Abstract
The co-ordination of T-cell motility, adhesion and activation remains poorly understood. It is also unclear how these functions are co-ordinated with external stimuli. Here we unveil a series of molecular interactions in cis at the surface of T lymphocytes with potent effects on motility and adhesion in these cells, and communicating with proliferative responses. These interactions were controlled by the signature cytokines of T helper subsets interleukin-2 (IL-2) and IL-4. Low-density lipoprotein receptor-related protein 1 (LRP1) was found to play a key role for T-cell motility by promoting development of polarized cell shape and cell movement. Endogenous thrombospondin-1 (TSP-1) enhanced cell surface expression of LRP1 through CD47. Cell surface expressed LRP1 induced motility and processing of TSP-1 while inhibiting adhesion to intercellular adhesion molecule 1 and fibronectin. Interleukin-2, but not IL-4, stimulated synthesis of TSP-1 and motility through TSP-1 and LRP1. Stimulation of the T-cell receptor (TCR)/CD3 complex inhibited TSP-1 expression. Inhibitor studies indicated that LRP1 regulated TSP-1 expression and promoted motility through JAK signalling. This LRP1-mediated motogenic signalling was connected to CD47/Gi protein signalling and IL-2-induced signalling through TSP-1. The motogenic TSP-1/LRP1 mechanism antagonized TCR/CD3-induced T-cell proliferation. These results indicate that LRP1 in collaboration with TSP-1 directs a counter-adhesive and counter-proliferative motogenic cascade. T cells seem programmed to prioritize movement before adhesion through this cascade. In conclusion, vital decision-making in T lymphocytes regulating motility, adhesive interactions and proliferation, are integrated through a molecular mechanism connecting different cell surface receptors and their signalling pathways.
Keywords: lipoprotein receptor-related protein 1, lymphocytes, MHC, migration, thrombospondin-1
Introduction
T lymphocytes play a pivotal role for the adaptive immune system through their uniquely high motility, which enables them to recognize foreign antigens throughout the organism and initiate specific responses while maintaining tolerance to self-antigens and allergens. T cells exhibit transient adhesive interactions with endothelial cells and antigen-presenting cells and therefore must co-ordinate motility with adhesion. The coordination of T-cell motility, T-cell adhesion and proliferation, and how these functions are influenced by external stimuli via adhesion molecules or cytokine receptors, remains poorly understood. The responsiveness of cells to external stimuli is generally assumed to reflect signalling from a preformed landscape of surface receptors to intracellular networks. We show here that this receptor landscape is much less pre-assembled than previously thought and part of a dynamic cell-intrinsic regulation of motility and adhesion. We therefore report a series of cytokine-controlled interactions between cell surface molecules in cis which are recruited to the cell surface to promote a motile response and regulate adhesion to intercellular adhesion molecule 1 (ICAM-1) and fibronectin. These interactions are differentially controlled by cytokines and counteract proliferative responses. The classical view of motogenic stimulation in T cells is that a chemokine induces migration via a Gi-mediated signalling pathway competing with stop signals delivered by T-cell receptor (TCR) engagement by antigen.1
Interleukin-2 (IL-2) is essential for the homeostasis and differentiation of CD4 T cells into T helper 1 (Th1), Th2, Th17 and regulatory T (Treg) cells.2 Interleukin-2 was originally considered as a growth factor for T cells. Subsequent research has elucidated that IL-2 is essential for down-regulation of immune responses through induction of T reg cells and also for maintenance of the active suppression.3–5 It therefore plays a pivotal role for the regulation of the adaptive immune system and maintenance of immune tolerance and contributes to suppression of autoimmunity6 and allergy and even induces acceptance of allografts.7 Interleukin-2 is also a potent stimulator of T-cell motility via IL-2 receptor β.8,9 Interleukin-4 has a crucial role for the differentiation of Th2 cells that are indispensable for immunity to extracellular parasites but inhibits Th1 cell differentiation.7 In contrast to the protective role of IL-2, IL-4 is coupled to adverse responses in the form of allergy and autoimmunity. The mechanisms by which IL-2 and IL-4 exert their actions are still poorly understood.
Although T cells migrate extensively throughout the organism and adhesive interactions play a pivotal role for T-cell function, the mechanisms regulating T-cell motility and adhesion remain unclear. T cells are therefore capable of high motility while down-regulating adhesion through an obscure mechanism.10 Thrombospondin-1 (TSP-1), a 450 000 molecular weight (MW) calcium-binding protein with binding sites for integrins, integrin-associated protein (CD47), CD36, low-density lipoprotein receptor-related protein 1 (LRP1) and calreticulin,11–16 has been implicated in the regulation of motility and adhesion in T cells.17,18 The LRP1 is a multifunctional 600 000 MW member of the LDL receptor family with a broad repertoire of ligand interactions including proteases, growth factors, and matrix proteins19,20 involved in the regulation of motility of non-lymphoid cells.21–23 Interestingly, LRP1 on T cells has been reported to predict unresponsiveness to anti-tumour necrosis factor therapy in patients with rheumatoid arthritis24 but its role for motility and other T-cell functions is unknown. CD47 is a membrane protein that cooperates with the TCR to induce T-cell activation25 but is also an inhibitory receptor that mediates inhibition of TCR-induced T-cell activation and promotes T-cell anergy and Treg cells.26–28 Calreticulin, a calcium-binding chaperone protein, is a co-receptor for LRP1.29
We examined the possible importance of LRP1 for T-cell motility and adhesion and also attempted to further clarify the role of TSP-1. Earlier studies of endogenous TSP-1 in the regulation of T-cell motility and adhesion were performed with T-cell blasts, did not include silencing experiments, or examine the influence of LRP1.18 However, understanding of basic motility probably requires the analysis of non-activated cells. The present experiments were performed with non-activated blood T cells from healthy individuals and a birch allergen-specific T-cell clone in type 1 collagen matrices. This is a well-established test system for analysis of lymphocyte motility.30–33 Adhesion was studied using surfaces coated with fibronectin and ICAM-1. Our results indicate that T-cell motility and adhesion are regulated by a molecular cascade comprising TSP-1, CD47, LRP1 and calreticulin controlled by IL-2 and IL-4 and antagonizing TCR/CD3-induced proliferative responses.
Materials and methods
Chemicals and antibodies
Human plasma fibronectin and rat tendon collagen type I were purified and prepared as described elsewhere.34,35 Poly-l-lysine (5300 MW) was purchased from Miles-Yeda Ltd (Rehovoth, Israel). Both IL-2 and IL-4 were from Genzyme Diagnostics (Cambridge, MA). Dynabeads were from Dynal Biotech (Oslo, Norway). ICAM-1 was from R&D Systems Ltd, Europe (Abingdon, UK). UO126 was from Promega (Madison, WI), wortmannin, pertussis toxin, and AG490 from Sigma-Aldrich (St Louis, MO) and GM6001 from Chemicon International (Temecula, CA). Cholera toxin was from List Biologic Laboratories (Campbell, CA). Receptor-associated protein (RAP) was obtained from Oxford Biomedical Research (Oxford, MI). Amiloride was from Calbiochem (Darmstadt, Germany). Anti-fibronectin (clone IST1, IgG1) was obtained from sera Lab (Loughborough, UK). Anti-CD3 (clone SK7, IgG1) and anti-CD4 (clone SK3, IgG1) were obtained from Becton Dickinson (Mountain View, CA). Mouse IgG, anti-CD8 (C8/144B, IgG1) and goat anti-mouse IgG were from Dacopatts A/S (Glostrup, Denmark). Anti-TSP-1 clone A6.1 (also called TSP-Ab-4, IgG1), clone C 6.7 (also called TSP-Ab-3, IgG1) and clone MBC 200.1 (also called TSP–Ab-9, IgG1) were from NEO-MARKERS (Fremont, CA). Anti-CD91/LRP1 (clone A2MRα2, IgG1) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CD18 (clone 7E4, IgG1) was obtained from Immunotech (Marseille, France). Anti-calreticulin (clone FMC75) was from Biosite (Täby, Sweden). Anti-CD47 (clone CIKm1) was from Chemicon International, and anti-CD47 (clone B6H12.2) was from NEO-MARKERS. Biotinylated peroxidase and avidin were from Vector Laboratories (Burlingame, CA). The birch antigen Bet v G75 was obtained from ALK (Hoersholm, Denmark). ELT GAA RKG SGR RLV KGP D (hepI) was synthesized by the Biomolecular Resource Facility (University of Lund, Sweden). RSK AGT LGE RDL KPG ARV G (scrambled hepI peptide), KRFYVVMWKK (4N1K) and KVFRWKYVMK (scrambled 4N1K) were synthesized by Tri pep (Novum Research Park, Huddinge, Sweden).
