Abstract
DNAX-activation protein 12 (DAP12), a transmembrane adapter, plays a major role in transducing activation signals in natural killer cells and various myeloid cells. Quantitative RT-PCR detected in normal mouse eyes considerable levels of DAP12 and multiple DAP12-coupled receptors, in particular TREM-1, Clec5a and SIRPb1. The role of DAP12 and its receptors in experimental autoimmune diseases has been controversial. Here, we analysed the effect of DAP12 deficiency on the capacity of mice to mount immunopathogenic cellular responses to the uveitogenic ocular antigen and interphotoreceptor retinoid-binding protein (IRBP), and to develop experimental autoimmune uveitis (EAU). Surprisingly, sequential analysis of EAU in mice deficient in DAP12 in two different animal facilities at first revealed enhanced disease as compared with wild-type mice, but when these mice were re-derived into a second, cleaner, animal facility, the response of control mice was essentially unchanged, whereas the DAP12 null mice were markedly hyporesponsive relative to controls in the new facility. Accordingly, when stimulated in vitro with IRBP, lymphocytes from the DAP12-deficient mice housed in the two facilities proliferated and produced opposite profiles of pro-inflammatory and anti-inflammatory cytokines, compared with their controls. These findings therefore demonstrate that the effects of DAP12 deficiency on development of autoimmune disease are dramatically affected by environmental factors.
Keywords: cytokines, lymphoid cells, ocular inflammation, TREM-2
Introduction
DNAX-activation protein 12 (DAP12), also known as killer-activating receptor-associated protein (KARAP), is a transmembrane adapter that plays a major role in transducing activation signals from an array of receptors expressed by natural killer cells, and a variety of myeloid cells including dendritic cells, granulocytes and macrophages.1–7 DAP12 contains a phosphorylated immunoreceptor signalling motif that provides signalling function via the Syk and ZAP-70 tyrosine kinase activation pathways.1,8 These immunoreceptor signalling motif-mediated signals, initiated after receptor stimulation, lead to amplification and diversification of signalling pathways needed to mediate the complex biological responses required in specific immune responses. Within the myeloid compartment, DAP12 has paradoxically been implicated in both pro-inflammatory and anti-inflammatory activities.4,9 In granulocytes, DAP12 couples to triggering receptor expressed in myeloid cells (TREM) -1 and co-stimulation with anti-TREM-1 antibody and endotoxin results in accentuated inflammatory cytokine production.10,11 Moreover, blockade of TREM-1 rescues mice from endotoxaemia11,12 and accordingly, mice lacking DAP12 (Tyrobp−/−) were also found to be resistant to endotoxaemia.13 In contrast to TREM-1, TREM-2 coupled to DAP12 attenuates macrophage activation. Hence, macrophages of Tyrobp−/− or Trem2−/− mice are hyper-responsive to lipopolysaccharide and repression of TREM-2 expression in microgial cells enhances their inflammatory potential.2,14,15 Similarly, when sensitized with d-galactosamine, Tyrobp−/− mice are more susceptible than their wild-type (WT) controls to fatal endotoxaemia.2,14,15 In another study, deficiency in DAP12 enhanced the immunopathology related to mycobacterial infection in mice.16 Of particular interest to the present study are reports showing the outcome of DAP12 deficiency on experimental autoimmune diseases. Hence, whereas mice deficient in DAP12 were initially found to be resistant to induction of experimental autoimmune encephalomyelitis,17 higher susceptibility to this disease was observed in another study in mice treated with antibody against TREM-218 and expression of TREM-2 on myeloid precursors ameliorated experimental autoimmune encephalomyelitis.19 Tyrobp−/− mice also showed enhanced susceptibility to immune-mediated diabetes, compared with WT controls.20
Animals immunized with certain retinal antigens may develop the inflammatory eye disease experimental autoimmune uveitis (EAU). This animal disease serves as a model for several inflammatory human eye diseases thought to be immune-mediated and grouped under the term ‘uveitis’.21–23 EAU is induced in mice by immunization with the retinal antigen interphotoreceptor retinoid-binding protein (IRBP). Here, we compared DAP12-deficient mice with WT controls for their susceptibility to EAU induction and for their capacity to develop cellular immunity against IRBP. Initially, DAP12-deficient animals exhibited higher susceptibility to the disease and superiority in their proliferation and pro-inflammatory cytokine profile in response to IRBP. However, after re-derivation into a second, cleaner, animal facility on the same campus, although the response of control mice was unchanged, DAP12-deficient mice were markedly hyporesponsive relative to controls. These data therefore suggest that DAP12-coupled receptors play a key role in responding to environmental factors and tuning subsequent inflammatory responses in the eye and that their activity is determined by environmental factors.
