Abstract
The gene encoding sortilin receptor 1 (SORL1) has been associated with Alzheimer’s disease risk. We examined 15 SORL1 variants and SNP-set risk scores in relation to longitudinal verbal, spatial, memory and perceptual speed performance, testing for age trends and sex-specific effects. Altogether, 1609 individuals from three population-based Swedish twin studies were assessed up to five times across 16 years. Controlling for APOE, multiple simple and sex-moderated associations were observed for spatial, episodic memory and verbal trajectories (p = 1.25E-03 to p = 4.83E-02). Five variants (rs11600875, rs753780, rs7105365, rs11820794, rs2070045) were associated across domains. Notably, in those homozygous for rs2070045 risk alleles, males demonstrated initially favorable performance but accelerating declines, while females showed overall lower performance. SNP-set risk scores predicted spatial (Card Rotations, p = 5.92E-03) and episodic memory trajectories (Thurstone Picture Memory, p = 3.34E-02), where higher risk scores benefitted men’s versus women’s performance up to age 75 but with accelerating declines. SORL1 is associated with cognitive aging, and may contribute differentially to change in men and women.
Keywords: cognitive decline, SORL1 association, sortilin receptor 1, SNP set risk scores, sex differences, aging
1. Introduction
The neuronal sorting receptor SORL1 (sortilin receptor 1) is involved in the trafficking of APP (precursor of Aβ peptides) (Rogaeva, et al., 2009) and may bind with lipoproteins and mediate endocytotic processes (Wollmer, 2010). In 2007, variants of the SORL1 gene were associated with Alzheimer’s disease (AD) risk (Rogaeva, et al., 2007). Subsequent replication efforts, though mixed, have been predominantly positive (Alexopoulos, et al., 2010,Bettens, et al., 2008,Cousin, et al., 2011,Feulner, et al., 2009,Kimura, et al., 2009,Kölsch, et al., 2009,Lee, et al., 2007,Lee, et al., 2008,Li, et al., 2008,Liu, et al., 2009,Meng, et al., 2007,Minster, et al., 2008,Patel, et al., 2010,Reitz, et al., 2011,Reynolds, et al., 2010,Schjeide, et al., 2009,Tan, et al., 2009,Webster, et al., 2008). Recently, we reported no significant association of six SORL1 markers with dementia risk, age-of-onset, CSF Aβ or tau (Reynolds, et al., 2010). However, meta-analysis of our findings with prior studies included in AlzGene (Bertram, et al., 2007) indicated a weak association for dementia risk. Likewise, a subsequent meta-analysis of all variants published to date (Reitz, et al., 2011) supports association with AD risk even when controlling for age, sex and APOE. A study of Italian AD patients reported that sex and APOE e4 status may moderate the association of SORL1 with AD risk (Cellini, et al., 2009). Although sex moderation may be a source of disparate findings, it has not received additional attention.
SORL1 has less often been examined for association with cognitive performance in non-demented adults, and to our knowledge never previously with change in cognition over time. A study of 705 stroke- and dementia-free individuals from the Framingham study reported two of seven SORL1 variants (rs1131497, rs726601) were associated with abstract verbal reasoning performance (p = 3.2 × 10−6) but not verbal memory, visuospatial memory, or attention composites (Seshadri, et al., 2007). A later study of the 1936 Lothian Birth Cohort reported that one of two SORL1 variants (rs3824968) was associated with a spatial span task (p = 0.029) (Houlihan, et al., 2009). Sex was statistically controlled for or residualized in these studies and not directly considered as an effect moderator.
In the current report we examine associations of SORL1 variants with cognitive decline trajectories, especially considering sex-specific trends. We note that this study, with up to 16 years of follow-up, is the first to report findings of SORL1 on longitudinal cognitive performance across multiple domains in a population-based sample.
2. Methods and Materials
2.1 Participants
Participants were drawn from three similarly-designed population-based studies originating from the Swedish Twin Registry (Lichtenstein, et al., 2002): the Swedish Adoption/Twin Study of Aging (SATSA) (Finkel and Pedersen, 2004,Pedersen, et al., 1991), the Origins of Variance in the Oldest-Old (OCTO-Twin) (McClearn, et al., 1997), and the Sex Differences in Health and Aging Study (GENDER) (Gold, et al., 2002). Across these studies, SORL1 genotypes, APOE genotype, covariates and cognitive outcomes were available for 1,609 individuals (680 males, 929 females) dementia-free at baseline, representing 919 pairs (690 complete, 229 incomplete). Eighty-three MZ twins without genotyping (17.0%) were assigned their cotwin’s SORL1 genotypes. Incident dementia was evaluated (Gatz, et al., 2005) and any cognitive data obtained post-onset were excluded from the current analyses. The median age at initial testing was 72.3 years (interquartile range = 15.9, range= 50.1 to 93.0) and the median follow-up time was 7.8 years (interquartile range = 6.0, range = 0 to 17.8).
The study was conducted with approvals from the Swedish Regional Ethical Review Board at the Karolinska Institute, and from the relevant local Institutional Review Boards of the investigators’ institutions.
2.2 Genotyping
All DNA samples underwent whole genome amplification (WGA) using standard kits (Phi29 DNA polymerase, Amersham). All subsequent genotyping was conducted at the Uppsala University SNP technology platform (http://www.medsci.uu.se/molmed/snpgenotyping/methods.htm). We previously reported high concordance (> 98%) for three ABCA1 genotypes obtained from these same amplified and non-amplified samples under different genotyping platforms (Reynolds, et al., 2009). These results are comparable to studies on the fidelity of WGA with respect to SNP genotyping published by other groups (Tzvetkov, et al., 2005,Xing, et al., 2008). Initially, seven SORL1 markers were typed using the Illumina GoldenGate assay system on Illumina BeadStation 500GX. Markers were selected based upon prior association findings, functional candidature, linkage disequilibrium (LD), and Illumina SNP design scores (Rogaeva, et al., 2007): rs668387, rs689021, rs641120, rs2070045, rs1699102, rs3824968, and rs2282649. SNP rs1699102 was excluded from analysis due to genotyping failure. Additional SORL1 markers were obtained from a custom designed Illumina iSelect array (Metabochip) with an analysis set of 184,189 SNPs. Additional markers were imputed using IMPUTE (Marchini, et al., 2007), retaining those with > 0.9 posterior probability estimates and missingness of ≤ 0.1, resulting in 528,184 total SNPs. Altogether, 49 markers in the SORL1 region were available (chr11: 120,828,171-121,009,681; NCBI36/hg18 build). Of these, 24 markers had minor allele frequencies > 1%. After accounting for perfect LD (i.e. perfect proxies with r2 = 1), including two previously typed markers, 10 markers remained: rs11600875, rs4631890, rs923893, rs17245976, rs12364988, rs753780, rs7105365, rs12285364, rs11512475, and rs11820794.