Cells
Blood lymphocytes were purified using Lymphoprep and depleted of monocytes by treatment with carbonyl iron and magnetic removal of phagocytic cells. The cell preparations obtained consisted of 82–93% CD3-positive cells. Further enrichment of T cells was accomplished by depleting CD56-, CD19- and CD14-positive cells using beads coated with the corresponding antibodies. In experiments with the birch-specific (Bet v) CD4-positive T-cell clone AF24 anti-CD19 coated beads were used to remove B lymphocytes used as antigen-presenting cells. AF24 was stimulated with anti-CD3 or specific antigen presented by autologous B cells. AF24 was cultured in the presence of 10 ng/ml IL-2. For stimulation and to investigate the influence of CD3 ligation on T-cell motility and cell surface expression of TSP-1 and LRP1 the cells were incubated on plastic coated with goat anti-mouse IgG (2 μg/ml) and anti-CD3 (50 ng/ml). In the knockdown experiments, cells were cultured in RPMI-1640 (Gibco Ltd., Paisley, UK) supplemented with 2 mm l-glutamine, 0.16% sodium bicarbonate, 10 000 U/ml benzylpenicillin, 10 000 μg/ml streptomycin and 10% fetal calf serum. In the other experiments the cells were cultured in serum-free AIM-V medium (Gibco). To maintain the cells in the free-floating state they were shaken on an IKAWERK KS 500 (IKA-Werk, Staufen, Germany) shaker at an agitation rate 150/min unless otherwise stated. To optimize the experimental conditions we also tested a STRG PLATFORM ROCKER and a Swelab MIXER 820 (Boule Medical, Stockholm, Sweden) as well as a flow system created using a Pharmacia (Bergman Labora, Danderyd, Sweden) peristaltic pump and attaching tubes.
Thrombospondin mRNA expression
Messenger RNA was extracted as previously described36 and the RNA/protein ratio was measured. The following primer pairs were used for PCR amplification of TSP-1. 5′-primer, 5′-CGT CCT GTT CCT GAT GCA TG-3′(99–118); 3′-primer, 5-GGC AGG ACA CCT TTT TGC AGA-3′(115–1135). Amplification product was mixed with 6× loading buffer (0·25% xylene cyanol, 30% glycerol in MQ-water) and separated on a 1·5% agarose gel containing 0·5 μg/ml ethidium bromide.
Small interfering RNA-mediated gene silencing
The expression of TSP-1 and LRP1 was suppressed using the human T-cell Nucleofector kit (Lonza, Köln, Germany) and a Nucleofector device (Amaxa biosystems, Köln, Germany) as previously described.37 Briefly, 5 × 106 T-enriched cells were resuspended in 100 μl nucleofector solution and transfected with 500 nm final concentration of small interfering RNA (siRNA) using protocol U14. The siRNA consisted of TSP-1 siRNA (human) (Alternative 1) (A: Sense: CCACGAUGAUGACAACGAUtt. Antisense: AUCGUUGUCAUCAUCGUGGtt.
B: Sense: CGAGACGAUUGUAUGAAGAtt. Antisense: UCUUCAUACAAUCGUCUCGtt. C: Sense: GAAGAAGCGUAAAGACUAUtt. Antisense: AUAGUCUUUACGCUUCUUCtt), LRP1 siRNA (Alternative 1) (human). Sense: AAGACUUGCAGCCCCAAGCAGtt. Antisense: CUGCUUGGGGCUGCAAGUCUUtt) and control siRNA (sc-37007) from Santa Cruz Biotechnology. TSP-1 siRNASuppl (human): (Sense: GCAUGACCCUCGUCACAUAtt. Antisense: UAUGUGACGAGGGUCAUGCca.) and LRP1 SiRNASuppl (human) (Sense: GCUGUGACAUGGACCAGUUtt. Antisense: AACUGGUCCAUGUCACAGCgg) were obtained from Applied Biosystems (Stockholm, Sweden). The degree of gene silencing and the influence of silencing on motility were determined 40 hr after introducing siRNAs.
Quantitative immunocytochemistry
The expression of various antigens was analysed in cells fixed in 2% paraformaldehyde at 4° for 20 min attached to glass slides coated with poly-l-lysine (10 μg/ml) at 4° overnight. Antigen expression was detected with monoclonal antibodies and a complex of biotinylated peroxidase and avidin (Vector Laboratories). For detection of intracellular antigens cells were fixed in 2% paraformaldehyde and permeabilized by 0·1% saponin. The cells were examined in a Nikon Eclipse E1000M (Nikon Instruments Inc., New York, NY) microscope. The intensity of the immunocytochemical staining was quantified using the image processing and analysis program imagej (NIH, Bethesda, MA).
Biotinylation and immunoprecipitation
The surface membrane of intact lymphocytes was labelled with d-biotinyl-e-aminocaproic acid-n-hydroxysuccinimide ester (biotin-7-NHS) as described by the manufacturer (Roche Molecular Biochemical, Bromma, Sweden). For immunoprecipitation, the adherent cells were biotinylated, released using a cell scraper and then immunoprecipitated. The reaction was stopped with 75 μl stop solution per tube after incubation for 15 min at room temperature and centrifuged at 300 g for 10 min. The supernatant was discarded and 5 ml cold PBS was added to each tube followed by centrifugation at 1500 rpm for 10 min. The cells were lysed in 1 ml lysis buffer (50 mm core buffer, 150 mm NaCl, 0·1 mg/ml PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1% Nonidet P-40 and 0·5% sodium deoxycholate) and incubated for 30 min on ice. After incubation for 15 min the cells were resuspended and centrifuged at 12 000 g for 10 min at 4° and the supernatants were transferred to clean Eppendorf tubes.
Immunoprecipitation was essentially carried out with protein G agarose beads as described by the manufacturers (Roche). The supernatants were mixed with 1 μg antibody at 4° overnight followed by centrifugation at 12 000 g at 4° for 20 seconds. Then the supernatants were discarded and the beads were resuspended in 1 ml washing buffer, and centrifuged again at 12 000 g at 4° for 20 seconds, the same procedure was repeated twice. After washing, 20 μl reducing buffer (2×, containing 0·15 g dithiothreitol in 5 ml 62.5 mM TRIS, 10% glycine and 2.5% SDS buffer) was mixed with the beads and heated at 95° for 4 min and subsequently centrifuged at 7000 g for 1 min to spin down the beads and the proteins were separated by SDS–PAGE. Proteins were transferred to the Hybond ECL membrane (GE Healthcare Biosciences, Uppsala, Sweden) and detected using the BMC chemiluminescence blotting kit (Roche).
Biosynthetic labelling, immunoprecipitation and gel electrophoresis
Biosynthetic labelling of polypeptides synthesized during a 3-hr culture period was carried out with [35S]methionine at 50 μCi/ml (Amersham: 54·6 TBq/mmol, 533 MBq/ml) in methionine-free medium supplemented with 10% complete medium. Material from equal numbers of live cells was immunoprecipitated. Immunoprecipitation was essentially carried out with protein G-Agarose (Pharmacia) reacted with goat anti-mouse immunoglobulin and mouse monoclonal antibodies. The immunoprecipitated material was solubilized in SDS–PAGE sample buffer containing 3% SDS. The proteins were separated on 6% SDS–PAGE gels. After drying of the gels, detection of 35S incorporated into antigenic material and present in gels was facilitated by use of fluorography. Bio-Rad (Hercules, CA) reference proteins were run simultaneously with the samples.
