | 172857 (48.1%) | |
| Class IV | 17315 (3.7%) | 2228 (2.1%) | 15087 (4.2%) | |
| Atrial Fibrillation | 156639 (33.8%) | 43502 (41.8%) | 113137 (31.4%) | 23.8 |
| Ventricular Tachycardia | 182901 (39.4%) | 60739 (60.5%) | 122162 (33.9%) | 50.6 |
| Abnormal Sinus Node Function | 125169 (27.0%) | 32959 (31.7%) | 92210 (25.6%) | 15.9 |
| Cerebrovascular Disease | 69256 (14.9%) | 16587 (16.0%) | 52669 (14.6%) | 3.6 |
| Chronic Lung Disease | 105656 (22.8%) | 22285 (21.4%) | 83371 (23.2%) | 1.8 |
| Diabetes | 170567 (36.8%) | 35492 (34.1%) | 135075 (37.5%) | 4.9 |
| Hypertension | 351661 (75.8%) | 75997 (73.1%) | 275664 (76.6%) | 6.8 |
| Renal Failure-Dialysis | 17797 (3.8%) | 2836 (2.7%) | 14961 (4.2%) | 5.6 |
| Diagnostic Studies | ||||
| Left ventricular ejection fraction % | 28.7 ± 11.6 | 32.6 ± 13.7 | 27.7 ± 10.8 | 25.3 |
| QRS Duration (ms) | 127.9 ± 35.2 | 137.6 ± 37.3 | 125.1 ± 34.0 | 41.2 |
| Atrioventricular Conduction | 47.0 | |||
| Normal | 193977 (41.8%) | 31448 (30.2%) | 162529 (45.1%) | |
| LBBB | 106736 (23.0%) | 14783 (14.2%) | 91953 (25.5%) | |
| RBBB | 39241 (8.5%) | 5931 (5.7%) | 33310 (9.3%) | |
| PACED | 69186 (14.9%) | 43493 (41.8%) | 25693 (7.1%) | |
| OTHER | 54838 (11.8%) | 8330 (8.0%) | 46508 (12.9%) | |
| Serum creatinine (mg/dL) | 1.374 ± 1.097 | 1.361 ± 0.905 | 1.378 ± 1.146 | 1.1 |
| Serum sodium (mEq/L) | 138.6 ± 3.5 | 138.9 ± 3.3 | 138.5 ± 3.5 | 8.6 |
| Systolic blood pressure (mmHg) | 130.5 ± 22.4 | 131.2 ± 22.4 | 130.4 ± 22.4 | 2.6 |
| ICD Procedure | ||||
| Reason for Admission | 57.5 | |||
| Admitted for this Procedure | 314909 (68.0%) | 96420 (92.8%) | 218489 (60.8%) | |
| Cardiac CHF | 48136 (10.4%) | 2204 (2.1%) | 45932 (12.8%) | |
| Cardiac Other | 87322 (18.8%) | 4131 (4.0%) | 83191 (23.1%) | |
| Noncardiac | 13036 (2.8%) | 1133 (1.1%) | 11903 (3.3%) | |
| ICD Indication | 37.3 | |||
| Primary Prevention | 365798 (78.8%) | 67655 (65.1%) | 298143 (82.8%) | |
| Secondary Prevention | 98180 (21.2%) | 36330 (34.9%) | 61850 (17.2%) | |
| ICD Type | 31.9 | |||
| Single Chamber | 100730 (21.7%) | 17609 (17.0%) | 83121 (23.1%) | |
| Dual Chamber | 189308 (40.9%) | 41522 (40.0%) | 147786 (41.1%) | |
| Biventricular | 173305 (37.4%) | 44704 (43.1%) | 128601 (35.8%) | |
| Discharge Medications | ||||
| ACE-Inhibitor | 275667 (60.5%) | 54620 (53.7%) | 221047 (62.5%) | 16.2 |
| Amiodarone | 62532 (13.7%) | 18675 (18.4%) | 43857 (12.4%) | 20.5 |
| ARB | 74563 (16.4%) | 18564 (18.3%) | 55999 (15.8%) | 6.0 |
| Aspirin | 304203 (66.8%) | 62592 (61.5%) | 241611 (68.3%) | 13.3 |
| Beta Blocker | 393938 (86.5%) | 85644 (84.2%) | 308294 (87.2%) | 6.5 |
| Coumadin | 131905 (29.0%) | 36368 (35.8%) | 95537 (27.0%) | 21.3 |
| Digoxin | 117963 (25.9%) | 33589 (33.0%) | 84374 (23.9%) | 21.8 |
| Diuretic | 289026 (63.5%) | 65096 (64.0%) | 223930 (63.3%) | 7.0 |
ICD = implantable cardioverter-defibrillator; CABG = coronary artery bypass grafting; CHF = congestive heart failure; LBBB = left bundle branch block; RBBB = right bundle branch block
Demographic variables included age, gender, and race (white vs. other). Clinical information included data from clinical history and diagnostic studies. History of the following cardiac conditions was collected: any ischemic heart disease, myocardial infarction, coronary artery bypass grafting, congestive heart failure, non-ischemic dilated cardiomyopathy (never, past 3 months, past 3–9 months, over 9 months), atrial fibrillation, ventricular tachycardia (any), and abnormal sinus node function. Functional status was rated using the New York Heart Association levels I-IV. Finally, the following comorbid conditions were ascertained: cerebrovascular disease, chronic lung disease, diabetes, hypertension, and renal failure or dialysis.