Materials and methods
Mice
DNAX-activation protein 12 deficient mice (Tyrobp−/−), described elsewhere,24 were backcrossed to C57BL/6 mice and screened by microsatellite marker analysis at Washington University before being maintained at the National Cancer Institute-Frederick. DAP12 null mice and age- and gender-matched control C57BL/6 mice, between the ages of 8 and 16 weeks, were used in this study. Mice developing ocular inflammation as a result of interleukin-1 (IL-1) expression in their lens were generated as detailed elsewhere.25 Ocular inflammation in the IL-1 transgenic mice developed spontaneously, apparently as a result of the potent pro-inflammatory activity of IL-1, expressed in these eyes. The WT littermates, used as controls, had no pathological changes in their eyes.25 The mice were housed in pathogen-free facilities and all experiments were carried out under protocols approved by the National Eye Institute, National Institutes of Health, Animal Care and Use Committee.
RNA isolation and quantitative RT-PCR
Cells were resuspended in Trizol and RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized from total RNA using Superscript III First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed using ABI Taqman Primer and Probe sets and normalization was performed against Hprt1. Data are the means of two quantitative PCR analyses of cDNA pooled from several mice.
Immunostaining of mouse eye sections
Conventional methods were used for immunostaining of frozen eye sections, to determine the expression of DAP12 and of CD11b, a marker for macrophages. The primary antibodies were rabbit anti-mouse DAP12 (AB4070, Chemicon, Temecula, CA), or rat anti-mouse CD11b (MCA711, AbD serotec, Raleigh, NC), whereas the corresponding secondary antibodies were goat anti-rabbit AlexaFluor 555 (A21428, Molecular Probes, Eugene, OR), or goat anti-rat Alexa Fluor 568 (A11077, Molecular Probes).
Induction and evaluation of EAU
Experimental autoimmune uveitis was induced in DAP12 null mice and in WT C57BL/6 controls housed within the same room of the same facility by immunization with whole bovine IRBP, at 150 μg/mouse, and IRBP 1–20 peptide, at 200 μg/mouse, emulsified in complete Freund's adjuvant. In addition, the mice were treated intraperitoneally with 0·15 μg pertussis toxin (List Biological Laboratories, Campbell, CA). Eyes were collected 14 days after immunization and examined histologically as detailed elsewhere.26 Severity of disease, on a scale of 0–4, in half-point increments, was scored as detailed elsewhere.27
Lymphocyte responses: cytokine production
Draining lymph nodes were collected 14 days after immunization and pooled within each group. Lymph node cells were cultured in 24-well plates at 5 × 106 cells/well in 1 ml RPMI-1640 medium, supplemented with 2% HL-1 serum replacement (Lonza, Walkersville, MD), antibiotics and 2-mercaptoethanol. The cultures were stimulated with whole IRBP at 10 μg/ml. Supernatants were collected following incubation for 48 hr and their cytokine levels were determined by Multiplex SearchLight technology (Pierce, Woburn, MA). Levels of interferon-γ, IL-17 and IL-10 were also determined by ELISA, using specific kits (R&D Systems, Minneapolis, MN).
Lymphocyte responses: proliferation assay
The lymph node cells were also tested for their proliferative response, as detailed elsewhere.26 Data were expressed as mean delta counts per minute.