Western blotting
The samples were separated by SDS–PAGE and blotted onto a nitrocellulose membrane (Amersham), blocked overnight with PBS, 4% BSA and 0·5% Tween. Filters were washed with PBS with 1·5% BSA and incubated with antibodies. ECL Western blotting detection reagents were used for detection with Hyperfilm TM (Amersham).
Cell motility
Collagen type 1 was diluted in serum-free RPMI-1640 and H2O (8/1/1), applied in plastic Petri dishes 1 ml/dish (30 mm; BD Biosciences, Franklin Lakes, NJ) and allowed to polymerize at room temperature. A total of 1·0 × 106 cells in AIM-V medium was added to each well and allowed to migrate for different times. Cytochalasin B, 10 μg/ml, prevented migration into the collagen showing that it is an active cellular process. The cells were fixed in 2·5% glutaraldehyde or for immunocytochemistry in 2% paraformaldehyde and washed twice with PBS. Cell morphology and cell migration were evaluated in nine fixed positions in each well and at 50-μm intervals throughout the gel by the use of an inverted microscope (Nikon Eclipse TE300) and a digital depth meter (Heidenhein ND221, Skärholmen, Sweden). The results are given as mean number of infiltrating cells/field (×20 objective) per infiltration depth (50 μm for the first two layers immediately beneath the gel surface and 100 μm for other layers further down), as total number of infiltrating cells throughout the gel (×20 objective) or as maximal infiltration depth. The infiltrating cells were identified in situ in the collagen gels using immunocytochemistry after fixation in paraformaldehyde.
Cell adhesion
To study cell adhesion, plastic Petri dishes (90 mm; Heger A/S, Svaddeveien, Norway) were coated with ICAM-1 (2 μg/ml), fibronectin (10 μg/ml) or poly-l-lysine (10 μg/ml) and extensively washed before use. The cells (10 000/position) in AIM-V medium were incubated on the substrates in a humidified CO2 incubator at 37° for 15 or 30 min. Cells were fixed in 2·4% cold glutaraldehyde for 10 min, unbound cells were removed by gentle aspiration or for identification using immunocytochemistry after fixation in 2% paraformaldehyde. The number of adherent cells per microscopic field (20× objective) was counted. Cell adhesion was evaluated in nine fixed positions.
Cell proliferation
Cell proliferation was determined as previously described.38
Statistical analysis
Staining intensity in immunocytochemistry experiments and number of migrating cells are presented as mean arbitrary units ± SD and the Mann–Whitney U-test was used to evaluate differences between groups. For determination of differences in cell proliferation and adhesion paired Student's t-test was used. Values of P < 0·05 were considered statistically significant.
Results
Silencing of TSP-1 and LRP1 differentially affects T-cell motility and adhesion
To examine the possible influence of LRP1 and TSP-1 on T-cell motility, siRNA-mediated gene silencing of these proteins was performed (see Supplementary material, Fig. S1). The silencing was performed in serum-containing medium whereas subsequent experiments were performed under serum-free conditions to exclude any interference of exogenous proteins and peptides, such as exogenous TSP-1. The exposure to the stimulatory effects of serum and two more days of culture before the experiments yielded a higher basic motility level than in the rest of the experiments. Cells transfected with scrambled control siRNA exhibited variable morphology (Fig. 1a). Transfection with LRP1 siRNA generally inhibited the variations in cell shape seen after transfection with scrambled control siRNA and > 80% of the cells exhibited apolar rounded morphology (Fig. 1a–c). LRP1 siRNA further inhibited migration of lymphocytes into collagen matrices (Fig. 1d). Since > 85% of the cells migrating into collagen generally were identified as CD3-positive the effects of siRNA silencing on motility were characteristic of T cells. Transfection with TSP-1 siRNA also inhibited migration in collagen matrices but to a lesser extent than transfection with LRP1 siRNA and in contrast to LRP1 siRNA-transfected cells and cells transfected with control siRNA many cells were polarized and elongated (Fig. 1a,b) indicating that TSP-1 counterbalances polarization. Transfection with both LRP1 siRNA and TSP-1 siRNA had a pronounced inhibitory effect on migration and the cells showed a rounded shape. The pronounced inhibition of migration by LRP1 siRNA (Fig. 1d) probably means that the LRP1-dependent polarization was necessary for migration into the collagen. However, TSP-1 also seems to have an important role for the regulation of motility.
Figure 1.
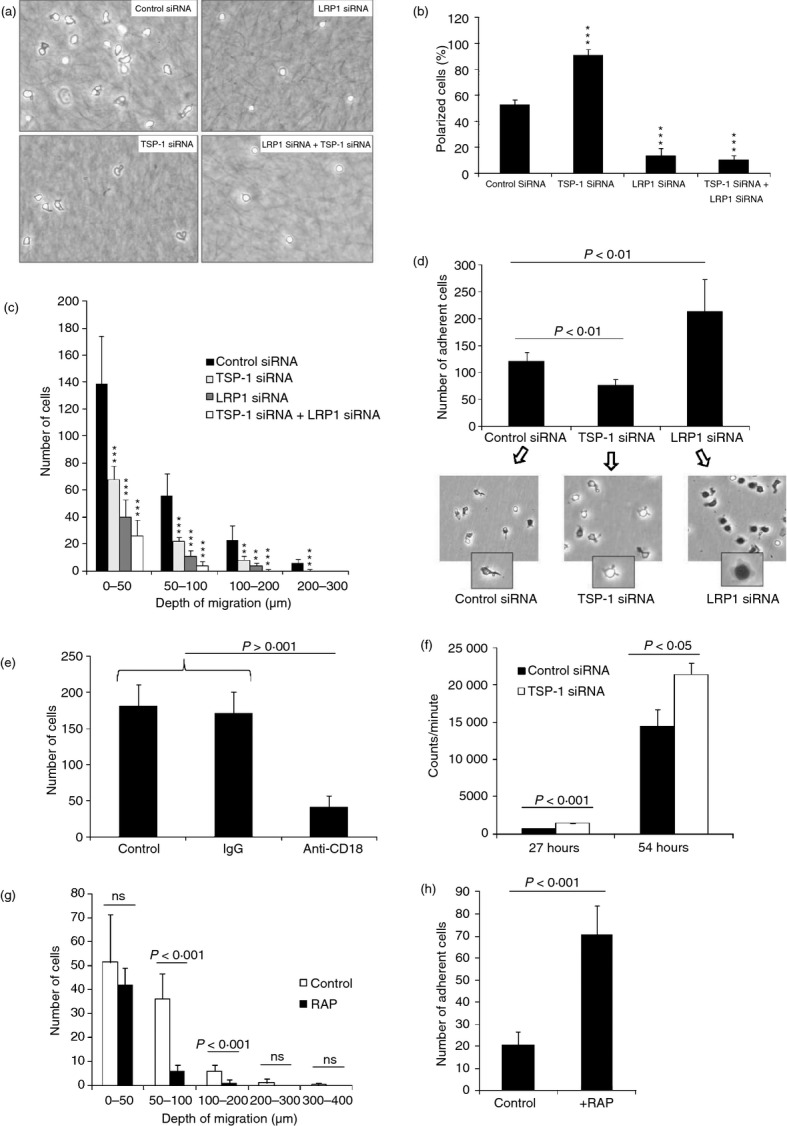
Silencing of thrombospondin 1 (TSP-1) and low-density lipoprotein receptor-related protein 1 (LRP1) differentially affects T-cell motility and adhesion. (a) The influence of transfection with scrambled control small interfering RNA (siRNA), LRP1 siRNA (alternative 1 see Materials and methods), TSP-1 siRNA (alternative 1 see Materials and methods) or both LRP1 siRNA and TSP-1 siRNA on the appearance of cells allowed to infiltrate a collagen gel for 30 min. (b) The influence of silencing, as described in (a), on cell shape expressed as per cent polarized cells. It is evident that silencing of LRP1 inhibits whereas silencing of TSP-1 enhances polarized cell shape. (c) Migration of cells into a collagen gel after transfection with scrambled control siRNA, LRP1 siRNA, TSP-1 siRNA or both LRP1 siRNA and TSP-1 siRNA. The number of cells at different depths was determined after 30 min. The results show that silencing of LRP1 as well as TSP-1 inhibits migration but that the silencing of LRP1 is more effective in this regard. (d) Adhesion of CD3-positive lymphocytes to intercellular adhesion molecule 1 (ICAM-1) and photographs showing pattern of spreading after transfection with control siRNA, TSP-1 siRNA and LRP1 siRNA as determined after 30 min. (e) Influence of an antibody to β2 integrin on adhesion of CD3-positive lymphocytes to ICAM-1 after transfection with LRP1 siRNA as determined after 30 min. (f) Influence of transfection with control siRNA and TSP-1 siRNA on anti-CD3-induced T-cell activation as determined by incorporation of radioactive thymidine after different times. (g, h) Influence of receptor-associated protein (50 μg/ml) on migration in collagen (g) and adhesion of CD3-positive cells to ICAM-1 (h). The results in the different figures represent mean values of three independent experiments or a representative experiment of three and four individual experiments (a, c, f). Between 86 and 98% of the cells in collagen in different experiments were identified as CD3-positive. **P < 0·01 versus control siRNA; ***P < 0·001 versus control siRNA.