Hardy-Weinberg equilibrium (HWE) tests of all SORL1 SNPs were performed (Table S1). We dropped rs4631890, which did not achieve HWE (p = 0.0247). Thus, there were a total of 15 SORL1 makers, 6 from the first and 9 from the second genotyping.
APOE markers (rs7412, rs429358) were genotyped separately using Illumina GoldenGate assays and were in HWE (p > 0.07).
2.3 Cognitive measures
The primary cognitive measures for the present analysis included: (a) Synonyms, representing the verbal domain; (b) Block Design, representing the spatial domain; (c) Thurstone Picture Memory, a test of episodic recognition memory; and (d) Symbol Digit, a measure of perceptual speed. Models were fitted to the four primary cognitive tests first. Additional cognitive tests were available for only two of the three studies and were followed up in analysis if a primary measure of a cognitive domain indicated possible association. These included: (a) verbal, Information (SATSA & OCTO-Twin); (b) spatial, Figure Logic (SATSA & OCTO-Twin) and Card Rotations (GENDER & SATSA); and (c) memory, Digit Span (SATSA & OCTO-Twin). All tests were rescaled to percent correct for comparability. Descriptive statistics are presented in Table 1.
Table 1.
Descriptive statistics for cognitive test scores scaled in percent correct (%) units.
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Test | Npersons× occasions |
M | SD | Npersons× occasions |
M | SD |
| SynonymsP | 1860 | 61.78 | 22.13 | 2620 | 61.23 | 20.13 |
| Information | 1484 | 75.84 | 19.53 | 2404 | 65.54 | 23.08 |
| Block DesignP | 1900 | 41.45 | 19.14 | 2726 | 39.27 | 17.98 |
| Figure Logic | 1389 | 58.97 | 15.84 | 2185 | 55.61 | 15.34 |
| Card Rotations | 1306 | 46.77 | 18.44 | 1607 | 37.96 | 16.89 |
| Thurstone Picture | ||||||
| MemoryP | 1779 | 70.57 | 18.03 | 2505 | 73.98 | 16.96 |
| Digit Span | 1471 | 55.52 | 13.32 | 2411 | 54.10 | 12.98 |
| Symbol DigitP | 1700 | 33.69 | 12.87 | 2417 | 33.23 | 12.61 |
Note. Means and standard deviations are computed across measurement waves.
Primary cognitive test, available in all three studies.
2.4 Analyses
Multilevel growth models were fitted to the cognitive data using SAS PROC MIXED 9.3 (SAS Institute, Inc., Raleigh, NC) with full information maximum likelihood estimation (Reynolds, et al., 2005). The model parameters included the expected cognitive performance level at 75 years (i.e., intercept, I), the linear slope at age 75 years (S) reflecting the ‘tilt’ of the curve at age 75, and the quadratic trend across age (Q), representing accelerating change. Participant ages were divided by five so that a 1-unit difference would reflect 5 elapsed years. Estimation included the average trajectory and variation in trajectories, respectively, by allowing for fixed and random effects for I, S, and Q. Between- and within-pair random effects were estimated to account for dependencies.
A baseline model (M1) was fitted with the covariates sex (−0.5 = male, +0.5 = female), study cohort (GENDER, OCTO-Twin, and SATSA as the referent), APOE genotype (e22, e23, e24 = −1; e33 = 0; e34, e44 = 1), educational attainment, and an illness sum score (cf. Reynolds, et al., 2011); hypertension, diabetes, angina, myocardial infarction, congestive heart failure, stroke, Parkinson disease, rheumatoid arthritis, emphysema and bronchitis) as predictors of I, S, and Q parameters. Lastly, we captured potential retest effects given exposure to cognitive testing (baseline = 0, other waves = 1). In the next model (M2), each SORL1 SNP was entered as a predictor of the set of I, S, and Q parameters, where the growth parameters for each of two genotypes were compared to those of a third referent genotype (e.g., Q C/C vs QT/T, QC/T vs QT/T). The last model (M3) included interaction terms of the SORL1 marker and sex as predictors of trajectory parameters. Model comparisons were conducted by calculating difference chi-square tests to evaluate (1) the simple association of the SNP with the I, S, and Q parameters (M1 vs M2; df = 6), (2) the addition of sex by SNP interaction terms to predict I, S, and Q (M2 vs M3; df=6), and (3) full association of the SNP plus sex by SNP interaction terms (M1 vs M3; df=12). For markers with minor allele frequencies (MAF) less than 10%, we collapsed the rare homozygote with the heterozygote to allow for sufficient data to test for sex moderation; thus, the degrees-of-freedom for model comparisons were halved (3 df, 3df, and 6 df, respectively).
We evaluated the overall contribution of SORL1 by estimating genotype risk scores, adapting a nested random effects pathway approach (Houwing-Duistermaat, et al., 2011,Tsonaka, et al., 2011). For purposes of this analysis, we limited the SNPs to 11 out of the 15 that tagged the SORL1 gene with r2 ≥ 0.89. SNPs were recoded to reflect the number of ‘risk’ alleles contributed (see Table S1). The SNP sets evaluated were based on the LD structure observed in Haploview 4.2 (Barrett, 2009,Barrett, et al., 2005) (Figure S1). We evaluated the number of SNP sets to be estimated from the 11 tag SNPs using the ‘lme’ package (Bates, et al., 2011) in R (R Development Core Team, 2011), fitting a multilevel logistic regression model, using data from one MZ and both DZ twins where available (N= 1,462). The nested random effects logistic model accounted for nesting of SNPs, individuals, and pairs (Houwing-Duistermaat et al., 2011;Tsonaka et al., 2012). The empirical Bayes estimates of the SNP risk sets generated from the best-fitting model were used as predictors of cognitive trajectories in SAS PROC MIXED, with evaluations similar to models M1-M3 above but where the risk set scores were treated as continuous. Higher risk score values are indicative of carrying a greater number of ‘risk’ alleles (cf. Houwing-Duistermaat, et al., 2011,Tsonaka, et al., 2011).
3. Results
Significant model comparisons (testing SNP, Sex*SNP, or Full association) were observed across the 15 SORL1 SNPs for three of the four primary cognitive tests: Synonyms, Thurstone Picture Memory, and Block Design (p = 6.13E-03 to p = 4.51E-02; Table 2). Model comparisons were not significant for Symbol Digit (not shown). For the additional cognitive tests, significant comparisons were observed for Card Rotations and Figure Logic (p = 1.25E-03 to p = 4.83E-02; Table 2), whereas significance was not observed for Information or Digit Span (not shown). Altogether, 35 of 360 model comparisons (9.7%) were significant, with 17 of these observed for Card Rotations alone (37.8%; see Table 2). Patterns observed in tests of single markers were: (1) markers towards the 5’ region tended to be associated with verbal trajectories while markers towards the 3’ end tended to be associated with episodic memory trajectories (Table 2); (2) spatial trajectories were associated with markers both towards the 5’ region and the 3’ end (Table 2); and (3) sex moderation was evident particularly for markers towards the 3’ end (Table 2, Figures 1–2).