The most recent diagnostic findings included the left ventricular ejection fraction (%), QRS duration (ms), atrioventricular conduction problem (none, left bundle branch block (LBBB), right bundle branch block (LBBB), paced, or other), serum creatinine (mg/dl), serum sodium (mEq/L), and systolic blood pressure (mmHg). Use of the following cardiac medications at time of discharge was recorded: ACE-inhibitor, amiodarone, aspirin, beta blocker, coumadin, digoxin, and a diuretic.
Procedure characteristics captured in the ICD registry included identification of new versus replacement ICD procedure. If the device was a replacement, the time elapsed since prior device implant was recorded and whether or not the procedure also included upgrade to a dual-chamber or biventricular system was identified. The reason for hospitalization during which the device was placed was categorized as follows: ICD placement, congestive heart failure (CHF), cardiac but not for CHF, and non-cardiac. Type of device (single chamber, dual chamber, or biventricular) and whether the device was for primary or secondary prevention were also ascertained. (In the ICD Registry, primary prevention indicates that the patient is at risk for but has not yet had an episode of sustained ventricular tachycardia, ventricular fibrillation, or resuscitated cardiac arrest. At the time of ICD replacement, a patient whose device was originally placed for primary prevention but subsequently experienced any of these events would be coded as secondary prevention.) Index admission complications (date of implant through hospital discharge) included: cardiac arrest, drug reaction, cardiac perforation, coronary venous dissection, lead dislodgment, hemothorax or pneumothorax, transient ischemic attack or stroke, myocardial infarction, pericardial tamponade, and infection related to the device. Missing data was present <0.3% of the time for all data elements, and only complete case analysis was used for the propensity matching.
Statistical Analysis
All baseline demographic data, clinical information, and procedural variables were described using frequencies for categorical variables and means/medians with SDs/interquartile ranges for continuous variables. Given the size of the population, the comparison of patient and procedural characteristics between subjects who received replacement and initial ICDs were described using percent standardized mean differences (SMD). An absolute value of ≥10 was considered a meaningful difference.15
Among subjects who received replacement ICDs, the time (years) from original ICD insertion was calculated and presented overall and stratified by initial device type (single chamber, dual chamber, and biventricular).
Unadjusted survival analysis compared the survival after device placement between subjects who received replacement ICDs and those who received new ICDs using the Kaplan-Meier method and the log-rank test. Median survival was calculated for each group. Hazard ratios (HR) and 95% confidence intervals (CI) were generated from these analyses
To further evaluate the influence of patient characteristics on survival, propensity score methods were used. Though not intended to model randomization between having received a replacement versus new ICD, the propensity score approach was selected as an alternative method to adjust for potential differences between these groups in order to minimize parametric assumptions regarding the relationship between covariates and outcomes while balancing treatment groups on all measured covariates. A propensity score for each patient was generated using a logistic regression model predicting replacement (versus new) ICD based on the demographic, clinical, and procedural characteristics presented in Table 2. We then sought to match patients with replacement and initial device insertions by performing a 1:1 nearest neighbor match with a caliper width of 0.2 of the standard deviation of the logit of the propensity score.16 Absolute SMDs were used to determine success of the matching where values less than 10 and close to 0 indicate a good match.15, 17 We then assessed the association of a replacement ICD with survival in the matched data using conditional proportional hazard regression.18 Additionally, we evaluated survival for both new and replacement ICD patients in the propensity matched cohort when stratified according to device type (single-chamber, dual-chamber, or biventricular).
Table 2.
Details of propensity matching.*
| Characteristic | RICD n = 72905 |
NICD n =72905 |
Absolute Standardized Difference (%) |
|---|---|---|---|
| Demographics | |||
| Age (years) | 69.8 ± 12.6 | 69.9 ± 12.4 | 1.0 |
| Male | 54636 (74.9%) | 54906 (75.3%) | 0.9 |
| Caucasian | 63017 (86.4%) | 62934 (86.3%) | 0.3 |
| Hispanic Ethnicity | 2928 (4.0%) | 3003 (4.1%) | 0.5 |