Transfection with LRP1 siRNA increased the number of CD3-positive cells adhering to ICAM-1 in comparison with cells transfected with scrambled control siRNA (Fig. 1e). Cells transfected with LRP1 siRNA also generally showed pronounced cytoplasmic spreading (dark when observed by phase-contrast microscopy owing to flattening of the nucleus and cytoplasm) compared with cells transfected with scrambled control siRNA and an apolar shape (Fig. 1d, inserts). This enhancement of adhesion could be inhibited with an anti-β2 integrin antibody (Fig. 1e). Cells transfected with scrambled control siRNA were indistinguishable from non-transfected cells with respect to tendency to adhesion and pattern of spreading (not shown). After transfection with TSP-1 siRNA, CD3-positive cells showed reduced adhesion measured both as number of adherent cells and cytoplasmic spreading in comparison with cells transfected with scrambled control siRNA (Fig. 1d).
Knockdown of TSP-1 increased anti-CD3-induced DNA synthesis (Fig. 1f). This strongly argues against the possibility that the inhibitory and stimulatory effects on motility and adhesion respectively in the silencing experiments were the result of toxicity and suggests that endogenous TSP-1 inhibits TCR/CD3-induced T-cell activation.
Additional support for the conclusion that LRP1 induces motility and modulates adhesion was obtained using RAP, an endoplasmic reticulum-resident protein that binds tightly to multiple sites in LRP1 and prevents ligand binding to this receptor.39 Hence, RAP inhibited migration into collagen gels and enhanced adhesion of CD3-positive cells to ICAM-1 (Fig. 1g,h).
The validity of the siRNA silencing experiments was further examined using separate LRP1 and TSP-1 siRNAs (see Supplementary material, Fig. S2). These yielded essentially the same results as the LRP1 and TSP-1 siRNAs used in the primary experiments, which argues against the possibility that off-target effects were responsible.40 Experiments performed to study the duration of siRNA silencing of TSP-1 and LRP1 led to the observation that IL-2, but not activation of the cells with anti-CD3, restored the expression of TSP-1 and LRP1 (Fig. 2a,b). Further studies of this effect showed that IL-2 was a specific driver of TSP-1 synthesis in T cells, as will be described below.
Figure 2.
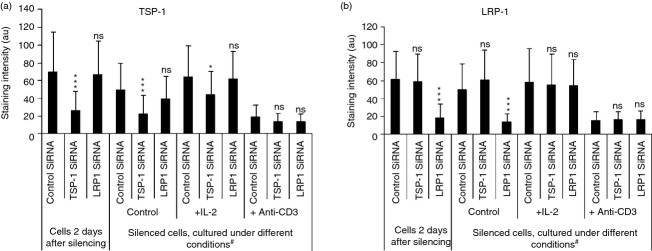
Silencing of thrombospondin 1 (TSP-1) and low-density lipoprotein receptor-related protein 1 (LRP1) using separate small interfering RNAs (siRNAs). (a, b) Influence of interleukin-2 (IL-2) and anti-CD3 on the expression of TSP-1 (a) and LRP1 (b) in permeabilized cells after transfection with scrambled control siRNA, TSP-1 siRNA and LRP1 siRNA as determined by immunocytochemistry. After transfection the cells were incubated for 2 days and subsequently in the presence and absence of IL-2 or anti-CD3 for 7 days (#). There was no hidden stimulatory effect of anti-CD3 using shorter incubation times. *P < 0·05 versus control siRNA; **P < 0·01 versus control siRNA; ***P < 0·001 versus control siRNA. The results in (a) and (b) show mean values of three independent experiments. The results in c, d show one representative experiment of two independent experiments.
IL-2 stimulates TSP-1 production and T-cell motility
In the light of the evidence that IL-2 stimulated TSP-1 production (Fig. 2c,d) we examined its influence on TSP-1 expression. Culture in the presence of IL-2 stimulated biosynthesis of TSP-1 as shown by the birch-specific T-cell clone AF24 (Fig. 3a). Culture in the presence of both IL-2 and IL-4 had a stimulatory effect on the biosynthesis of TSP-1 that was virtually the same as or weaker than that of IL-2 alone, showing that IL-4 did not contribute to TSP-1 synthesis (Fig. 3a). AF24 cells stimulated with anti-CD3 and IL-2 plus IL-4 for 5 days exhibited significant TSP-1 production not observed after stimulation with anti-CD3 alone and culture in the presence of anti-CD3 even decreased TSP-1 synthesis in comparison with cells in medium only. The RT-PCR confirmed that IL-2 stimulated whereas anti-CD3 inhibited TSP-1 mRNA expression as determined over a 7-day period (Fig. 3b). After 7 days, IL-2 seemed to overcome the inhibitory effect of anti-CD3 (Fig. 3b) consistent with the results in Fig. 2(a) that anti-CD3 plus IL-2 plus IL-4 yielded a substantially higher TSP-1 synthesis than anti-CD3 only. It is also evident that AF24 exhibited a markedly increased IL-2-dependent rate of synthesis of TSP-1 with time detectable as release of protein into the medium (Fig. 3c).
Figure 3.

Interleukin-2 (IL-2) stimulates thrombospondin 1 (TSP-1) expression in T cells and motility through TSP-1 and low-density lipoprotein receptor-related protein 1 (LRP1). (a) Gel analysis (6% SDS–PAGE) showing immunoprecipitation of biosynthetically labelled TSP-1 from the birch antigen-specific T-cell clone AF24 ([35S]methionine; 3-hr pulse) after culture under different conditions for 5 days. IL-2 and IL-4 were used at a concentration of 10 ng/ml. (b) Agarose gel electrophoresis showing RT-PCR-amplified TSP-1 and β-actin fragments using mRNA isolated from AF24 cells cultured for different times with IL-2 and IL-2 and anti-CD3 as templates. Fragments were visualized by ethidium bromide staining and UV illumination. (c) Western blotting of material from conditioned medium of unprecipitated material from AF24 cells cultured with IL-2 for different times and separated on a 6% SDS–PAGE gel. After different times the cells were cultured in AIM-V medium over night. It is evident that the synthesis of TSP-1 increases with time and is IL-2-dependent. (d, e) Interleukin-2 stimulates cell surface expression of TSP-1 whereas IL-4 rather inhibits TSP-1 expression but stimulates LRP1 expression. The cells were incubated in the presence of IL-2 (10 ng/ml) or IL-4 (20 ng/ml) for 30 min and the cell surface expression of TSP-1 and LRP1 was subsequently determined using immunoprecipitation of material from surface biotinylated cells (d) or quantitative immunocytochemistry (e). (f) Influence of IL-2 (10 ng/ml) and IL-4 (10 ng/ml) on T-cell migration into a collagen matrix. Motility was determined after 30 min. (g) Silencing of TSP-1 and LRP1 abrogates the stimulatory effect of IL-2 on T-cell motility. Migration into a collagen gel was determined after transfection with control siRNA, TSP-1 siRNA (Alternative 1), or LRP1 siRNA (Alternative 1). The number of cells at different depths was determined after 30 min. (a–e) One representative experiment of three to six independent experiments; (f) mean values of three independent experiments and (g) one representative experiment of two independent experiments.