Results
DAP12 and TREM2 participate in the pathogenic process of immune-mediated ocular inflammation
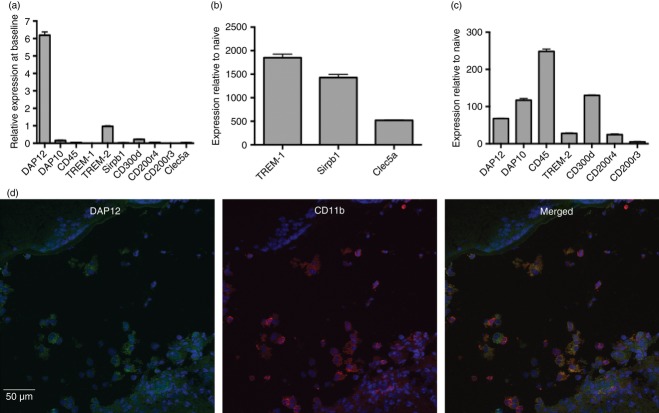
We first assessed by quantitative RT-PCR the expression of Tyrobp (DAP12), Hcst (DAP10) and several DAP12-coupled receptors in the eyes of naive mice, to evaluate which of these proteins might play a critical role in EAU. Although DAP12 and TREM-2 are considered to be relatively rare in naive C57BL/6 mouse eyes, our analysis showed that Tyrobp was abundantly expressed and that Trem2 had the highest level of expression of the panel of receptors tested (Fig. 1a). We next assessed the expression of these same receptors in the inflamed eyes of C57BL/6 mice that had been immunized with IRBP to induce EAU. The influx of leucocytes into the eye was reflected by a nearly 250-fold increase in expression of Ptprc (CD45) in inflamed eyes relative to controls (Fig. 1c). Increases in expression of the tested receptors and signalling chains in eyes with EAU are recorded in Fig. 1(b,c), as levels of each gene normalized to the level of that gene in naive eyes. The lowest normalized value was observed with Cd200r3, which increased by only about fivefold; other molecules increased more dramatically. Highest increases were recorded for Trem1 (1850·6), SIRPb1 (1427·7) and Clec5a (520·7) (Fig. 1b); lower levels were measured with Tyrobp (DAP12, 67·9), hcst (DAP10, 117·1), Trem2 (27·9), Cd300d (130·2) and Cd200r4 (24·7) (Fig. 1c). To confirm the expression of DAP12 in inflamed eyes we further tested the expression of this molecule by immunohistochemical analysis of eyes with ‘spontaneous’ inflammation due to transgenic local expression of IL-1. The ocular inflammation in the IL-1 transgenic mice develops spontaneously, apparently as a result of the potent pro-inflammatory activity of IL-1, expressed in these eyes. Wild-type C57BL/6 controls had no pathological changes in their eyes.25 As seen in Fig. 1(d), in the inflamed eye, DAP12 is expressed consistently in infiltrating cells that are also stained for CD11b, a marker for monocytes/macrophages and neutrophils.
Figure 1.
(a–c) Expression of DNAX-activation protein 12 (DAP12) and related molecules is remarkably enhanced in inflamed eyes. Total RNA from C57BL/6 naive control mice (a), or from inflamed eyes of mice with experimental autoimmune uveitis (EAU), 14 days following immunization with interphotoreceptor retinoid-binding protein (IRBP) (b and c), was assayed for DAP12, CD45 and DAP12-coupled receptors using specific TaqMan quantitative PCR probe sets. In (a), data are presented as fold of TREM2, the highest receptor we detected. In (b) and (c), levels of each gene were normalized to the level of that gene in naive eyes. All data were normalized using Hprt1 as a house-keeping gene. Data represent the mean of triplicate determinations from cDNA of pools of animals. (d) DAP12 is expressed by eye-infiltrating inflammatory cells. Immunostaining of an eye section of interleukin-1 (IL-1) transgenic mice, with severe ocular inflammaion,26 identifying DAP12 (green) in infiltrating cells in the vitreous, along with CD11b (red), a marker for monocytes/macrophages. In certain positive cells, the two molecules are expressed at different cell loci, whereas in other cells, DAP12 and CD11b are expressed at the same loci, yielding the yellow staining when merged.
Environmental factors control susceptibility to EAU in DAP12-deficient mice
Having documented an increase in DAP12 and DAP12-coupled receptor expression in inflamed eyes, we next examined the effect of DAP12 deficiency on the development of EAU by comparing the disease development in DAP12-deficient mice and in their WT controls. We immunized groups of mice of the two lines with the uveitogenic protein, IRBP, as described above, and 14 days later we collected the mouse eyes and evaluated the severity of ocular changes by histological examination.27 The tested mice were on the C57BL/6 background, a mouse strain that is only moderately susceptible to EAU induction28 and requires high doses of IRBP, as well as the additional adjuvant effect of pertussis toxin. The WT C57BL/6 mice also exhibit high variability in their capacity to develop EAU (Fig. 2a and refs. 29 and 30).
Figure 2.