| Clinical Factors | |||
| Any Ischemic Heart Disease | 48796 (66.9%) | 48784 (66.9%) | 0.0 |
| Prior myocardial infarction | 39940 (54.8%) | 39692 (54.4%) | 0.7 |
| Previous CABG | 27749 (38.1%) | 27893 (38.3%) | 0.4 |
| Prior percutaneous coronary intervention | 23358 (32.0%) | 23496 (32.2%) | 0.4 |
| Congestive Heart Failure | 53033 (72.7%) | 52973 (72.7%) | 0.2 |
| Non-Ischemic Dilated Cardiomyopathy | 9.4 | ||
| No | 52512 (72.1%) | 52558 (72.1%) | |
| Yes Within the past 3 months | 737 (1.0%) | 3622 (5.0%) | |
| Yes 3 to 9 months | 493 (0.7%) | 3222 (4.4%) | |
| Yes Greater than 9 months | 19129 (26.3%) | 13475 (18.5%) | |
| NYHA Class | 1.8 | ||
| Class I | 13816 (19.0%) | 13622 (18.7%) | |
| Class II | 29454 (40.4%) | 28904 (39.6%) | |
| Class III | 27904 (38.3%) | 28547 (39.2%) | |
| Class IV | 1731 (2.4%) | 1832 (2.5%) | |
| Atrial Fibrillation | 27554 (37.8%) | 28229 (38.7%) | 1.9 |
| Ventricular Tachycardia (VT) | 7.3 | ||
| No | 35691 (49.0%) | 34085 (46.8%) | |
| Yes VT, Non Sustained | 19591 (26.9%) | 27192 (37.3%) | |
| Yes Monomorphic Sustained VT | 14756 (20.3%) | 8999 (12.3%) | |
| Yes Polymorphic Sustained VT | 2811 (3.9%) | 2613 (3.6%) | |
| Sinus Node Function | 3.4 | ||
| Normal | 51246 (70.3%) | 50117 (68.7%) | |
| Abnormal | 21659 (29.7%) | 22788 (31.3%) | |
| Cerebrovascular Disease | 11160 (15.3%) | 11272 (15.5%) | 0.4 |
| Chronic Lung Disease | 15643 (21.5%) | 15833 (21.7%) | 0.6 |
| Diabetes | 25092 (34.4%) | 24955 (34.2%) | 0.4 |
| Hypertension | 53709 (73.7%) | 53960 (74.0%) | 0.8 |
| Renal Failure-Dialysis | 2163 (3.0%) | 2188 (3.0%) | 0.2 |
| Diagnostic Studies | |||
| Left ventricular ejection fraction % | 31.1 ± 12.3 | 31.1 ± 12.1 | 0.2 |
| QRS Duration (ms) | 133.2 ± 36.3 | 133.7 ± 38.5 | 1.3 |
| Atrioventricular Conduction | 0.8 | ||
| Normal | 27318 (37.5%) | 27292 (37.4%) | |
| LBBB | 13581 (18.6%) | 13179 (18.1%) | |
| RBBB | 5276 (7.2%) | 5348 (7.3%) | |
| PACED | 19398 (26.6%) | 19708 (27.0%) | |
| OTHER | 7332 (10.1%) | 7378 (10.1%) | |
| Serum creatinine (mg/dL) | 1.353 ± 0.887 | 1.360 ± 1.173 | 0.6 |
| Serum sodium (mEq/L) | 138.9 ± 3.3 | 138.8 ± 3.4 | 1.1 |
| Systolic blood pressure (mmHg) | 131.3 ± 22.5 | 131.1 ± 22.0 | 0.8 |
| ICD Procedure | |||
| Reason for Admission | 3.6 | ||
| Admitted for this Procedure | 65632 (90.0%) | 64672 (88.7%) | |
| Cardiac CHF | 2138 (2.9%) | 2523 (3.5%) | |
| Cardiac Other | 4054 (5.6%) | 4557 (6.3%) | |
| Noncardiac | 1081 (1.5%) | 1153 (1.6%) | |
| ICD Indication | 2.9 | ||
| Primary Prevention | 52629 (72.2%) | 53573 (73.5%) | |
| Secondary Prevention | 20276 (27.8%) | 19332 (26.5%) | |
| ICD Type | 1.7 | ||
| Single Chamber | 14108 (19.4%) | 13289 (18.2%) | |
| Dual Chamber | 29689 (40.7%) | 30419 (41.7%) | |
| Biventricular | 29108 (39.9%) | 29197 (40.0%) | |
| Discharge Medications | |||
| ACE-Inhibitor | 40457 (55.5%) | 43458 (59.6%) | 8.3 |
| Amiodarone | 12217 (16.8%) | 8999 (12.3%) | 12.5 |
| ARB | 12879 (17.7%) | 11968 (16.4%) | 3.3 |
| Aspirin | 45971 (63.1%) | 48615 (66.7%) | 7.6 |
| Beta Blocker | 61914 (84.9%) | 62368 (85.5%) | 1.8 |
| Coumadin | 23725 (32.5%) | 22104 (30.3%) | 4.8 |
| Digoxin | 22886 (31.4%) | 16033 (22.0%) | 21.4 |
| Diuretic | 46851 (64.3%) | 43740 (60.0%) | 8.8 |
Discharge medications were not matched.
This project was deemed exempt by the institutional review boards at Beth Israel Deaconess Medical Center and the Hebrew SeniorLife Institute for Aging Research.19
Results
Baseline Characteristics
Among 533,817 procedures entered into the ICD Registry™ during the study period, 463,978 unique patients were eligible for these analyses (Figure 1). In this sample, 22.4% (N = 103,985) of subjects received a replacement ICD and 77.6% received a new ICD (N = 359,993). Median follow-up times for the replacement and new ICD patients were 2.04 years (25–75% IQR 1.37–3.00) and 2.54 years (25–75% IQR 1.58–3.70), respectively.
Demographic, clinical and procedural characteristics of the overall cohort and stratified by replacement versus new ICD are presented in Table 1. In unadjusted analyses, subjects receiving replacement versus new ICDs were significantly older (median age 70.7 years versus 67.5; SMD 21.0%) and more frequently white (87.6% versus 81.5%, SMD 15.3%). With respect to cardiac history, a greater proportion of subjects receiving replacement versus new ICDs had atrial fibrillation (41.8% versus 31.4%, SMD 23.8%), ventricular tachycardia (60.5% versus 33.9%, SMD 50.7%), sinus node dysfunction 31.7% versus 25.6%, SMD 15.9%), and coronary artery bypass surgery (39.8% versus 33.3%, SMD 14.4%). Subjects getting ICD replacement had relatively better functional status as measured NYHA classifications. Other comorbid conditions including chronic obstructive lung disease, cerebrovascular disease, diabetes, and end-stage renal disease did not differ between the two groups.