Table 2.
SORL1 marker associations with cognitive trajectories.
| Association tests | |||||||
|---|---|---|---|---|---|---|---|
| Cognitive Testa |
SNP (*3df; 6df) |
Sex × SNP (*3df; 6df) |
Full (*6df; 12df) |
||||
| SNP | Δχ2 | p | Δχ2 | p | Δχ2 | p | |
| Synonymsp | rs11600875* | 12.40 | 6.13E-03 | 1.40 | 7.05E-01 | 13.80 | 3.20E-02 |
| rs923893i | 7.06 | 3.16E-01 | 4.65 | 5.89E-01 | 11.71 | 4.69E-01 | |
| rs17245976*i | 12.35 | 6.27E-03 | 1.73 | 6.31E-01 | 14.08 | 2.88E-02 | |
| rs12364988 | 7.38 | 2.87E-01 | 4.79 | 5.71E-01 | 12.17 | 4.32E-01 | |
| rs668387 | 4.33 | 6.32E-01 | 4.82 | 5.67E-01 | 9.15 | 6.90E-01 | |
| rs753780* | 7.76 | 5.13E-02 | 5.60 | 1.33E-01 | 13.36 | 3.77E-02 | |
| rs689021 | 5.72 | 4.56E-01 | 3.88 | 6.93E-01 | 9.60 | 6.51E-01 | |
| rs7105365i | 4.38 | 6.25E-01 | 6.20 | 4.02E-01 | 10.58 | 5.65E-01 | |
| rs641120 | 4.25 | 6.43E-01 | 4.11 | 6.62E-01 | 8.35 | 7.57E-01 | |
| rs12285364* | 1.65 | 6.48E-01 | 3.69 | 2.98E-01 | 5.34 | 5.01E-01 | |
| rs11512475* | 2.86 | 4.15E-01 | 1.70 | 6.37E-01 | 4.56 | 6.02E-01 | |
| rs11820794*i | 2.51 | 4.73E-01 | 6.90 | 7.51E-02 | 9.41 | 1.52E-01 | |
| rs2070045 | 4.65 | 5.90E-01 | 13.60 | 3.45E-02 | 18.25 | 1.08E-01 | |
| rs3824968 | 9.77 | 1.35E-01 | 7.60 | 2.69E-01 | 17.37 | 1.36E-01 | |
| rs2282649 | 8.01 | 2.37E-01 | 8.70 | 1.91E-01 | 16.71 | 1.61E-01 | |
| Block Designp |
rs11600875* | 6.02 | 1.11E-01 | 5.11 | 1.64E-01 | 11.13 | 8.45E-02 |
| rs923893i | 6.36 | 3.84E-01 | 5.08 | 5.34E-01 | 11.44 | 4.92E-01 | |
| rs17245976*i | 4.52 | 2.11E-01 | 4.97 | 1.74E-01 | 9.49 | 1.48E-01 | |
| rs12364988 | 5.84 | 4.42E-01 | 5.59 | 4.71E-01 | 11.43 | 4.93E-01 | |
| rs668387 | 3.87 | 6.95E-01 | 11.25 | 8.08E-02 | 15.12 | 2.35E-01 | |
| rs753780* | 3.37 | 3.38E-01 | 9.87 | 1.97E-02 | 13.24 | 3.94E-02 | |
| rs689021 | 5.55 | 4.75E-01 | 9.01 | 1.73E-01 | 14.56 | 2.66E-01 | |
| rs7105365i | 5.67 | 4.61E-01 | 1.77 | 9.40E-01 | 7.44 | 8.27E-01 | |
| rs641120 | 4.81 | 5.68E-01 | 8.90 | 1.80E-01 | 13.71 | 3.20E-01 | |
| rs12285364* | 3.00 | 3.91E-01 | 0.36 | 9.48E-01 | 3.36 | 7.62E-01 | |
| rs11512475* | 5.02 | 1.71E-01 | 0.88 | 8.30E-01 | 5.90 | 4.35E-01 | |
| rs11820794*i | 1.98 | 5.77E-01 | 3.17 | 3.66E-01 | 5.15 | 5.25E-01 | |
| rs2070045 | 7.06 | 3.15E-01 | 14.76 | 2.22E-02 | 21.82 | 3.96E-02 | |
| rs3824968 | 13.01 | 4.29E-02 | 8.37 | 2.12E-01 | 21.38 | 4.51E-02 | |
| rs2282649 | 9.56 | 1.45E-01 | 6.51 | 3.68E-01 | 16.07 | 1.88E-01 | |
| Card Rotations |
rs11600875* | 8.25 | 4.12E-02 | 1.67 | 6.44E-01 | 9.92 | 1.28E-01 |
| rs923893i | 20.10 | 2.65E-03 | 3.75 | 7.11E-01 | 23.85 | 2.13E-02 | |
| rs17245976*i | 5.07 | 1.67E-01 | 2.20 | 5.31E-01 | 7.28 | 2.96E-01 | |
| rs12364988 | 16.82 | 9.98E-03 | 4.41 | 6.21E-01 | 21.23 | 4.71E-02 | |
| rs668387 | 11.19 | 8.28E-02 | 8.85 | 1.82E-01 | 20.04 | 6.63E-02 | |
| rs753780* | 6.28 | 9.87E-02 | 2.54 | 4.67E-01 | 8.83 | 1.84E-01 | |
| rs689021 | 12.68 | 4.83E-02 | 7.66 | 2.64E-01 | 20.34 | 6.09E-02 | |
| rs7105365i | 16.00 | 1.37E-02 | 3.43 | 7.53E-01 | 19.44 | 7.85E-02 | |
| rs641120 | 12.85 | 4.55E-02 | 7.28 | 2.95E-01 | 20.13 | 6.46E-02 | |
| rs12285364* | 3.07 | 3.81E-01 | 8.49 | 3.69E-02 | 11.56 | 7.26E-02 | |
| rs11512475* | 1.19 | 7.54E-01 | 2.03 | 5.66E-01 | 3.22 | 7.80E-01 | |
| rs11820794*i | 15.79 | 1.25E-03 | 2.48 | 4.79E-01 | 18.27 | 5.59E-03 | |
| rs2070045 | 5.68 | 4.61E-01 | 17.40 | 7.92E-03 | 23.08 | 2.71E-02 | |
| rs3824968 | 10.34 | 1.11E-01 | 15.61 | 1.60E-02 | 25.95 | 1.09E-02 | |
| rs2282649 | 8.50 | 2.03E-01 | 18.24 | 5.67E-03 | 26.74 | 8.42E-03 | |
| Figure Logic |
rs11600875* | 6.02 | 1.11E-01 | 5.37 | 1.47E-01 | 11.39 | 7.72E-02 |
| rs923893i | 5.88 | 4.37E-01 | 10.18 | 1.17E-01 | 16.06 | 1.89E-01 | |
| rs17245976*i | 5.96 | 1.14E-01 | 6.18 | 1.03E-01 | 12.14 | 5.89E-02 | |
| rs12364988 | 6.68 | 3.52E-01 | 10.80 | 9.47E-02 | 17.48 | 1.32E-01 | |
| rs668387 | 3.24 | 7.78E-01 | 8.77 | 1.87E-01 | 12.00 | 4.45E-01 | |
| rs753780* | 5.92 | 1.15E-01 | 10.93 | 1.21E-02 | 16.86 | 9.83E-03 | |
| rs689021 | 3.52 | 7.42E-01 | 8.02 | 2.37E-01 | 11.54 | 4.83E-01 | |
| rs7105365i | 9.44 | 1.50E-01 | 9.80 | 1.33E-01 | 19.24 | 8.29E-02 | |