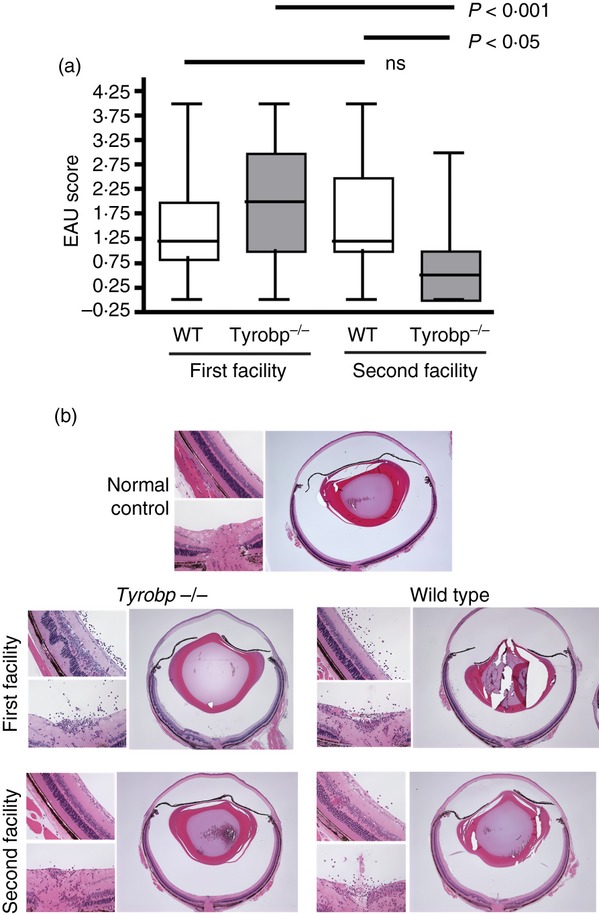
DNAX-activation protein 12 (DAP12) -deficient mice developed more severe experimental autoimmune uveitis (EAU) in the first facility compared with their wild-type (WT) controls, but less severe disease in the second facility. Groups of DAP12-deficient mice and their WT controls, five to eight mice per group, housed in either the first or second animal facility, were immunized with interphotoreceptor retinoid-binding protein (IRBP) and the development of EAU was determined by histological examination. (a) Mean EAU severity levels of DAP12-deficient (Tyrobp−/−) and WT mice, accumulated in seven different experiments in animals housed in the first facility and in five experiments in animals from the second facility. P-values represent the results of a one-way analysis of variance. (b) Representative histological sections of eyes developing EAU from DAP12-deficient mice and their matched WT controls from the two facilities. Similar changes are seen in the eyes from the control mice of the two facilities (scored at ‘2’), that mainly include foci of inflammatory cell infiltration in the retina and other ocular tissues. Even more severe changes are seen in the eye of the DAP12-deficient (Tyrobp−/−) mouse of the first facility (scored at ‘2·5’), that also include retinal detachment with folding and proteinaceous exudate in the sub-retinal space. In contrast, the eye of the DAP12-deficient mouse from the second facility shows minimal histological changes (scored at ‘0·5’), that only include small numbers of inflammatory cells infiltrating the optic nerve head and limbus. A section of an eye of a normal C57BL/6 control is also included in the figure, for comparison.
During the course of our study the DAP12-deficient mice and their WT controls were re-derived into a second animal facility. Like the first, this new facility is also pathogen free. Caging, light cycles, chow and water supplies are all the same, but the second facility is more stringently maintained, as indicated by its being free of Helicobacter infestation, whereas the original facility, like many facilities throughout the country, was Helicobacter contaminated. This situation gave us the unique opportunity to assess the phenotype of DAP12-deficient mice in two different environmental situations. Interestingly, data collected in our first seven independent experiments, performed with mice housed in the first facility, indicated that although the severity of disease varied among experiments, in six out of seven experiments, disease severity was higher in the group of DAP12-deficient mice than in their controls and when the experiments were combined, the difference between the two genotypes appeared highly significant by direct comparison (P = 0·0055, Student's t-test, compared with WT, not shown) (Fig. 2a). In contrast, in five consecutive, additional experiments, using mice reared in the newer cleaner facility, DAP12-deficient mice developed statistically less severe EAU than their WT controls (Fig. 2a). It is important to note that the response of WT animals to immunization was similar between the two facilities; only the phenotype of the DAP12-deficient mice was affected (Fig. 2a, one-way analysis of variance between all groups).