In terms of baseline diagnostic studies, subjects who received replacement versus new ICDs had significantly higher left ventricular ejection fractions (32.6% versus 27.7%, SMD 25.3%), wider QRS duration, and were more likely to have paced rhythms. Finally, with respect to the procedural details, the vast majority (92.8%) of replacement patients were admitted to the hospital for the implant procedure specifically, compared with only 60.8% of initial ICD patients (SMD 57.5%). At the time of ICD replacement, 6540 (6.3%) of these patients also had an upgrade / lead addition, of which 4912 (75.2%) entailed upgrade to a biventricular system. Patients getting their ICDs replaced also more commonly received biventricular devices (43.1% versus 35.8%, SMD 31.9%) and were more like to have secondary prevention of sudden death recorded as the indication for the procedure (34.9% versus 17.2%, SMD 31.9%).
Adverse events during the index admission were relatively uncommon in both groups (Table 3), but were significantly more common among patients receiving new versus replacement ICDs (3.2% versus 0.9%, SMD 10.3%). The most common complication following ICD replacement was a hematoma (0.4%), whereas lead dislodgements was the most common complication among those receiving new ICDs (1.0%).
Table 3.
Complications of ICD implantation procedures for the overall cohort and recipients of replacement or new ICDs.
| Event | Total n = 463978 |
Replacement ICDS n = 103985 |
New ICDs n = 359993 |
Absolute Standardized Difference (%) |
|---|---|---|---|---|
| Any adverse event | 12453 (2.684%) | 945 (0.909%) | 11508 (3.197%) | 10.3 |
| Cardiac arrest | 1225 (0.264%) | 101 (0.097%) | 1124 (0.312%) | 2.0 |
| Drug reaction | 365 (0.079%) | 50 (0.048%) | 315 (0.088%) | 0.8 |
| Cardiac perforation | 289 (0.062%) | 10 (0.010%) | 279 (0.078%) | 2.1 |
| Coronary venous dissection | 511 (0.110%) | 32 (0.031%) | 479 (0.133%) | 2.4 |
| Hematoma | 3554 (0.766%) | 368 (0.354%) | 3186 (0.885%) | 4.3 |
| Lead Dislodgement | 3959 (0.853%) | 197 (0.189%) | 3762 (1.045%) | 7.5 |
| Hemothorax or Pneumothorax | 2081 (0.449%) | 138 (0.133%) | 1943 (0.540%) | 4.8 |
| Transient ischemic attack or stroke | 310 (0.067%) | 25 (0.024%) | 285 (0.079%) | 1.4 |
| Myocardial infarction | 107 (0.023%) | 4 (0.004%) | 103 (0.029%) | 1.2 |
| Pericardial tamponade | 349 (0.075%) | 15 (0.014%) | 334 (0.093%) | 2.4 |
| Infection Related to Device | 100 (0.022%) | 10 (0.010%) | 90 (0.025%) | 0.1 |
Continuous variables compared using Student's T-test.
Categorical variables compared using χ2 or Fisher's exact test.
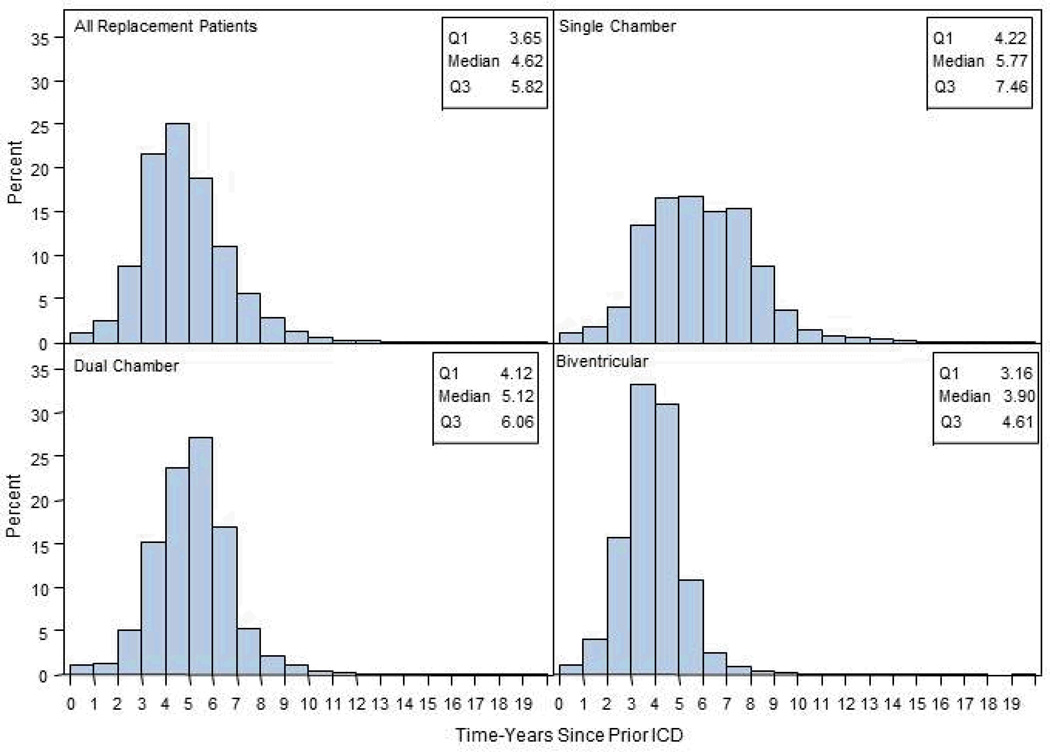
The median time to replacement was only 4.6 years (25–75% IQR 3.7–5.8) for all replaced devices, 5.8 (25–75% IQR 4.2–7.5) for single-chamber, 5.1 (25–75% IQR 4.1–6.1) for dual-chamber, and 3.9 (25–75% IQR 3.2–4.6) years for biventricular devices. (Figure 2).