We next compared the influence of IL-2 and IL-4 on the cell surface expression of TSP-1 and LRP1 in blood T cells. Interleukin-2 was found to increase cell surface TSP-1 within 30 min, whereas IL-4 did not (Fig. 3d,e). In contrast, IL-4 seemed to increase cell surface LRP1. The IL-2 also stimulated T-cell migration into a three-dimensional collagen matrix (Fig. 3f). Interleukin-4 inhibited T-cell migration into collagen, which as far as we have been able to determine has not been described before. The siRNA silencing of LRP1 and TSP-1 abrogated the stimulatory effect of IL-2 on T-cell motility (Fig. 3g) indicating that IL-2 stimulates T-cell motility through TSP-1 and LRP1.
TSP-1 stimulates T-cell motility through CD47 and LRP1
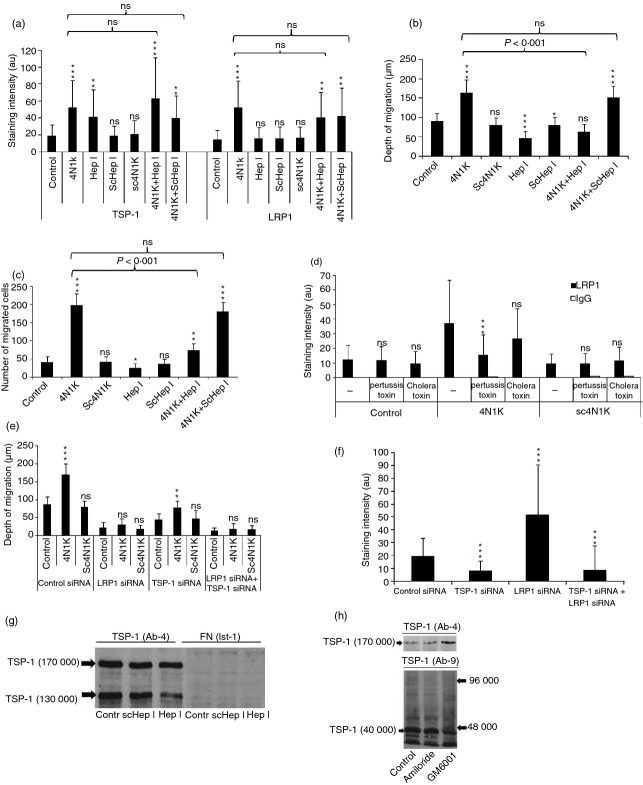
To further elucidate the role of LRP1 and TSP-1 for motility we examined whether peptides mimetic of the N-terminal calreticulin-binding site (hepI) and the C-terminal CD47-binding site in TSP-1 (4N1K) as well as scrambled control peptides affected the expression of endogenous TSP-1 and LRP1 and motility.11,41 4N1K increased the cell surface expression of TSP-1 and LRP1 and also motility substantially (Fig. 4a–c) in agreement with the evidence in Fig. 1 that LRP1 and TSP-1 are motogens. Pertussis toxin inhibited the 4N1K-induced cell surface expression of LRP1 indicating involvement of a CD47/Gi protein complex (Fig. 4d) but did not inhibit the expression of TSP-1 (not shown). The increased TSP-1 expression induced by 4N1K probably reflects inhibition of interaction of endogenous TSP-1 with CD47. 4N1K did not stimulate motility after knockdown of LRP1 and did so to a lesser extent after knockdown of TSP-1 (Fig. 4e). Knockdown of LRP1 also markedly increased the cell surface expression of TSP-1, further indicating that cell surface-expressed TSP-1 disappears through LRP1 (Fig. 4f). Together with the evidence that 4N1K increased cell surface expression of TSP-1 (Fig. 4a) this suggested that TSP-1 interacts with CD47 and with LRP1. HepI increased TSP-1 expression on the cell surface and inhibited motility (Fig. 4a,b). This probably means that hepI inhibited an interaction that promotes disappearance of TSP-1 and suggests a role of LRP1-dependent processing of TSP-1 for motility.
Figure 4.
Thrombospondin 1 (TSP-1) stimulates T-cell motility through CD47 and low-density lipoprotein receptor-related protein 1 (LRP1). (a) The influence of peptides mimetic of the CD47-binding site in TSP-1 (4N1K), the calreticulin-binding site of TSP-1 (hepI), and scrambled (sc) control peptides on the cell surface expression of TSP-1 and LRP1 as determined after 30 min using quantitative immunocytochemistry. (b, c) The influence of 4N1K, sc4N1K, hepI and sc hepI on T-cell motility measured as maximal depth of migration into a collagen gel (b) and number of cells at different levels throughout the gel (c) after 30 min. (d) Influence of incubation with pertussis toxin (10 μg/ml) or cholera toxin (10 μg/ml) on 4N1K-induced cell surface expression of LRP1 as determined after 30 min by quantitative immunocytochemistry. The results show mean values of three independent experiments. (e) The influence of 4N1K and sc4N1K on T-cell motility measured as maximal infiltration depth into a collagen gel after transfection with scrambled control small interfering (si) RNA, TSP-1 siRNA, LRP1 siRNA or both LRP1 siRNA and TSP-1 siRNA. The cells were allowed to migrate for 30 min. (f) The influence of transfection with scrambled control siRNA, TSP-1 siRNA, LRP1 siRNA or both LRP1 siRNA and TSP-1 siRNA on the cell surface expression of TSP-1. (g) Western blotting (6% SDS–PAGE) of material from lysed cells showing that incubation with hepI, but not a scrambled control peptide, for 15 min inhibits a 130 000 MW TSP-1 fragment. The reactivity with anti-fibronectin antibodies is shown as a control. (h) Western blotting (6% SDS–PAGE) of material from lysed cells demonstrating a 40 000 MW N-terminal fragment with a monoclonal antibody specific for the N-terminal domain (AB-9). It can further be seen that GM6001 (10 μm) but not amiloride (50 μm) inhibits this fragment and increases the intensity of intact 170 000 MW TSP-1 as demonstrated using another TSP-1 antibody (Ab-4). The results in (a–e) represent mean values of three individual experiments; (f–h) are representative of four or five individual experiments. *, **, *** refer to statistical comparisons with control cells.
Western blotting of whole cell material indicated that hepI prevented the appearance of a 130 000 MW TSP-1 fragment, suggesting that interaction with calreticulin/LRP1 induces cleavage of TSP-1 (Fig. 4g). Western blotting of whole cell material further demonstrated that the cells contained a 40 000 MW N-terminal fragment of TSP-1, the appearance of which was decreased by a 15-min exposure to the metalloproteinase inhibitor GM6001 whereas intact TSP-1 concomitantly increased, further suggesting that TSP-1 undergoes cleavage (Fig. 4h). The results in Fig. 4(a–h) indicated that interaction of the C-terminal domain of TSP-1 with CD47 stimulates cell surface expression of LRP1 and motility and that subsequent LRP1-dependent processing of TSP-1 further stimulates motility.