Figure 2(b) shows representative eye sections of mice of WT controls and DAP12-deficient mice from the two facilities. In both sets of experiments, the characteristic changes seen in the WT control eyes (scored at ‘2’) mainly include foci of inflammatory cell infiltration in the retina and other ocular tissues. More severe changes are seen in the eye of the DAP12-deficient mouse of the first facility (scored at ‘2·5’), that include retinal detachment with folding and proteinaceous and cellular exudates in the sub-retinal space. In contrast, the eye of the DAP12-deficient mouse from the second facility shows minimal histological changes (scored at ‘0·5’) that include small numbers of inflammatory cells infiltrating the optic nerve head and limbus.
Production of pro-inflammatory cytokines is preferentially enhanced or reduced in DAP12-deficient mice reared in the two facilities
Like the majority of other experimental autoimmune diseases, EAU is induced by the complex process of cell-mediated inflammation.22,23 This process is mediated by cytokines released by cells of the myeloid lineage, as well as by a variety of lymphoid cells, particularly T helper cells.31,32 We hypothesized, therefore, that modified patterns of change in the eyes of DAP12-deficient mice might be the result, to a large extent, to altered profiles of cytokine production during the immunopathogenic process. To examine this notion, we collected lymphoid cells from the draining lymph nodes of DAP12-deficient and control mice, 14 days following immunization with IRBP, and measured the levels of six major cytokines secreted into the culture medium after 48 hr of incubation with IRBP. Figure 3(a) shows the mean cytokine levels of repeated experiments with culture supernatants of the first and second animal facilities, using the Multiplex SearchLight technology. The data indicate opposite patterns of differences between cells of the DAP12-deficient and WT control mice of the two facilities in their production of IL-10 and IL-17, the signature anti-inflammatory and pro-inflammatory cytokines, respectively. Hence, whereas in cultures of the first facility the levels of IL-10 were lower and those of IL-17 were higher in deficient mice than in their WT controls, the opposite pattern was seen in cultures of mice from the second facility. The production pattern of tumour necrosis factor-α, another pro-inflammatory cytokine, resembled that of IL-17, but the higher level in the WT cultures was only marginally higher in mice of the second facility. No specific pattern was seen in the levels of interferon-γ, IL-4 and IL-6 in cultures of cells from the two facilities. Supernatants of cell cultures of mice from the second facility were also tested by ELISA and the data of repeated experiments are summarized in Fig. 3(b). A good correlation was found between the two assays in showing the opposite patterns of IL-10 and IL-17 production by cells of the two facilities. It is important to note that the opposite observations made with production of pro-inflammatory and anti-inflammatory cytokines are in line with our findings concerning EAU development in mice reared in the two facilities (Fig. 2).
Figure 3.
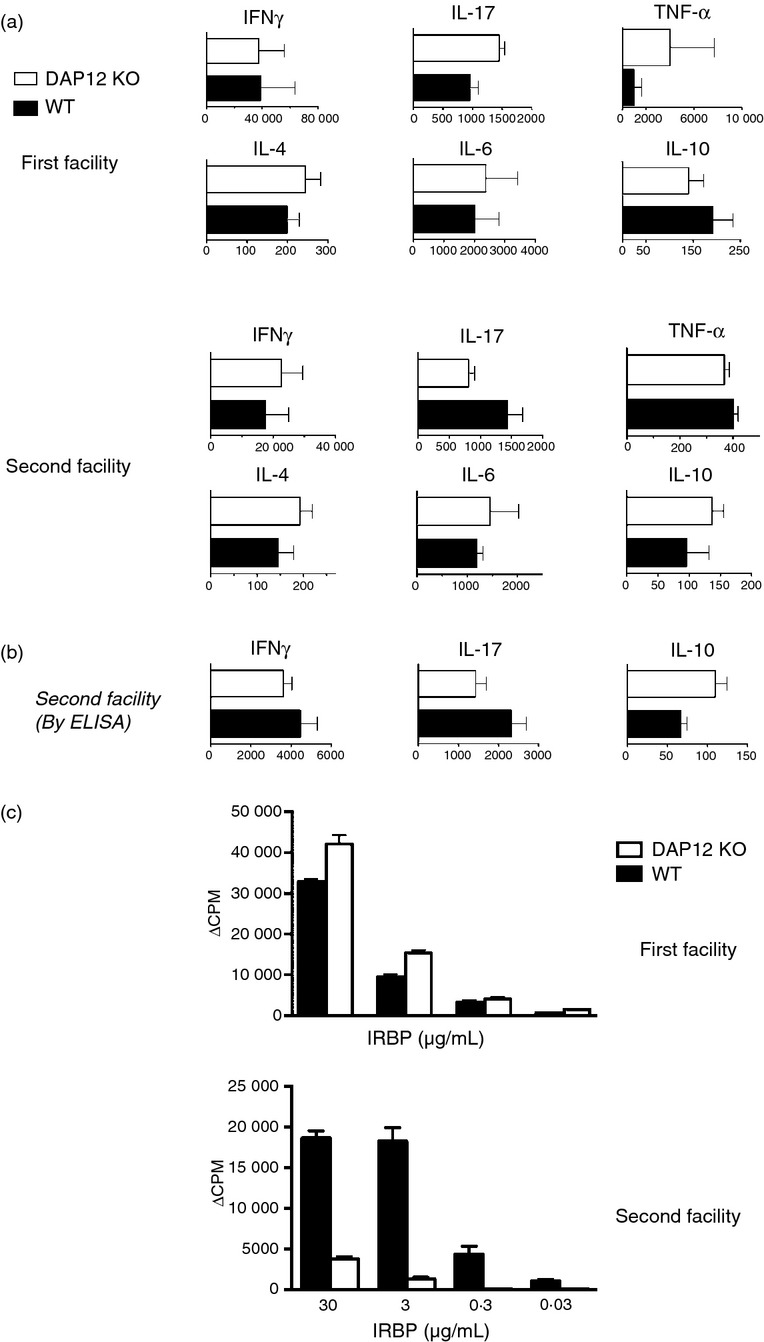