| rs641120 | 3.54 | 7.39E-01 | 7.44 | 2.82E-01 | 10.97 | 5.31E-01 | |
| rs12285364* | 0.45 | 9.29E-01 | 3.54 | 3.15E-01 | 4.00 | 6.77E-01 | |
| rs11512475* | 0.89 | 8.27E-01 | 1.57 | 6.66E-01 | 2.47 | 8.72E-01 | |
| rs11820794*i | 2.62 | 4.53E-01 | 6.57 | 8.69E-02 | 9.20 | 1.63E-01 | |
| rs2070045 | 8.30 | 2.17E-01 | 3.60 | 7.31E-01 | 11.90 | 4.54E-01 | |
| rs3824968 | 7.39 | 2.86E-01 | 6.75 | 3.45E-01 | 14.14 | 2.92E-01 | |
| rs2282649 | 9.54 | 1.45E-01 | 5.06 | 5.36E-01 | 14.60 | 2.64E-01 | |
| Thurstone Memoryp |
rs11600875* | 2.33 | 5.07E-01 | 3.85 | 2.78E-01 | 6.18 | 4.04E-01 |
| rs923893i | 9.27 | 1.59E-01 | 5.01 | 5.43E-01 | 14.28 | 2.83E-01 | |
| rs17245976*i | 1.31 | 7.28E-01 | 2.37 | 4.99E-01 | 3.68 | 7.20E-01 | |
| rs12364988 | 11.28 | 8.02E-02 | 7.84 | 2.50E-01 | 19.12 | 8.57E-02 | |
| rs668387 | 5.09 | 5.32E-01 | 9.00 | 1.74E-01 | 14.09 | 2.95E-01 | |
| rs753780* | 1.71 | 6.35E-01 | 2.11 | 5.49E-01 | 3.82 | 7.00E-01 | |
| rs689021 | 6.16 | 4.06E-01 | 9.35 | 1.55E-01 | 15.51 | 2.15E-01 | |
| rs7105365i | 15.12 | 1.94E-02 | 10.48 | 1.06E-01 | 25.59 | 1.22E-02 | |
| rs641120 | 5.82 | 4.43E-01 | 8.23 | 2.22E-01 | 14.05 | 2.98E-01 | |
| rs12285364* | 4.90 | 1.79E-01 | 4.89 | 1.80E-01 | 9.79 | 1.34E-01 | |
| rs11512475* | 4.37 | 2.24E-01 | 3.87 | 2.76E-01 | 8.24 | 2.21E-01 | |
| rs11820794*i | 0.05 | 9.97E-01 | 8.57 | 3.56E-02 | 8.62 | 1.96E-01 | |
| rs2070045 | 7.81 | 2.52E-01 | 13.07 | 4.19E-02 | 20.88 | 5.21E-02 | |
| rs3824968 | 9.10 | 1.68E-01 | 7.78 | 2.54E-01 | 16.88 | 1.54E-01 | |
| rs2282649 | 7.70 | 2.61E-01 | 7.28 | 2.96E-01 | 14.97 | 2.43E-01 | |
No associations were observed for Information, Digit Span or Symbol Digit (not shown).
Imputed SNP.
MAF < 10%: Rare homozygotes were combined with heterozygotes and the degrees of freedom (df) adjusted.
Primary cognitive test, available in all three studies.
Table 3.
Three SORL1 risk set scores and cognitive trajectories: full model parameter estimates.
| Card Rotations | B | LL | UL | p | B.sex | LL | UL | p | |
|---|---|---|---|---|---|---|---|---|---|
| I75 | 34.09 | 32.31 | 35.87 | *** | −6.97 | −8.82 | −5.12 | *** | |
| risk1 | −4.42 | −16.97 | 8.13 | −0.42 | −25.46 | 24.63 | |||
| risk2 | 16.19 | −3.70 | 36.09 | 7.86 | −30.05 | 45.77 | |||
| risk3 | 0.91 | −5.42 | 7.24 | −13.92 | −26.66 | −1.19 | * | ||
| S75 | −4.97 | −5.64 | −4.29 | *** | 0.84 | −0.14 | 1.82 | t | |
| risk1 | −9.92 | −16.82 | −3.03 | ** | 3.77 | −9.99 | 17.53 | ||
| risk2 | −8.16 | −18.10 | 1.78 | 25.67 | 6.17 | 45.17 | ** | ||
| risk3 | −1.33 | −4.73 | 2.07 | −0.83 | −7.65 | 6.00 | |||
| Q | −0.12 | −0.32 | 0.07 | 0.11 | −0.18 | 0.39 | |||
| risk1 | −1.91 | −3.88 | 0.06 | t | 0.19 | −3.76 | 4.13 | ||
| risk2 | −2.11 | −5.00 | 0.78 | 0.06 | −5.66 | 5.78 | |||
| risk3 | −0.81 | −1.79 | 0.17 | 0.72 | −1.24 | 2.68 | |||
|
Thurstone Picture Memory |
B | LL | UL | P | B.sex | LL | UL | P | |
| I75 | 70.66 | 68.97 | 72.36 | *** | 5.11 | 3.49 | 6.74 | *** | |
| risk1 | −5.75 | −16.69 | 5.19 | 1.69 | −20.10 | 23.48 | |||
| risk2 | −1.08 | −18.79 | 16.63 | 4.02 | −29.50 | 37.54 | |||
| risk3 | 1.20 | −4.38 | 6.79 | 2.60 | −8.60 | 13.80 | |||
| S75 | −2.91 | −3.66 | −2.17 | *** | −0.24 | −1.04 | 0.57 | ||
| risk1 | −1.63 | −6.96 | 3.69 | −5.71 | −16.30 | 4.88 | |||
| risk2 | −1.43 | −9.61 | 6.75 | 21.97 | 5.73 | 38.20 | ** | ||
| risk3 | 1.31 | −1.46 | 4.09 | −3.65 | −9.16 | 1.86 | |||
| Q | −0.44 | −0.64 | −0.23 | *** | −0.10 | −0.34 | 0.14 | ||
| risk1 | 0.55 | −1.08 | 2.18 | −3.48 | −6.74 | −0.22 | * | ||
| risk2 | −1.26 | −3.74 | 1.21 | 2.51 | −2.40 | 7.42 | |||
| risk3 | 0.14 | −0.69 | 0.97 | −2.32 | −3.98 | −0.65 | ** | ||
Note. I= intercept, or performance level at age 75 years; S=linear slope at age 75 years; Q=quadratic trend over age; risk1 – risk3 = risk set scores estimated from mixed effects logistic regression model. The cognitive performance outcomes were additionally adjusted for study, retest, APOE genotype, education level, and baseline illness sum (hypertension, diabetes, angina, myocardial infarction, congestive heart failure, stroke, Parkinson disease, rheumatoid arthritis, emphysema and bronchitis.)