A JAK/STAT pathway inhibitor mimics silencing of LRP1 in its effect on cell surface TSP-1, motility and adhesion
To elucidate which signalling pathways that regulate T-cell motility were dependent on LRP1 and TSP-1 we applied various inhibitors. Striking effects were obtained using AG490, which inhibits the Janus kinase–signal transducer and activator of transcription 1 (JAK-STAT) signalling pathway, and UO126, which inhibits extracellular-signal regulated kinase (ERKs),42 a family of mitogen-activated protein kinases. AG490 inhibited T-cell motility (Fig. 5a). In contrast, AG490 increased adhesion to fibronectin and ICAM-1 substantially (Fig. 5b) and induced apolar cytoplasmic spreading almost indistinguishable from cells after knockdown of LRP1 or exposed to RAP (not shown). Notably, AG490 increased the cell surface expression of TSP-1 (Fig. 5c). In contrast to AG490, UO126 decreased the expression of TSP-1 (Fig. 5c). UO126 had a suggested stimulatory effect on motility and an inhibitory effect on adhesion (Fig. 5b,c). The results in Fig. 5 indicated that TSP-1 undergoes processing as a consequence of interaction with LRP1/calreticulin and that this is coupled to motility. The effect of AG490 on cell surface expression of TSP-1, motility and adhesion therefore mimicked the effect of silencing of LRP1.
Figure 5.
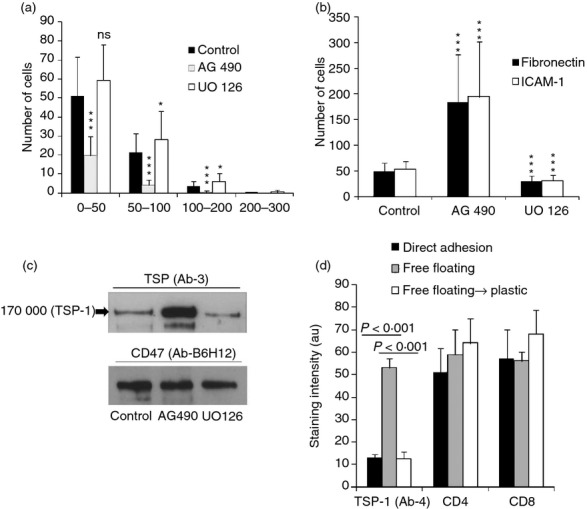
T-cell motility and cell surface expression of thrombospondin 1 (TSP-1) depends on Janus kinase (JAK) and extracellular signal-regulated kinase (ERK) signalling and flow. (a) Influence of AG490 and UO126 on T-cell migration into collagen as determined after 30 min. (b) Influence of AG490 and UO126 on T-cell adhesion to intercellular adhesion molecule 1 (ICAM-1) and fibronectin as determined after 30 min. (c) Gel analysis (6% SDS–PAGE) of material from AG490- and UO126-treated surface biotinylated cells (30 min) immunoprecipitated with anti-TSP-1 and anti-CD47. It is evident that AG490 enhances whereas UO126 inhibits TSP-1 expression. (d) Influence of incubation of cells for 10 min under different conditions on the cell surface expression of different antigens. It is evident from the results that the free-floating state induces cell surface expression of TSP-1 and that this increased expression is reversible after incubation for 30 min under static conditions. The results in (a) and (b) show mean values of three independent experiments, (c) shows a representative experiments of three independent experiments and (d) one representative experiment of two independent experiments.
Based on the evidence that flow stimulates T-cell adhesion,10 we examined the possible influence of the free-floating state on TSP-1 expression. Free-floating cells exhibited a marked up-regulation of the cell surface expression of TSP-1 (Fig. 5d). As previously reported,10 they also showed increased adhesion to ICAM-1 (not shown). This suggested that the free-floating state affects the motogenic TSP-1/LRP1 mechanism and is consistent with the finding using AG490 that up-regulation of cell surface TSP-1 supports adhesion as well as with the evidence from knockdown experiments that TSP-1 stimulates adhesion.
The motogenic TSP-1/LRP1 mechanism inhibits TCR/CD3-induced proliferation
To further examine the evidence in Fig. 1(f) that the motogenic TSP-1/LRP1 mechanism suppresses TCR-induced T-cell proliferation, we examined the influence of stimulation of this mechanism through CD47 using 4N1K. In addition, we examined the influence of a monoclonal anti-CD47 antibody (C1Km1) that we found to stimulate motility compared with another antibody (B6H12) that did not stimulate motility (Fig. 6a). 4N1K caused a marked inhibition of TCR-induced DNA synthesis in the birch allergen-specific CD4-positive T-cell clone AF24, which was most pronounced at the initial determination, whereas control peptides did not inhibit DNA synthesis (Fig. 6b, c). C1Km1 also inhibited DNA synthesis whereas B6H12 did not inhibit it. The results in Fig. 4(b) therefore indicated that stimulation of motility correlates with inhibition of T-cell proliferation. In addition, culture of the T-cell clone in the presence of an antibody to TSP-1 increased DNA synthesis (Fig. 6c) consistent with the evidence in Figs 1(f) and 4(b) that inhibition of participation of TSP-1 in motility would stimulate proliferation. There was no significant difference between the viability of the cells exposed to the peptides in Fig. 6(b) (not shown). These results further strengthened the conclusion that the motogenic TSP-1/LRP1 mechanism antagonizes TCR-induced T-cell proliferation.
Figure 6.

The motogenic thrombospondin 1 (TSP-1)/ low-density lipoprotein receptor-related protein 1 (LRP1) mechanism inhibits Tcell receptor-induced T-cell proliferation. (a) The anti-CD47 antibody C1Km1 stimulates whereas B6H12 inhibits T-cell motility as determined by maximal depth of migration into a collagen gel. Migration was determined after 30 min. (b) 4N1K and C1Km1 inhibit proliferation. AF24 T cells were stimulated with antigen in the presence of various peptides and antibodies. The values show [3H]thymidine incorporation. (c) Anti-TSP-1 antibodies (Ab-3) stimulate anti-CD3-induced activation of AF24 as determined by [3H]thymidine incorporation. (a) Mean values of three independent experiments; (b, c) representative experiments of two or three independent experiments.
Discussion
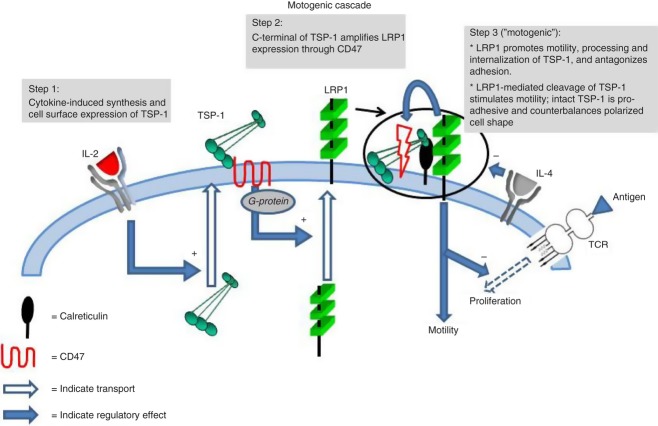
These results provide new information about the action of the major cytokines IL-2 and IL-4 and unveil a mechanism for integrated regulation of T-cell motility and adhesion to ICAM-1 and fibronectin. Knockdown experiments and the effect of RAP first indicated that LRP1, with the support of TSP-1, are of crucial importance for T-cell motility and adhesion, LRP1 by promoting polarized cell shape and TSP-1 through a counter-polarizing effect. Peptides mimetic of TSP-1 binding sites for CD47 (4N1K) and calreticulin (hepI) together with various inhibitors confirmed and extended these findings, indicating that a cell surface cascade comprising TSP-1, CD47, calreticulin and LRP1 combines the regulation of motility and adhesion. This cascade antagonizes TCR/CD3-induced proliferation, seems to coordinate distinct signalling pathways and mediates inter-regulation of IL-2 and IL-4, as depicted in the model in Fig. 7. Interleukin-2 stimulated synthesis of TSP-1 as well as motility through TSP-1/LRP1 interaction in cis, whereas IL-4 inhibited motility.
Figure 7.