Different profiles of cellular immune responses against interphotoreceptor retinoid-binding protein (IRBP) of DNAX-activation protein 12 (DAP12) -deficient (Tyrobp−/−) mice and their wild-type (WT) controls from the two animal facilities. (a) Draining lymph node lymphocytes collected 14 days post-immunization were cultured with IRBP (10 μg/ml) for 48 hr and their supernatants were analysed for the indicated cytokines by Multiplex SearchLight technology. The data, in pg/ml, are means ± SEM of three experiments with mice of the first facility and two experiments with mice of the second facilty. (b) Cytokine levels (pg/ml) measured by ELISA. The bars present means ± SEM of four independent experiments. (c) Opposite profiles of proliferative responses against IRBP in cultures of DAP12-deficient mice and their WT controls from the two facilities. The draining lymph node lymphocytes were stimulated with IRBP at the indicated concentrations and their response measured by [3H]thymidine incorporation. The data shown are of representative experiments; similar patterns of response were observed in two additional experiments with cells of mice from each of the two facilities.
Cellular immune responses in DAP12-deficient mice from the two facilities are higher or lower than in their WT controls
As a final examination of this unexpected shift in phenotype, we compared the cellular immune response of the DAP12-deficient and control mice in the two facilities by measuring the proliferative response of their lymph node cells against IRBP. The data of a representative experiment from each facility are shown in Fig. 3(c) and demonstrate that lymphocyte responses to IRBP again mirrored the switch in EAU phenotype between the two animal facilities. This observation is again in line with the notion that DAP12-coupled receptors regulate the cellular immune response by interacting with environmental factors.
Discussion
This study analysed the involvement of the DAP12 system in the immunopathogenic process of EAU, an animal model for several uveitic conditions in humans.21–23 Analysis of DAP12 and its receptors showed low-level expression of these molecules in naive mouse eyes, presumably expressed physiologically by myeloid cells such as microglia. A striking increase in the levels of DAP12 and related molecules was observed in inflamed mouse eyes, apparently produced by the invading inflammatory cells. These findings are similar to those of Piccio et al.18 in the central nervous system of mice with experimental autoimmune encephalomyelitis. In the case of EAU, however, the dramatic increase in Clec5a and Sirpb1, even when compared with the increase in Ptprc, suggests a pronounced neutrophilic infiltrate, consistent with the Th17 involvement in this disease.30
The major observation of the present study is the dramatic differences between the immunopathogenic response of the DAP12-deficient mice of the same line, when reared and housed in the two animal facilities. Whereas the deficient mice in the first facility were superior to their WT controls in their development of EAU and immune response to IRBP, the opposite was seen when the same two lines of mice were re-derived and transferred into the second animal facility. EAU is an animal model for uveitic conditions in humans21–23 and our findings therefore suggest that DAP12-coupled receptors clearly play a role in controlling immunopathogenic processes in the eye and the activity of these molecules is determined to a large extent by the environment.