p < .10
p < .05
p < .01
p<.001
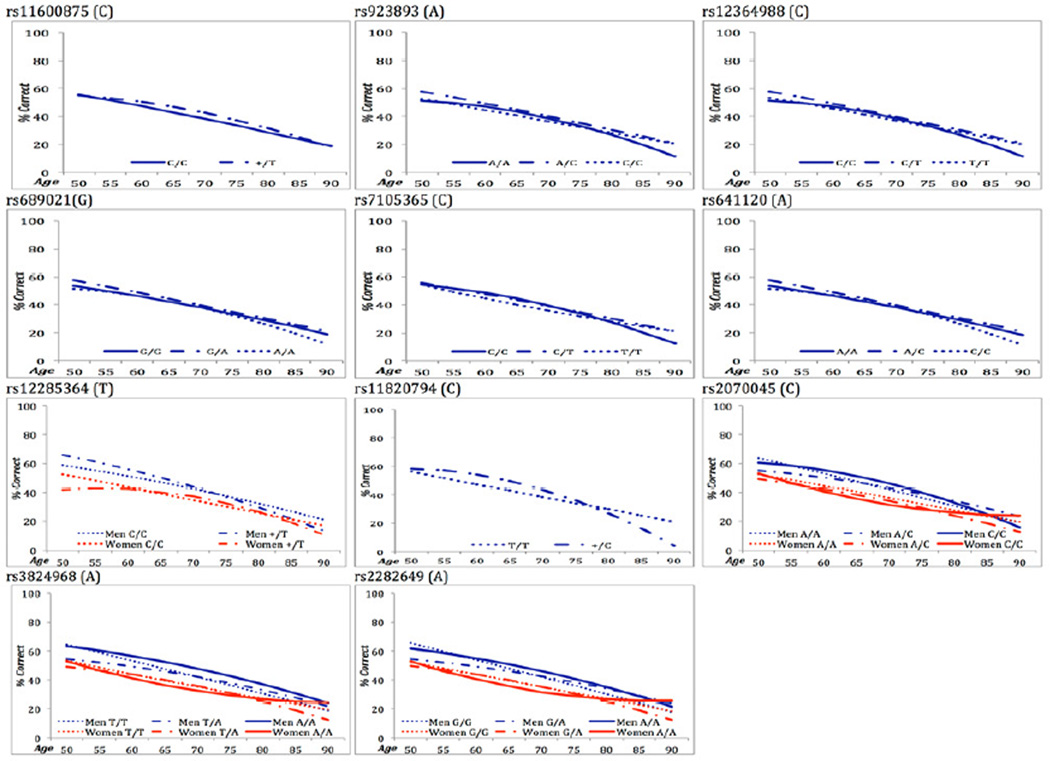
Figure 1.
SNP variant effects on trajectory patterns for Card Rotations.
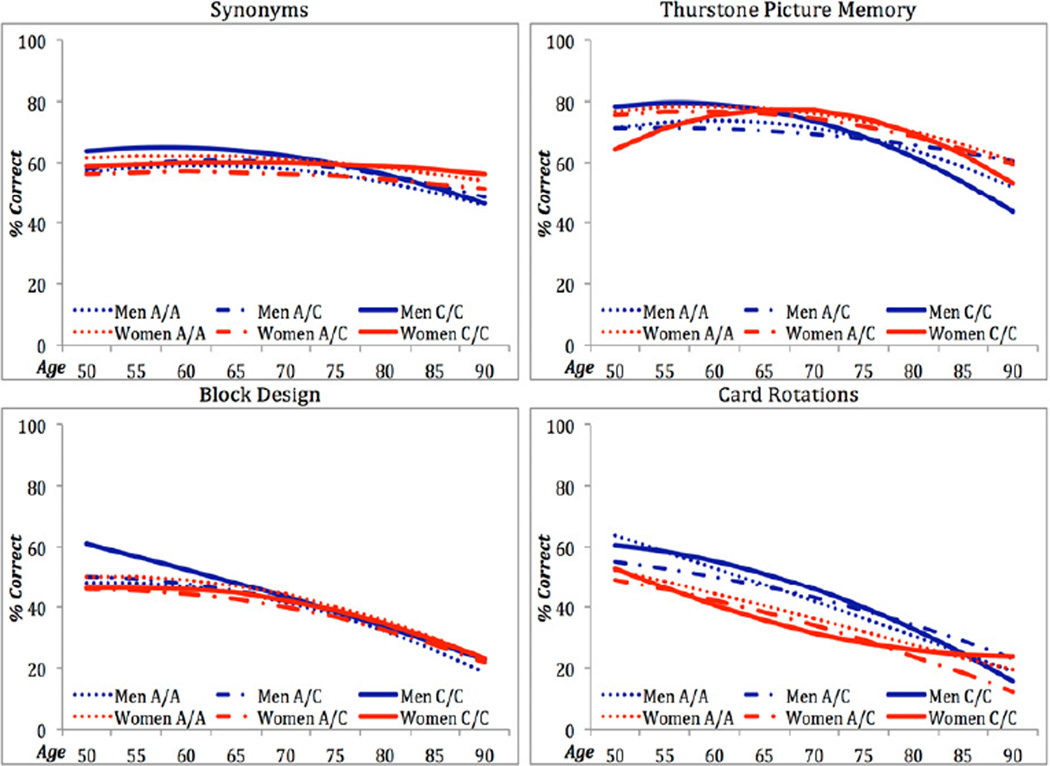
Figure 2.