A cell surface cascade for integrated regulation of T-cell motility, adhesion and T-cell receptor (TCR)- and cytokine-induced responses. Motility is directed by cell surface-expressed low-density lipoprotein receptor-related protein 1 (LRP1) and thrombospondin 1 (TSP-1). LRP1 expression at the cell surface promotes the processing of TSP-1 and probably also polarized cell shape and migration through Janus kinase (JAK) signalling while concomitantly inhibiting adhesion to intercellular adhesion molecule 1 (ICAM-1) and fibronectin. Extracellular-signal regulated kinase (ERK) inhibits this motogenic effect. Cell surface expression of LRP1 is enhanced by interaction of the C-terminal domain of endogenous TSP-1 with CD47, whereupon interaction of the N-terminal domain with LRP1 further enhances the motogenic effect of LRP1. Interleukin-2 (IL-2) stimulates synthesis and cell surface expression of TSP-1 and hence up-regulates the motogenic mechanism; IL-4 inhibits the motogenic mechanism. The motogenic mechanism inhibits TCR-induced T-cell proliferation. TCR-induced proliferative responses and IL-4 inhibit the motogenic mechanism. + denotes stimulatory effect; – denotes inhibitory effect.
The effects of AG490 (Fig. 5) indicated that LRP1 promotes TSP-1 processing and probably also T-cell motility while inhibiting adhesion to ICAM-1 and fibronectin via JAK signalling. In contrast, ERK-dependent protection of TSP-1 seems to support adhesion while inhibiting motility. LRP1 signalling through JAK and suppression of the ERK pathway is consistent with data from non-lymphoid cells.43–46 TSP-1 enhances cell surface expression of LRP1 through a CD47/Gi protein complex. The fact that inhibition of TSP-1 processing stimulates whereas knockdown of TSP-1 inhibits adhesion further indicates that TSP-1 promotes adhesion. Furthermore, the results point to the possibility that the free-floating vascular state inhibits the motogenic JAK-pathway, while protecting TSP-1, which enhances adhesion to ICAM-1. Hence, our results indicate that T cells are endowed with a cell surface cascade connecting signalling via cytokine receptors with CD47/Gi protein signalling and LRP1 signalling through JAK coupled to inhibition through ERK. The trimolecular TSP-1 structure with binding sites for CD47 and calreticulin/LRP1 at different ends of the molecule may be essential for this.
The extreme flattening of adherent cells by LRP1 knockdown, and the enhancement of adhesion by RAP or JAK inhibition, probably reflect the disappearance of a counteradhesive LRP1 effect. Therefore, the TSP-1/CD47/calreticulin/LRP1 cascade may also be considered as a potent mechanism for regulation of adhesion. It is an intriguing possibility that this cascade may endow the T-cell with its migratory phenotype by prioritizing motility over adhesion whereas inhibition of the cascade promotes transient up-regulation of adhesion. In agreement with the present evidence that LRP1 counteracts adhesion, knockdown of LRP1 increases adhesion of carcinoma cells to matrix substrates.47
The TSP-1/CD47/calreticulin/LRP1 cascade may account for the high motility of T cells and their constant repositioning within the organism. This autocrine regulation of motility and adhesion by endogenous components, which are not anchored to other cells or the extracellular matrix and hence do not restrict movement, may be a prerequisite for this behaviour. The role of LRP1 as a major T-cell motogen is consistent with the fact that it is a transmembrane protein capable of transducing a cell surface signal to cytoskeletal components.20
Our findings may explain several unresolved therapeutic effects as well as effects induced through cell surface receptors reported in the literature by putting them into a broader mechanistic context. Accordingly, it is possible that the inhibition of graft-versus-host disease, type 1 diabetes and experimental autoimmune encephalomyelitis by AG490 treatment48–50 depends on inhibition of the motogenic cascade and infiltration of effector cells into target organs. The inhibitory effect on TCR-induced T-cell activation of exogenous TSP-1 via CD4726,28 may also be accounted for by stimulation of this cascade. Inhibition of the motogenic cascade by shear force may account for the capacity of T cells to undergo rapid adhesion to endothelial cells.10 It is also conceivable that the generation of Treg cells by TSP-1 via CD47, as well as by IL-2 and the ERK inhibitor UO126,26,27,42 may reflect up-regulation of the motogenic cascade, so enhancing the capacity to execute suppression by moving in close contact with other cells. Another possibility is that IL-2-induced TSP-1 maintains the function of T-cell subsets such as Treg cells. In support of this contention, migration is required for efficient suppression of effector cell responses51 and maintenance of the Treg cell effect requires presence of IL-2.2
The antagonistic effect of the motogenic cascade on TCR-induced proliferative responses may play a critical balancing role for immune regulation. This antagonistic effect is consistent with the idea that T-cell migration and activation leading to division are distinct and incompatible steps of the immune response.52 The motogenic cascade may therefore play an important physiological role through inhibition of antigen-induced T-cell proliferation. In this context our finding that T cells produce TSP-1 and that this production can be up-regulated and enhance the motogenic cascade may also have bearing on the fact that defective expression of TSP-1 promotes inflammation in most tissues whereas over-expression of TSP-1 protects against inflammation.53,54 Up-regulation and dominance of the motogenic cascade over the effects of TCR-induced T-cell activation and IL-4 may explain why IL-2 promotes tolerance and protects against autoimmune diseases and allergy.2,7,55 The motogenic cascade may therefore endow the immune system with an immunoregulatory pathway in addition to the tolerance-promoting interactions of programmed cell death 1 and cytotoxic T-lymphocyte antigen 4 with their ligands.56,57 Interleukin-2-induced TSP-1 expression may also regulate immune responses through transforming growth factor-β (TGF-β), since TSP-1 is an activator of TGF-β.53 This may play a critical role in the down-regulation of immune responses, as illustrated by development of multi-organ autoimmunity in TGF-β-deficient mice.58,59 The inhibition of T-cell motility by IL-4 probably reflects an effect on LRP1 but it is also possible that IL-4 affects TSP-1 and hence indirectly LRP1 and motility. This suggests that IL-2 and IL-4 inter-regulate and balance Th1 and Th2 responses through antagonistic effects on TSP-1 and LRP1 consistent with the evidence that Th1 and Th2 responses may co-exist in vivo although one response dominates functionally.60
In conclusion, T-cell regulation through the TSP-1/CD47/calreticulin/LRP1 cascade may provide rapid controlled adaptation to different needs and demands. The finding that a cell surface cascade directs T-cell functions also has bearing on the plasticity of T-cell subsets and their regulation is often discussed in relation to T17 and Treg cells.61 The present results provide a conceptual background for better understanding of T-cell regulation and adaptive immune responses.
Acknowledgments
This work was supported by the Swedish Research Council the Konsul Th. C. Bergh's Foundation, and the Centre for Allergy Research at Karolinska Institute. SEB analysed data and contributed to the design of the study and to the writing of the manuscript. EB performed research work. KGS designed the study, performed research work, analysed data and wrote the manuscript.
Disclosures
We have no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Western blot analysis (4–12% SDS–PAGE gels) of expression of low-density lipoprotein receptor-related protein 1 (LRP1) and thrombospondin 1 (TSP-1) after transfection with scrambled control small interfering RNA (siRNA), LRP1 siRNA (alternative 1 see Materials and methods) and TSP-1 siRNA (alternative 1 see Materials and methods).
Figure S2. (a) Expression of low-density lipoprotein receptor-related protein 1 (LRP1) and thrombospondin 1 (TSP-1) in permeabilized cells after transfection with scrambled control small interfering RNA (siRNA), TSP-1 siRNA suppl (see Materials and methods), LRP1 siRNA suppl (see Materials and methods) or both LRP1 siRNA and TSP-1 siRNA and incubation with brefeldin A for 30 min before detection using quantitative immunocytochemistry. (b) Migration of cells into a collagen gel after transfection as in (a). The number of cells at different depths was determined after 30 min. The results show that silencing of LRP1 as well as TSP-1 inhibits migration but that the silencing of LRP1 is much more effective.