Figure 2.
Distribution of time (in years) from initial implant for patients receiving replacement ICDs, divided by original device type (single-chamber, dual-chamber, or biventricular ICD).
Survival
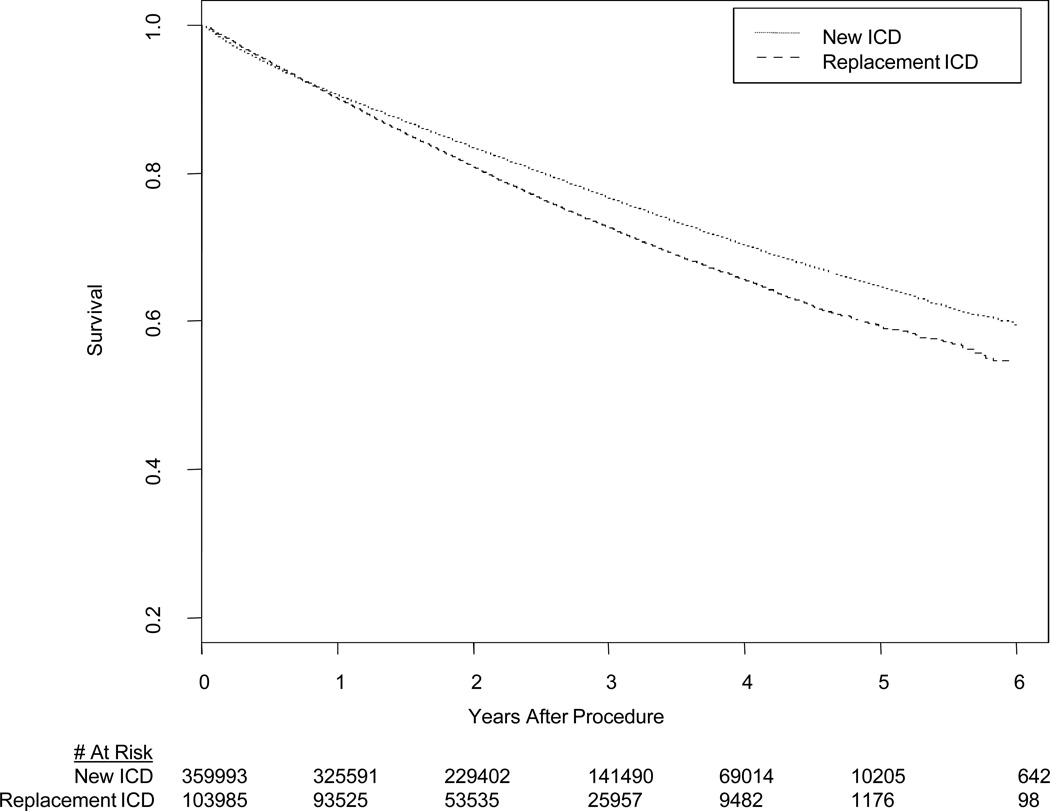
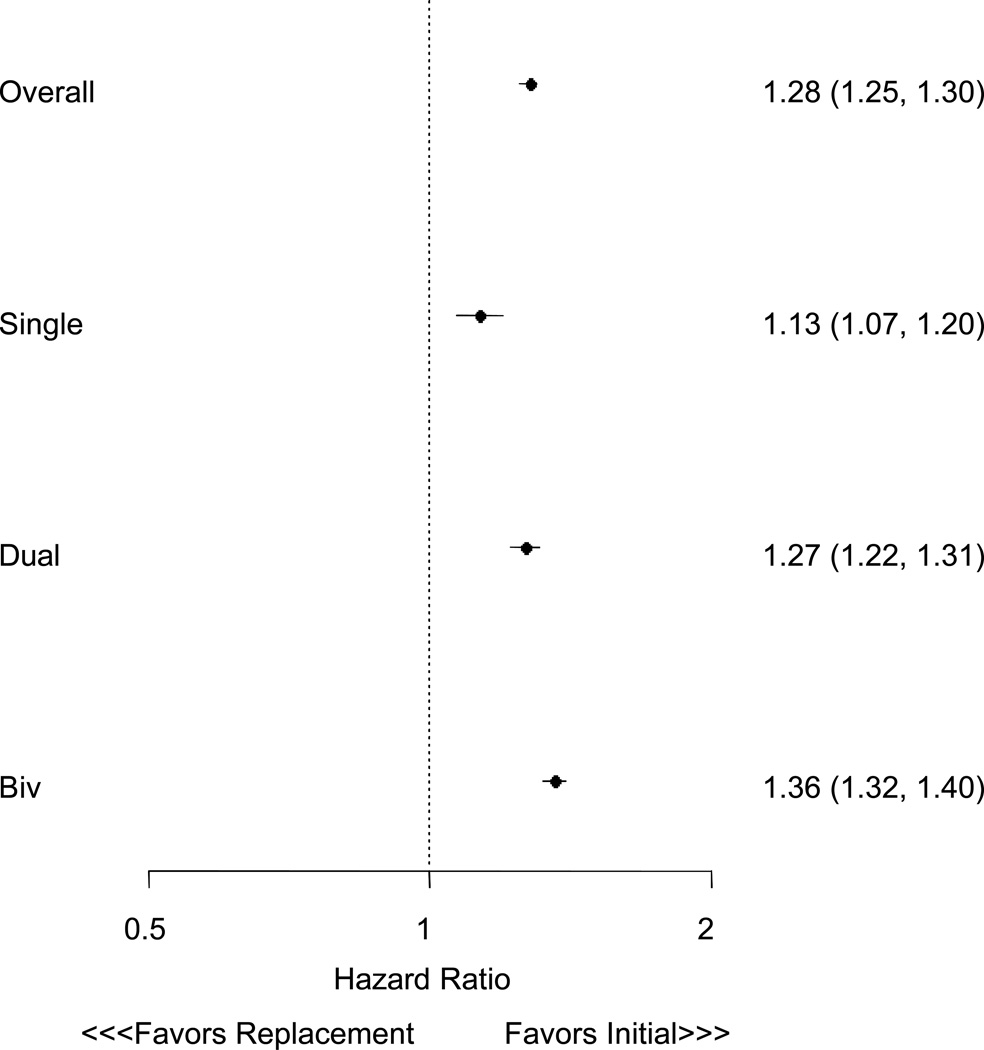
The median survival of patients receiving replacement ICDs was 2.0 years (25–75% IQR 1.6–3.7), compared with 2.5 years (25–75% IQR 1.6–3.7) for those receiving new ICDs. Mortality at 1 year for those receiving replacement ICDs was 9.9% versus 9.4% following new ICD implantation). At 3 years, mortality for replacement ICD patients was 27.4% compared with 23.5% for those receiving new ICDs. The unadjusted HR comparing survival among patients getting replacement versus new ICDs was 1.18 (95% CI 1.16–1.19, P < 0.0001, Figure 3). Propensity score matching successfully paired 72,905 new and replacement patients, with all variables in the propensity score having a SMD <10% (Table 2). Survival after ICD replacement versus initial implant remained worse in the propensity match analysis ((HR of 1.28, 95% CI 1.25 to 1.30, P < 0.0001). Hazard ratios for survival for both new and replacement ICD patients stratified by device type are presented in Figure 4. Overall, these illustrate differences in survival between these three groups, with greatest risk for death in patients with biventricular ICDs, and a consistent hazard for patients after ICD replacement versus initial implant.
Figure 3.
Unadjusted KM curve for survival for patients receiving new ICDs (solid line) or replacement ICDs (dotted line).
Figure 4.
Hazard ratios for survival for propensity matched patients receiving new and replacement ICDs stratified by device type.
Discussion
To our knowledge, this is the largest and most complete description of replacement ICD patients and their outcomes including long-term survival, and the most substantive direct comparison of replacement and new ICD patients. We found that patients getting replacement ICDs are older and have greater history of arrhythmias compared to those getting new devices. While index admission complication rates were lower among patients undergoing device replacement, survival post-procedure was worse. After propensity matching, replacement ICD patients remained at greater risk for death compared with new ICD patients (HR 1.28, 95% CI 1.25 to 1.30, P < 0.0001).). In addition, we found that the mean battery life of new ICDs was less than 5 years; a duration much shorter than previously reported.20, 21 Taken together, these data highlight differences in clinical features and outcomes for new and replacement ICD recipients, reinforce the disconnect between patient and device longevity, and provide the context for further prospective studies evaluating the clinical benefits of ICD replacement.