DNAX-activation protein 12 and its cofactors, expressed by dendritic cells and other myeloid cells, indirectly regulate the immune response mainly by controlling the level and type of cytokine production, as well as other activities of lymphocytes.6 The activities of DAP12 and its cofactors are affected by environment-derived Toll-like receptor ligands,6 and it is plausible, therefore, that these activities are influenced by the animal facility's environment. The DAP12 affects the immunopathogenic response by controlling processes responsible for the balance between pro-inflammatory and anti-inflammatory responses, perhaps via regulation of their interactions with environmental factors. It is of note that a direct immunosuppressive capacity of TREM2 was reported by Takahashi et al.19 who demonstrated suppressive effects of TREM2- expressing cells and the data of Hall et al.20 showing that the level of regulatory cells was reduced by DAP12 deficiency. Also of note is the study by Whittaker et al.33 showing that by altering signalling in macrophage pathways used by TREM2, Toll-like receptor-induced IL-10 is increased at the expense of IL-12p40. Lastly, Weber et al.34 recently described the expression of TREM2 in gut phagocytes where they would be in a position to regulate the host–microbiome interaction. Taken together with our data we would propose that DAP12-coupled receptors, perhaps TREM2, interact with environmental factors such as the microbiome and regulate the outcome of subsequent immune challenge.
Our findings demonstrate, for the first time, that the immunopathogenic response of DAP12-deficient mice, compared with that of WT controls, could differ dramatically in different environmental conditions. A growing body of data has recently revealed the considerable extent to which the intestinal microbiome affects the immune response.35–37 Considering the relationship between the two animal facilities, including the tight monitoring of several potential murine pathogens, we conclude that the most likely environmental variable between the two facilities is the resulting gut flora of the mice. Even genetically identical mice from different sources or housed in different rooms within a given animal facility can have significantly different microbiomes.36 As our mice were re-derived using pseudopregnant mothers for nursing while being transferred from the first facility to the second, it is conceivable that the gut floras of the mice in the two facilities were not the same. Unfortunately, our first animal facility is undergoing decontamination and renovation, making it impossible to compare the floras of mice from the two sources. More studies are needed, therefore, to examine the exact effects of the gut flora on the mode of DAP12 activity.
In addition to suggesting a new avenue for the study of DAP12-coupled receptors, our data may provide a possible explanation for the more than a decade old enigma concerning the effect of DAP12 deletion on the immunopathogenic response: whereas Bakker et al.17 reported that DAP12-deficient mice were inferior to WT controls in their capacity to develop experimental autoimmune encephalomyelitis, several other groups reported the opposite observation, of increase in immunopathogenicity in DAP12-deficient or TREM2-deficient mice.18–20 In view of our findings here we hypothesize that the difference between these studies was a result of differences in the facilities and, consequently, the gut flora in mice of the various facilities were not the same and differently affected their immune responsiveness.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Eye Institute and the Intramural Research Program of the Center for Cancer Research of the National Cancer Institute. The authors are grateful to Dr Wayne Yokoyama of Washington University, St Louis, for the gift of DAP12-deficient mice on the C57BL/6 background. We thank Dr Robert Fariss, Biological Imagining Core, NEI, for digitized microscopy and Mehak K. Aziz and Osato Ogbeifun for superb professional help. V.M, L.Q., B.P.V., K.C.B. and L.F.N. performed the experimental work and contributed to the interpretation of data and preparation of the manuscript. T.T. generated the DAP12-deficient mice. D.W.M. and I.G. designed the experiments, contributed to data interpretation and wrote the paper.
Glossary
- DAP12
DNAX-activation protein 12
- EAU
experimental autoimmune uveitis
- IRBP
interphotoreceptor retinoid-binding protein
- TREM
triggering receptor expressed in myeloid cells
- WT
wild-type
Disclosures
The authors declare no financial and commercial conflicts of interest.