References
- 1.Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21:305–14. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, Rivera J. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity. 2011;35:562–71. doi: 10.1016/j.immuni.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 5.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2012;185:6426–30. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 7.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pryce G, Male D, Campbell I, Greenwood J. Factors controlling T-cell migration across rat cerebral endothelium in vitro. J Neuroimmunol. 1997;2:84–94. doi: 10.1016/s0165-5728(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson PC, Newman I. Chemoattractant activity of IL-2 for human lymphocytes: a requirement for the IL-2 receptor β-chain. Immunology. 1994;82:134–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Woolf E, Grigorova I, Sagiv A, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–85. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 11.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–4. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Tan K, Duquette M, Liu JH, Zhang R, Joachimiak A, Wang JH, Lawler J. The structures of the thrombospondin-1 N-terminal domain and its complex with a synthetic pentameric heparin. Structure. 2006;14:33–42. doi: 10.1016/j.str.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, Roberts DD. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by α6β1 integrin. J Biol Chem. 2003;278:40679–87. doi: 10.1074/jbc.M302014200. [DOI] [PubMed] [Google Scholar]
- 14.Goicoechea S, Pallero MA, Eggleton P, Michalak M, Murphy-Ullrich JE. The anti-adhesive activity of thrombospondin is mediated by the N-terminal domain of cell surface calreticulin. J Biol Chem. 2002;277:37219–28. doi: 10.1074/jbc.M202200200. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Calzada MJ, Sipes JM, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Interactions of thrombospondins with α4β1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol. 2002;157:509–19. doi: 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J Cell Sci. 2003;14:2917–27. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- 17.Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood. 2006;108:3112–20. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Christensson M, Forslow A, De Meester I, Sundqvist KG. A CD26-controlled cell surface cascade for regulation of T cell motility and chemokine signals. J Immunol. 2009;183:3616–24. doi: 10.4049/jimmunol.0804336. [DOI] [PubMed] [Google Scholar]
- 19.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–27. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao C, Lawrence DA, Li Y, et al. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006;25:1860–70. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng CF, Fan J, Fedesco M, et al. Transforming growth factor α (TGFα)-stimulated secretion of HSP90α: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFβ-rich environment during wound healing. Mol Cell Biol. 2008;28:3344–58. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of Schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28:11571–82. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksson C, Rantapaa-Dahlqvist S, Sundqvist KG. T-cell expression of CD91 – a marker of unresponsiveness to anti-TNF therapy in rheumatoid arthritis. APMIS. 2010;118:837–45. doi: 10.1111/j.1600-0463.2010.02677.x. [DOI] [PubMed] [Google Scholar]
- 25.Rebres RA, Green JM, Reinhold MI, Ticchioni M, Brown EJ. Membrane raft association of CD47 is necessary for actin polymerization and protein kinase C θ translocation in its synergistic activation of T cells. J Biol Chem. 2001;276:7672–80. doi: 10.1074/jbc.M008858200. [DOI] [PubMed] [Google Scholar]
- 26.Avice MN, Rubio M, Sergerie M, Delespesse G, Sarfati M. Role of CD47 in the induction of human naive T cell anergy. J Immunol. 2001;167:2459–68. doi: 10.4049/jimmunol.167.5.2459. [DOI] [PubMed] [Google Scholar]
- 27.Grimbert P, Bouguermouh S, Baba N, et al. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25– T cells in response to inflammation. J Immunol. 2006;177:3534–41. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, He L, Wilson K, Roberts D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol. 2001;166:2427–36. doi: 10.4049/jimmunol.166.4.2427. [DOI] [PubMed] [Google Scholar]
- 29.Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161:1179–89. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haston WS, Shields JM, Wilkinson PC. Lymphocyte locomotion and attachment on two-dimensional surfaces and in three-dimensional matrices. J Cell Biol. 1982;92:747–52. doi: 10.1083/jcb.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundqvist KG, Otteskog P. Anchorage and lymphocyte function: collagen and the maintenance of motile shape in T cells. Immunology. 1986;58:365–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Schor SL, Allen TD, Winn B. Lymphocyte migration into three-dimensional collagen matrices: a quantitative study. J Cell Biol. 1983;96:1089–96. doi: 10.1083/jcb.96.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–9. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 34.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–37. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.Bidere N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, Senik A. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278:31401–11. doi: 10.1074/jbc.M301911200. [DOI] [PubMed] [Google Scholar]
- 38.Sundqvist KG, Wanger L, Ensgstom W. Permissive effect of cytochalasin B on DNA synthesis in concanavalin-A-treated lymphocytes. J Cell Sci. 1984;66:155–66. doi: 10.1242/jcs.66.1.155. [DOI] [PubMed] [Google Scholar]
- 39.Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/α2-macroglobulin receptor. J Biol Chem. 1991;266:21232–8. [PubMed] [Google Scholar]
- 40.Schultz N, Marenstein DR, De Angelis DA, et al. Off-target effects dominate a large-scale RNAi screen for modulators of the TGF-β pathway and reveal microRNA regulation of TGFBR2. Silence. 2011;2:3. doi: 10.1186/1758-907X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Hook M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268:26784–9. [PubMed] [Google Scholar]
- 42.Luo X, Zhang Q, Liu V, Xia Z, Pothoven KL, Lee C. Cutting edge: TGF-β-induced expression of Foxp3 in T cells is mediated through inactivation of ERK. J Immunol. 2008;180:2757–61. doi: 10.4049/jimmunol.180.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 44.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–24. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 45.Guttman M, Betts GN, Barnes H, Ghassemian M, van der Geer P, Komives EA. Interactions of the NPXY microdomains of the low density lipoprotein receptor-related protein 1. Proteomics. 2009;9:5016–28. doi: 10.1002/pmic.200900457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM, Gonias SL. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol. 2002;159:1061–70. doi: 10.1083/jcb.200207070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langlois B, Perrot G, Schneider C, Henriet P, Emonard H, Martiny L, Dedieu S. LRP-1 promotes cancer cell invasion by supporting ERK and inhibiting JNK signaling pathways. PLoS One. 2010;5:e11584. doi: 10.1371/journal.pone.0011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behbod F, Erwin-Cohen RA, Wang ME, et al. Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J Immunol. 2001;166:3724–32. doi: 10.4049/jimmunol.166.6.3724. [DOI] [PubMed] [Google Scholar]
- 49.Constantin G, Laudanna C, Brocke S, Butcher EC. Inhibition of experimental autoimmune encephalomyelitis by a tyrosine kinase inhibitor. J Immunol. 1999;162:1144–9. [PubMed] [Google Scholar]
- 50.Davoodi-Semiromi A, Wasserfall CH, Xia CQ, Cooper-DeHoff RM, Wabitsch M, Clare-Salzler M, Atkinson M. The tyrphostin agent AG490 prevents and reverses type 1 diabetes in NOD mice. PLoS One. 2012;7:e36079. doi: 10.1371/journal.pone.0036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–69. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berg LP, James MJ, Alvarez-Iglesias M, Glennie S, Lechler RI, Marelli-Berg FM. Functional consequences of noncognate interactions between CD4+ memory T lymphocytes and the endothelium. J Immunol. 2002;168:3227–34. doi: 10.4049/jimmunol.168.7.3227. [DOI] [PubMed] [Google Scholar]
- 53.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–70. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 54.Velasco P, Huegel R, Brasch J, et al. The angiogenesis inhibitor thrombospondin-1 inhibits acute cutaneous hypersensitivity reactions. J Invest Dermatol. 2009;129:2022–30. doi: 10.1038/jid.2008.447. [DOI] [PubMed] [Google Scholar]
- 55.Malek TR, Pugliese A. Low-dose IL-2 as a therapeutic agent for tolerance induction. Immunotherapy. 2011;3:1281–4. doi: 10.2217/imt.11.120. [DOI] [PubMed] [Google Scholar]
- 56.Kamradt T, Goggel R, Erb KJ. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 2005;26:260–7. doi: 10.1016/j.it.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 58.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wurtz O, Bajenoff M, Guerder S. IL-4-mediated inhibition of IFN-γ production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int Immunol. 2004;16:501–8. doi: 10.1093/intimm/dxh050. [DOI] [PubMed] [Google Scholar]
- 61.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–80. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.