Our results build on prior attempts to characterize the clinical course of patients following ICD implantation. Haines et al used ICD Registry™ data from 2006 through 2008 with a focus only on index admission complications including death.2 They reported an overall complication rate of 3.05%, and developed a risk score that stratified this risk from <1.0% to ≥17%. They did not, however, report outcomes for routine ICD replacement specifically and did not characterize survival after the index admission. Poole et al reported outcomes up to 6 months for 871 ICD replacements in the REPLACE registry of both ICD and pacemaker patients, with the majority (96.7%) of procedures performed for battery depletion.4 In agreement with our data, they found only a <1% risk of periprocedural events (defined as < 24 hours). Extending their follow-up to 6 months, however, identified a 4.0% risk of major and 7.4% risk of minor complications, consistent with retrospective studies focusing on replacements performed for device malfunction and recalls.22, 23 REPLACE did not report mortality for the ICD patients alone, and only 8 deaths for both pacemaker and ICD patients were reported within six months (half due to attempted surgical placement of an LV lead). Similarly, Krahn et al evaluated 1081 patients undergoing ICD replacement as part of the Ontario ICD Registry and found an 4.3% rate of complications at 45 days. Krahn et al also noted that replacement ICD patients were older (by a mean of 1.8 years) and had improved LV function compared to new ICD recipients.3 However, additional characteristics and survival between these groups was not described. In sum, this suggests that our findings of a <1% risk for procedural complications for replacement ICD patients would likely grow (perhaps as much as four-fold) if longer-term follow-up were available to document these intermediate or late events, such as infections or more gradual hematomas
Our findings of median battery life for single-chamber, dual-chamber, and biventricular ICDs prior to replacement contrast with prior reports, particularly for biventricular devices. Thijssen et al reported a mean battery life of 5.5 ± 0.1 years for a relatively large group of 1072 ICDs replaced due to battery depletion.20 Biventricular devices in this cohort (N = 373) had a mean battery life of 4.7 ± 0.1 years, compared with our finding of a median of 3.90 and mean of 3.92 for these ICDs. Our much larger study therefore further strengthens advocacy to improve the longevity of these devices, particularly for patients receiving biventricular systems.21 Cost-effectiveness analyses of ICDs have demonstrated exquisite sensitivity to battery life, with replacement after 3 years rather than 5 years increasing the cost per quality-adjusted life year by tens of thousands of dollars.24 Similar analysis of CRT are even more sensitive to battery life.25
Our survival data for replacement ICD patients and direct comparison to new ICD patients further clarifies the picture of post-replacement clinical experiences. Seminal clinical trials have described annual mortality rates of patients with ICDs ranging from 5% (in SCD-HeFT)26 to 8.5% (MADIT-II)27 to 12% (COMPANION)28, with variable absolute reductions in mortality with device-based therapy. Our study does not have a control group of non-ICD patients for comparison, and so we are unable to evaluate the mortality advantage ICD replacement provides compared with a non-replacement strategy. Similarly, these data are not intended to identify a causal relationship between ICD replacement and an increased risk for death. Nevertheless, the comparatively higher mortality rate in the replacement ICD patients compared with new ICD patients – even after propensity matching for age and other covariates – suggests that directly extrapolating the benefits from these clinical trials to replacement ICD patients may not be straightforward. Again, though our analysis does not compare ICD replacement with a non-replacement strategy (either abandonment of the device or replacement of the ICD with a pacemaker generator), and thus cannot directly address the risks and benefits of replacement versus non-replacement. Yet these data do reinforce calls for clinical trials specifically evaluating this very common clinical decision.11
In addition, our results raise questions about what contributes to the excess hazard for replacement ICD patients. Late complications such as infections were not captured by this database, and residual confounding from unmeasured variables (such as more precise estimations of heart failure severity) may in part explain these findings. Patients receiving ICD replacements are necessarily farther along their disease course, and this may influence their survival and susceptibility to sudden arrhythmic death in particular. Our determination of vital status does not include cause of death, so it remains uncertain whether the excess mortality arises from progression of cardiovascular disease or non-cardiac causes such as malignancies. It is of interest that despite having more arrhythmia, replacement ICD patients had, on average, higher left ventricular ejection fractions and less severe heart failure. This may indicate that these patients were sicker when their devices were initially placed and recovered, that the devices themselves may have contributed to clinical improvement, indicate some selection bias on the part of operators avoiding replacements on specific patients, or represent a selected survivor bias.
Our study results should be interpreted within the context of several potential limitations. Though our study population was largely white and male, the ICD Registry™ itself is representative of the population receiving ICDs in the United States. Our analytic approach contrasted replacement ICD patients and new ICD patients whose implants of interest occurred during the same time period, meaning that the initial implants for replacement ICD patients occurred approximately 4 years earlier on average. Thus, it is possible that this difference in timing relative to publication of pivotal clinical trials and updated guidelines may have contributed in unmeasured ways to differences between the patient groups. For example, significantly more patients in the replacement ICD group were characterized as secondary prevention, but from these data we cannot determine whether or not these patients were survivors of qualifying events prior to their first ICD procedure, or if these events occurred after an initial primary prevention device was placed. Lastly, it is possible that some patients may have been eligible for ICD replacement during the study period but declined (due to comorbidity or for other reasons). However, this would tend to bias the replacement ICD group towards healthier patients, further strengthening the observation that survival following replacement is worse than following initial implantation.
In conclusion, the paucity of data on the features and clinical course of patients following ICD replacement poses significant challenges for developing clinical guidelines or promoting informed decision-making surrounding these procedures.11 Patients undergoing ICD replacement differ from those receiving initial ICD implants in several important ways, and require new generators due to declining battery life more quickly than previously reported. Following ICD replacement, patients are at an elevated risk for death compared to those receiving new ICDs. These estimates may provide context for patient and clinician expectations surrounding ICD implantation and replacement, but clinical trials are necessary to rigorously evaluate the clinical benefits of ICD replacement.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Kramer is the Lois Green Scholar at the Hebrew SeniorLife Institute for Aging Research, and is additionally supported by an award from the John A. Hartford Foundation and a career development award from the Harvard Clinical and Translational Science Center. Dr. Mitchell is supported by NIH-NIA K24AG033640.
Conflict of Interest Disclosures: This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. ICD Registry™ is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society.