Trajectory patterns for rs2070045 across cognitive domains.
Model comparisons for SNP variants rs11600875, rs753780, rs7105365, rs11820794, rs2070045 were significant across multiple cognitive domains (Tables 2, S3). The majority of significant effects on particular growth parameters were on the change parameters, i.e., S and Q (Table S3). For markers rs11600875 (men and women) and rs753780 (women), common homozygous C/C individuals showed earlier performance decrements in tests of verbal (Synonyms) and spatial abilities (Card Rotations, or Block Design & Figure Logic, respectively) than T carriers, who showed later decrements such that their advantage diminished in late life (Tables S3-S4). For markers rs7105365 and rs11820794, carrying the C risk allele was initially advantageous to spatial (Card Rotations) and memory performance (Thurstone Picture Memory) but detrimental in later life due to accelerating declines (cf. Table S3, Figure 1). Lastly, marker rs2070045 showed association across four tests from three cognitive domains: verbal (Synonyms), spatial (Block Design, Card Rotations) and memory (Thurstone Picture Memory). Specifically, males homozygous for the rare allele (C/C) initially showed a performance advantage over A/A and A/C males, but their advantage diminished with faster rates of decline; C/C and A/C females tended to show lower performance across age compared to A/A females, although C/C performance plateaus after age 75 (Figure 2; Tables S3-S4). Altogether for these 5 markers, the largest absolute effect size differences among genotypes for change in performance were observed for Card Rotations (M = |0.39|) and Thurstone Picture Memory (M = |0.61|) (Table S4) (Feingold, 2009).
We examined the summative effect of SORl1 SNPs by considering the association of empirical Bayes estimates of SORL1 risk scores for Card Rotations and Thurstone Picture Memory. The best-fitting model according to AIC and BIC fit criteria suggested that three risk sets best fitted the 11 tag markers (Table S2). The three risk set scores were entered simultaneously in growth models for Card Rotations, and sex moderation and full association were evaluated via model comparisons. Collectively, the risk set scores demonstrated significant sex-moderation [Δχ2(9)=21.24, p =1.16E-02] and full association [Δχ2 (18)=36.59, p =5.92E-03]. A similar result followed for Thurstone Picture Memory for sex-moderation [Δχ2 (9)=18.15, p = 3.34E-02], while the full association test was not significant [Δχ2(18)=25.84, p = 1.03E-01], consistent with single marker analyses. Final model results suggest that an accumulating number of SORL1 risk alleles across the three risk sets are relatively advantageous to men’s versus women’s longitudinal performance up to age 75, but with accelerating declines (Table 3).
4. Discussion
Our findings support the conclusion that SORL1 is related to cognitive change trajectories across multiple domains and furthermore may contribute differentially to change in men and women. Several SORL1 SNPs were associated with cognitive trajectories in adults aged 50 to 93 with up to 16 years of follow-up, primarily in the spatial domain but also episodic memory and verbal abilities. Five SNPs were significant across more than one cognitive domain (rs11600875, rs753780, rs7105365, rs11820794, and rs2070045). Eight additional SNPs were significant but specific to single cognitive tests or domains. Lastly, the analysis of three SNP-set risk scores suggests unique and cumulative effects of SNPs spanning SORL1.
In this longitudinal study with a larger sample that included more SORL1 SNPs, we found greater evidence of association compared to two previous cross sectional studies of cognitive performance (Houlihan, et al., 2009,Seshadri, et al., 2007). The results for the intercept, i.e. performance at age 75, are most comparable to those studies. Markers rs3824968 and rs2282649 represent the marker or a nearby marker associated previously with spatial span (Houlihan, et al., 2009) or a verbal reasoning performance (WAIS Similarities) (Seshadri, et al., 2007), respectively. In our study, the rare alleles for these markers were associated with better Card Rotations (spatial) performance among men and relatively poorer performance among women. The current study offers important insights on the impact of SORL1 on age-related change in cognitive abilities, not simply performance differences: SNP associations were observed twice as often with cognitive change than with performance level (cf. Tables S3, S5).
Our results further suggest that sex moderation of SORL1 may be important, although rarely considered. A female-specific effect of SORL1 markers and AD risk has been reported (Cellini, et al., 2009), particularly rs661057, not typed in the current study, and weaker effects for rs12364988 and rs641120. Limited sex moderation of a marker between rs12364988 and rs641120 was observed in the current study, i.e., rs753780. Sex moderation was most evident for rs2070045, with similar patterns for downstream markers rs3824968 and rs2282649. Possible protective effects of the rs2070045 rare homozygote were observed for men’s verbal, episodic memory and spatial performance at least before age 75, but detrimental effects for women’s performance. A recent study reported that a SORL1 haplotype in the 3' gene region, formed from rs1699102 (unavailable to the current study) and rs2070045, alters transcription of SORL1 and results in differential efficiency of receptor expression in the brains of AD patients (Caglayan, et al., 2012). While sex moderation was not evaluated, it may be important to note that 67% of the autopsied brains were from female AD patients and that the correlation between genotype and expression reportedly increased when statistically adjusting for main effects of sex, age and APOE genotype (Caglayan, et al., 2012).
Our findings for variant rs11600875 and nearby markers with verbal and spatial trajectories are particularly interesting given recent findings of the rs11600875 association with exon2-skipping in human frontal cortex (McCarthy, et al., 2010). Carriers of the rarer rs11600875 allele (+T) were observed to have twice the delta-2 SORL1-mRNA levels in frontal cortex than common homozygotes. A similar but non-significant pattern was observed in hippocampal tissue. Other 5’ variants, not typed in the current study, were associated with temporal cortex mRNA levels but not rs11600875. Moreover, mRNA expression in the frontal and temporal cortices was uncorrelated. Thus, different SORL1 variants are likely to be important to region-specific SORL1 expression (McCarthy, et al., 2010).
Our findings are broadly consistent with findings of MRI measured cerebrovascular and neuropathological changes (Bralten, et al., 2011,Cuenco, et al., 2008). White matter hyperintensities were associated with markers towards the 5’ region (Cuenco, et al., 2008), while markers towards the 3’ region were associated with cerebral (Cuenco, et al., 2008) and hippocampal atrophy (Bralten, et al., 2011,Cuenco, et al., 2008). We observed that SORL1 variants differentially overlap in their associations across cognitive domains, i.e. markers towards 5’ with verbal and spatial processing and markers toward 3’ with episodic memory and spatial processing.