References
- 1.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–7. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 2.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomasello E, Vivier E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur J Immunol. 2005;35:1670–7. doi: 10.1002/eji.200425932. [DOI] [PubMed] [Google Scholar]
- 4.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7:155–61. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 5.Hu X, Chen J, Wang L, Ivashkiv LB. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J Leukoc Biol. 2007;82:237–43. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 6.Chu CL, Yu YL, Shen KY, Lowell CA, Lanier LL, Hamerman JA. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRγ. Eur J Immunol. 2008;38:166–73. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–60. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McVicar DW, Taylor LS, Gosselin P, et al. DAP12-mediated signal transduction in natural killer cells. A dominant role for the Syk protein-tyrosine kinase. J Biol Chem. 1998;273:32934–42. doi: 10.1074/jbc.273.49.32934. [DOI] [PubMed] [Google Scholar]
- 9.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRγ and DAP12 in myeloid cells. Immunol Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–5. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 11.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–7. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 12.Gibot S, Kolopp-Sarda MN, Bene MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–26. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull IR, McDunn JE, Takai T, Townsend RR, Cobb JP, Colonna M. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med. 2005;202:363–9. doi: 10.1084/jem.20050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–57. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–5. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 16.Divangahi M, Yang T, Kugathasan K, et al. Critical negative regulation of type 1 T cell immunity and immunopathology by signaling adaptor DAP12 during intracellular infection. J Immunol. 2007;179:4015–26. doi: 10.4049/jimmunol.179.6.4015. [DOI] [PubMed] [Google Scholar]
- 17.Bakker AB, Hoek RM, Cerwenka A, et al. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–53. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 18.Piccio L, Buonsanti C, Mariani M, Cella M, Gilfillan S, Cross AH, Colonna M, Panina-Bordignon P. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2007;37:1290–301. doi: 10.1002/eji.200636837. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:e124. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall HT, Sjolin H, Brauner H, Tomasello E, Dalod M, Vivier E, Hoglund P. Increased diabetes development and decreased function of CD4+CD25+ Treg in the absence of a functional DAP12 adaptor protein. Eur J Immunol. 2008;38:3191–9. doi: 10.1002/eji.200838259. [DOI] [PubMed] [Google Scholar]
- 21.Gery I, Mochizuki M, Nussenblatt RB. Retinal specific antigens and immunopathogenic processes they provoke. Prog Retinal Res. 1986;5:75–109. [Google Scholar]
- 22.Nussenblatt RB, Gery I. Experimental autoimmune uveitis and its relationship to clinical ocular inflammatory disease. J Autoimmun. 1996;9:575–85. doi: 10.1006/jaut.1996.0077. [DOI] [PubMed] [Google Scholar]
- 23.Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23–35. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaifu T, Nakahara J, Inui M, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111:323–32. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai JC, Wawrousek EF, Sipe JD, Whitecup SM, Gery I. Reduced susceptibility to IL-1 and endotoxin in transgenic mice expressing IL-1 in their lens. Cytokine. 1996;8:288–93. doi: 10.1006/cyto.1996.0038. [DOI] [PubMed] [Google Scholar]
- 26.Takase H, Yu CR, Liu X, Fujimoto C, Gery I, Egwuagu CE. Induction of suppressors of cytokine signaling (SOCS) in the retina during experimental autoimmune uveitis (EAU): potential neuroprotective role of SOCS proteins. J Neuroimmunol. 2005;168:118–27. doi: 10.1016/j.jneuroim.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Chan CC, Caspi RR, Ni M, Leake WC, Wiggert B, Chader GJ, Nussenblatt RB. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun. 1990;3:247–55. doi: 10.1016/0896-8411(90)90144-h. [DOI] [PubMed] [Google Scholar]
- 28.Avichezer D, Silver PB, Chan CC, Wiggert B, Caspi RR. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest Ophthalmol Vis Sci. 2000;41:127–31. [PubMed] [Google Scholar]
- 29.Fujimoto C, Klinman DM, Shi G, et al. A suppressive oligodeoxynucleotide inhibits ocular inflammation. Clin Exp Immunol. 2009;156:528–34. doi: 10.1111/j.1365-2249.2009.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi R, Laron A, Weiner HL. Role of the innate immune system in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2010;221:7–14. doi: 10.1016/j.jneuroim.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–13. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 33.Whittaker GC, Orr SJ, Quigley L, Hughes L, Francischetti IM, Zhang W, McVicar DW. The linker for activation of B cells (LAB)/non-T cell activation linker (NTAL) regulates triggering receptor expressed on myeloid cells (TREM)-2 signaling and macrophage inflammatory responses independently of the linker for activation of T cells. J Biol Chem. 2010;285:2976–85. doi: 10.1074/jbc.M109.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber B, Saurer L, Schenk M, Dickgreber N, Mueller C. CX3CR1 defines functionally distinct intestinal mononuclear phagocyte subsets which maintain their respective functions during homeostatic and inflammatory conditions. Eur J Immunol. 2011;41:773–9. doi: 10.1002/eji.201040965. [DOI] [PubMed] [Google Scholar]
- 35.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–67. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–95. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]