The authors report the following additional disclosures:
JAS: Reports a contract from the American College of Cardiology Foundation to provide analytic support for the National Cardiovascular Data Registry.
AEB: St. Jude Medical - honoraria for serving on DSMB for a clinical trial (unrelated to ICDs); Medtronic - honoraria for speaking at educational symposia and serving on Events committee for a clinical trial (not related to ICDs); Boston Scientific - honoraria for serving on Events Committee for a clinical trial (not related to ICDs); GE Healthcare - consultant (for imaging, nothing related to ICDs)
MEJ: Medtronic -- honoraria for educational courses and small advisory input;Biotronik honoraria for educational courses
MRR: Medtronic – consulting contract
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DBK, KFK, PAN, SLN, PJZ, SLM: none
References
- 1.Hammill SC, Kremers MS, Stevenson LW, Heidenreich PA, Lang CM, Curtis JP, Wang Y, Berul CI, Kadish AH, Al-Khatib SM, Pina IL, Walsh MN, Mirro MJ, Lindsay BD, Reynolds MR, Pontzer K, Blum L, Masoudi F, Rumsfeld J, Brindis RG. Review of the registry's fourth year, incorporating lead data and pediatric icd procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–1345. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Haines DE, Wang Y, Curtis J. Implantable cardioverter-defibrillator registry risk score models for acute procedural complications or death after implantable cardioverter-defibrillator implantation. Circulation. 2011;123:2069–2076. doi: 10.1161/CIRCULATIONAHA.110.959676. [DOI] [PubMed] [Google Scholar]
- 3.Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV. Predictors of short-term complications after implantable cardioverter-defibrillator replacement: Results from the ontario icd database. Circ Arrhythm Electrophysiol. 2011;4:136–142. doi: 10.1161/CIRCEP.110.959791. [DOI] [PubMed] [Google Scholar]
- 4.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: Results from the replace registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. Acc/aha/hrs 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the acc/aha/naspe 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): Developed in collaboration with the american association for thoracic surgery and society of thoracic surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole-Wilson PA, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: Lessons from the mustt study. J Am Coll Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 9.Kramer DB, Friedman PA, Kallinen LM, Morrison TB, Crusan DJ, Hodge DO, Reynolds MR, Hauser RG. Development and validation of a risk score to predict early mortality in recipients of implantable cardioverter-defibrillators. Heart Rhythm. 2012;9:42–46. doi: 10.1016/j.hrthm.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Parkash R, Stevenson WG, Epstein LM, Maisel WH. Predicting early mortality after implantable defibrillator implantation: A clinical risk score for optimal patient selection. Am Heart J. 2006;151:397–403. doi: 10.1016/j.ahj.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Kramer DB, Buxton AE, Zimetbaum PJ. Time for a change--a new approach to icd replacement. N Engl J Med. 2012;366:291–293. doi: 10.1056/NEJMp1111467. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed uly 31, 2012];National cardiovascular data registry - icd registry website. Available at https://www.Ncdr.Com/webncdr/icd/default_ssl.Aspx.
- 13.Al-Khatib SM, Hellkamp A, Curtis J, Mark D, Peterson E, Sanders GD, Heidenreich PA, Hernandez AF, Curtis LH, Hammill S. Non-evidence-based icd implantations in the united states. JAMA. 2011;305:43–49. doi: 10.1001/jama.2010.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fein AS, Wang Y, Curtis JP, Masoudi FA, Varosy PD, Reynolds MR. Prevalence and predictors of off-label use of cardiac resynchronization therapy in patients enrolled in the national cardiovascular data registry implantable cardiac-defibrillator registry. J Am Coll Cardiol. 2010;56:766–773. doi: 10.1016/j.jacc.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Normand SL. Some old and some new statistical tools for outcomes research. Circulation. 2008;118:872–884. doi: 10.1161/CIRCULATIONAHA.108.766907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Assessing balance in measured baseline covariates when using many-to-one matching on the propensity-score. Pharmacoepidemiol Drug Saf. 2008;17:1218–1225. doi: 10.1002/pds.1674. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 19.United states department of health and human services. [Accessed may 13, 2013];Code of federal regulations 45cfr46.102(f) Available at: Http://www.Hhs.Gov/ohrp/humansubjects/guidance/45cfr46.Html#46.102.
- 20.Thijssen J, Borleffs CJ, van Rees JB, Man S, de Bie MK, Venlet J, van der Velde ET, van Erven L, Schalij MJ. Implantable cardioverter-defibrillator longevity under clinical circumstances: An analysis according to device type, generation, and manufacturer. Heart Rhythm. 2012;9:513–519. doi: 10.1016/j.hrthm.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Hauser RG. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol. 2005;45:2022–2025. doi: 10.1016/j.jacc.2005.02.077. [DOI] [PubMed] [Google Scholar]
- 22.Gould PA, Krahn AD. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006;295:1907–1911. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- 23.Costea A, Rardon DP, Padanilam BJ, Fogel RI, Prystowsky EN. Complications associated with generator replacement in response to device advisories. J Cardiovasc Electrophysiol. 2008;19:266–269. doi: 10.1111/j.1540-8167.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 25.Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, Taylor RS. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: Systematic review and economic model. Health Technol Assess. 2007;11:iii–iv. ix–248. doi: 10.3310/hta11470. [DOI] [PubMed] [Google Scholar]
- 26.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 27.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 28.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.