Limitations of the current study include multiple testing concerns. Considering the LD structure among SNPs would suggest significance thresholds of 6.39E-03 (Li and Ji, 2005). In this case five SNPs would survive testing (Synonyms: rs11600875, rs17245976; Card Rotations: rs923893, rs11820794, rs2282649). We note that the findings of the SNP set analyses, which evaluated SORL1 contributions across the region genotyped, support unique and cumulative effects of SNPs spanning SORL1.
In summary, using a large sample with longitudinal data assessed on multiple occasions spanning more than 16 years, we observed associations of SORL1 variants with verbal, spatial and episodic memory trajectories. Moreover, we extended earlier findings reporting sex moderation of SORL1 and AD risk, with results suggesting that sex-specific effects of SORL1 may be important to maintenance or decline in cognitive abilities in latelife. Lastly, unique contributions of markers across the SORL1 region to cognitive trajectories were evident. Future studies are warranted on sex-moderation and patterns of SORL1 effects on cognitive abilities in earlier adulthood periods.
Supplementary Material
Acknowledgements
This work was supported by the US National Institutes of Health (AG028555, AG08724, AG04563, AG10175, AG08861); and the Swedish Research Council (2007–2722).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors declare no conflicts of interest.
References
- Alexopoulos P, Kurz A, Lewczuk P, Kornhuber J, Wiltfang J, Maier W, Förstl H, Perneczky R. The sortilin-related receptor SORL1 and the amyloid cascade: A possible explanation for the concurrent elevation of CSF soluble APPα and APPβ in Alzheimer's disease. International Journal of Geriatric Psychiatry. 2010;25(5):542–543. doi: 10.1002/gps.2349. [DOI] [PubMed] [Google Scholar]
- Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc. 2009;2009(10) doi: 10.1101/pdb.ip71. pdb ip71. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. 2011 [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Bettens K, Brouwers N, Engelborghs S, De Deyn PP, Van Broeckhoven C, Sleegers K. SORL1 is genetically associated with increased risk for late-onset Alzheimer disease in the Belgian population. Hum Mutat. 2008;29(5):769–770. doi: 10.1002/humu.20725. [DOI] [PubMed] [Google Scholar]
- Bralten J, Arias-Vasquez A, Makkinje R, Veltman JA, Brunner HG, Fernandez G, Rijpkema M, Franke B. Association of the Alzheimer's Gene SORL1 With Hippocampal Volume in Young, Healthy Adults. Am J Psychiatry. 2011;168(10):1083–1089. doi: 10.1176/appi.ajp.2011.10101509. [DOI] [PubMed] [Google Scholar]
- Caglayan S, Bauerfeind A, Schmidt V, Carlo AS, Prabakaran T, Hubner N, Willnow TE. Identification of Alzheimer disease risk genotype that predicts efficiency of SORL1 expression in the brain. Arch Neurol. 2012;69(3):373–379. doi: 10.1001/archneurol.2011.788. [DOI] [PubMed] [Google Scholar]
- Cellini E, Tedde A, Bagnoli S, Pradella S, Piacentini S, Sorbi S, Nacmias B. Implication of Sex and SORL1 Variants in Italian Patients With Alzheimer Disease. Archives of Neurology. 2009;66(10):1260. doi: 10.1001/archneurol.2009.101. [DOI] [PubMed] [Google Scholar]
- Cousin E, Macé S, Rocher C, Dib C, Muzard G, Hannequin D, Pradier L, Deleuze JF, Génin E, Brice A. No replication of genetic association between candidate polymorphisms and Alzheimer's disease. Neurobiology of Aging. 2011;32(8):1443–1451. doi: 10.1016/j.neurobiolaging.2009.09.004. others. [DOI] [PubMed] [Google Scholar]
- Cuenco KT, Lunetta KL, Baldwin CT, McKee AC, Guo J, Cupples LA, Green RC, St George-Hyslop PH, Chui H, DeCarli C, Farrer LA. Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch Neurol. 2008;65(12):1640–1648. doi: 10.1001/archneur.65.12.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14(1):43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feulner TM, Laws SM, Friedrich P, Wagenpfeil S, Wurst SHR, Riehle C, Kuhn KA, Krawczak M, Schreiber S, Nikolaus S. Examination of the current top candidate genes for AD in a genome-wide association study. Mol Psychiatry. 2009;15:756–766. doi: 10.1038/mp.2008.141. others. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition Special Issue: Longitudinal studies of cognitive aging. 2004;11(2–3):325–345. [Google Scholar]
- Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer JA, Reynolds CA, Fiske A, Pedersen NL. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging. 2005;26(4):439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and health: a study of older unlike-sex twins. J Gerontol B Psychol Sci Soc Sci. 2002;57(3):S168–S176. doi: 10.1093/geronb/57.3.s168. [DOI] [PubMed] [Google Scholar]
- Houlihan LM, Harris SE, Luciano M, Gow AJ, Starr JM, Visscher PM, Deary IJ. Replication study of candidate genes for cognitive abilities: the Lothian Birth Cohort 1936 Genes. Brain and Behavior. 2009;8(2):238–247. doi: 10.1111/j.1601-183X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- Houwing-Duistermaat JJ, Uh HW, Tsonaka R. Pathway analysis for family data using nested random-effects models. BMC Proc. 2011;9(5 Suppl):S22. doi: 10.1186/1753-6561-5-S9-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Yamamoto M, Morihara T, Akatsu H, Kudo T, Kamino K, Takeda M. SORL1 is genetically associated with Alzheimer disease in a Japanese population. Neuroscience letters. 2009;461(2):177–180. doi: 10.1016/j.neulet.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Kölsch H, Jessen F, Wiltfang J, Lewczuk P, Dichgans M, Teipel SJ, Kornhuber J, Fr\ölich L, Heuser I, Peters O. Association of SORL1 gene variants with Alzheimer's disease. Brain Research. 2009;1264:1–6. doi: 10.1016/j.brainres.2009.01.044. others. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cheng R, Schupf N, Manly J, Lantigua R, Stern Y, Rogaeva E, Wakutani Y, Farrer L, St George-Hyslop P, Mayeux R. The association between genetic variants in SORL1 and Alzheimer disease in an urban, multiethnic, communitybased cohort. Arch Neurol. 2007;64(4):501–506. doi: 10.1001/archneur.64.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Shibata N, Cheng R, Mayeux R. Possible association between SORL1 and Alzheimer disease? Reanalysing the data of Shibata et al3 Dement Geriatr Cogn Disord. 2008;26(5):482. doi: 10.1159/000167792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95(3):221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- Li Y, Rowland C, Catanese J, Morris J, Lovestone S, O'Donovan MC, Goate A, Owen M, Williams J, Grupe A. SORL1 variants and risk of late-onset Alzheimer's disease. Neurobiol Dis. 2008;29(2):293–296. doi: 10.1016/j.nbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252(3):184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Ikram MA, Janssens ACJW, Schuur M, de Koning I, Isaacs A, Struchalin M, Uitterlinden AG, den Dunnen JT, Sleegers K. A Study of the SORL1 Gene in Alzheimer's Disease and Cognitive Function. Journal of Alzheimer's Disease. 2009;18(1):51–64. doi: 10.3233/JAD-2009-1137. others. [DOI] [PubMed] [Google Scholar]
- Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nature genetics. 2007;39(7):906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Saith S, Linnertz C, Burke JR, Hulette CM, Welsh-Bohmer KA, Chiba-Falek O. The Alzheimer's associated 5' region of the SORL1 gene cis regulates SORL1 transcripts expression. Neurobiol Aging. 2010;33(7):1485.e1–1485.e8. doi: 10.1016/j.neurobiolaging.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276(5318):1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- Meng Y, Lee JH, Cheng R, St George-Hyslop P, Mayeux R, Farrer LA. Association between SORL1 and Alzheimer's disease in a genome-wide study. Neuroreport. 2007;18(17):1761–1764. doi: 10.1097/WNR.0b013e3282f13e7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minster RL, DeKosky ST, Kamboh MI. No association of SORL1 SNPs with Alzheimer's disease. Neurosci Lett. 2008;440(2):190–192. doi: 10.1016/j.neulet.2008.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Rees SD, Kelly MA, Bain SC, Barnett AH, Thalitaya D, Prasher VP. Association of variants within APOE, SORL1, RUNX1, BACE1 and ALDH18A1 with dementia in Alzheimer's disease in subjects with Down syndrome. Neurosci Lett. 2010;487(2):144–148. doi: 10.1016/j.neulet.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, DeFaire U. The Swedish Adoption Twin Study of Aging: an update. Acta Genet Med Gemellol (Roma) 1991;40(1):7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, Zou F, Bettens K, Sleegers K, Tan EK, Kimura R, Shibata N, Arai H, Kamboh MI, Prince JA, Maier W, Riemenschneider M, Owen M, Harold D, Hollingworth P, Cellini E, Sorbi S, Nacmias B, Takeda M, Pericak-Vance MA, Haines JL, Younkin S, Williams J, van Broeckhoven C, Farrer LA, St George-Hyslop PH, Mayeux R. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol. 2011;68(1):99–106. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, McArdle JJ, Gatz M, Berg S, Pedersen NL. Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Dev Psychol. 2005;41(1):3–16. doi: 10.1037/0012-1649.41.1.3. [DOI] [PubMed] [Google Scholar]
- Reynolds CA, Gatz M, Pedersen NL, Prince JA. An assessment of CETP sequence variation in relation to cognitive decline and dementia risk. Int J Mol Epidemiol Genet. 2011;2(2):122–129. [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Hong MG, Eriksson UK, Blennow K, Bennet AM, Johansson B, Malmberg B, Berg S, Wiklund F, Gatz M, Pedersen NL, Prince JA. A survey of ABCA1 sequence variation confirms association with dementia. Hum Mutat. 2009;30(9):1348–1354. doi: 10.1002/humu.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Hong MG, Eriksson UK, Blennow K, Johansson B, Malmberg B, Berg S, Gatz M, Pedersen NL, Bennet AM, Prince JA. Sequence variation in SORL1 and dementia risk in Swedes. Neurogenetics. 2010;11(1):139–142. doi: 10.1007/s10048-009-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee J, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin C, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta K, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples L, Cuenco K, Green R, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland R, Inzelberg R, Hampe W, Bujo H, Song Y, Andersen O, Willnow T, Graff-Radford N, Petersen R, Dickson D, Der S, Fraser P, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer L, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nature Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Mayeux R, Farrer LA, George-Hyslop PS. Intracellular Traffic and Neurodegenerative Disorders. Springer; 2009. The sortilin-related receptor SORL1 is functionally and genetically associated with Alzheimer's disease; pp. 157–165. [Google Scholar]
- Schjeide BMM, McQueen MB, Mullin K, DiVito J, Hogan MF, Parkinson M, Hooli B, Lange C, Blacker D, Tanzi RE. Assessment of Alzheimer’s disease case–control associations using family-based methods. Neurogenetics. 2009;10(1):19–25. doi: 10.1007/s10048-008-0151-3. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, DeStefano AL, Au R, Massaro JM, Beiser AS, Kelly-Hayes M, Kase CS, D'Agostino RB, Sr, Decarli C, Atwood LD, Wolf PA. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EK, Lee J, Chen CP, Teo YY, Zhao Y, Lee WL. SORL1 haplotypes modulate risk of Alzheimer's disease in Chinese. Neurobiol Aging. 2009;30(7):1048–1051. doi: 10.1016/j.neurobiolaging.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Tsonaka R, Helm-van Mil AH, Houwing-Duistermaat JJ. A two-stage mixed-effects model approach for gene-set analyses in candidate gene studies. Stat Med. 2011 doi: 10.1002/sim.4370. [DOI] [PubMed] [Google Scholar]
- Tzvetkov MV, Becker C, Kulle B, Nurnberg P, Brockmoller J, Wojnowski L. Genome-wide single-nucleotide polymorphism arrays demonstrate high fidelity of multiple displacement-based whole-genome amplification. Electrophoresis. 2005;26(3):710–715. doi: 10.1002/elps.200410121. [DOI] [PubMed] [Google Scholar]
- Webster JA, Myers AJ, Pearson JV, Craig DW, Hu-Lince D, Coon KD, Zismann VL, Beach T, Leung D, Bryden L, Halperin RF, Marlowe L, Kaleem M, Huentelman MJ, Joshipura K, Walker D, Heward CB, Ravid R, Rogers J, Papassotiropoulos A, Hardy J, Reiman EM, Stephan DA. Sorl1 as an Alzheimer's disease predisposition gene? Neurodegener Dis. 2008;5(2):60–64. doi: 10.1159/000110789. [DOI] [PubMed] [Google Scholar]
- Wollmer MA. Cholesterol-related genes in Alzheimer's disease. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2010;1801:762–773. doi: 10.1016/j.bbalip.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Xing J, Watkins WS, Zhang Y, Witherspoon DJ, Jorde LB. High fidelity of whole-genome amplified DNA on high-density single nucleotide polymorphism arrays. Genomics. 2008;92(6):452–456. doi: 10.1016/j.ygeno.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.