Introduction
According to the American Heart Association (AHA), over 5 million men and women live with the diagnosis of heart failure (HF). Despite the national trend improving outcomes in cardiovascular diseases in general, one of every eight deaths in the U.S. is in some way attributable to HF with no improvement since the early 1990’s1. Beyond concerns about high prevalence and mortality, HF is consistently characterized by high symptom burden, diminished quality of life (QOL), and high costs to the health care system. Although the 2010 Comprehensive Heart Failure Practice Guideline2 advocates for palliative care, symptom management, referral to hospice and end of life support for HF families and their caregivers, little empirical evidence is available to effectively guide this type of palliative care.
When the hospice movement began, the majority of patients receiving care were persons with end-stage cancer. As recently as 1995, 80% of hospice patients had a cancer diagnosis3. However, a shift has occurred such that the proportion of hospice patients with cancer has decreased to less than half of all admissions4. A growing group of hospice patients are those with HF; in fact this is the second largest disease group receiving hospice care. Nationally, more than 1.5 million patients received hospice services in the U.S. in 2010, and the proportion of these patients who were being treated in hospice for HF was up to 12% of all hospice admissions by 2004, 13.2% by 2005; and 14.3% by 20104–5. It is generally known that these patients experience a wide array of symptoms that can have a negative impact on QOL6. However, only very limited research has been conducted with HF patients in hospice care.
Hospices provide palliative care to persons who are dying and supportive care to their informal family caregivers with a goal of improved QOL for both4,7. As death approaches, the family caregiver may be increasingly responsible for the majority of caregiving tasks, including emotional support, assistance with activities of daily living, administration of medication, provision of nutrition, and assistance with other physical aspects of care8–9. If the caregiver is not adequately prepared to provide the needed care, the patient’s QOL may suffer, and the caregiver may experience feelings of inadequacy, anxiety, or depression10–11.
The COPE method of supporting family caregivers of patients with advanced cancer was developed by Bucher, Houts, and Ades12. The COPE acronym stands for Creativity, Optimism, Planning, and Expert Information. This approach was modified to focus on caregivers of hospice patients with HF by the authors to include symptoms most commonly experienced by patients with HF. Following revision, a pilot study was conducted to determine its usefulness for this group. Thus, the purpose of this study was to pilot test the HF COPE intervention for caregivers of HF patients in hospice care in a small randomized clinical trial focusing on selected variables including: caregiver burden, QOL, depression and anxiety, caregiver knowledge, patient QOL, and Dyadic ER visits and hospitalizations.
Conceptual Framework
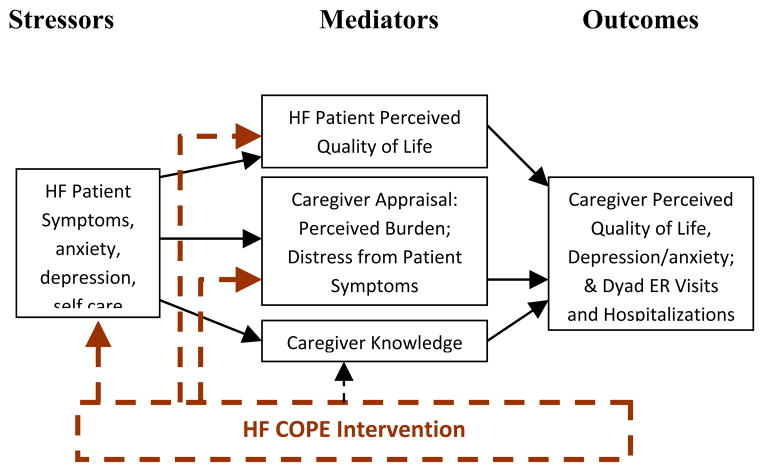
The transactional stress process model developed by Lazarus and Folkman13, with its emphasis on the influences of stress, appraisals, and resources, has been revised by a number of scholars to fit the special context of family caregiver stress and coping. In our conceptual model, patient symptoms are viewed as stressors with the potential to worsen caregiver well-being (Figure 1). However, just as important as these stressors are the caregivers’ internal resources, some of which can be modifiable by intervention. We targeted caregiver appraisals of burden, patient QOL, and caregiver knowledge as potentially modifiable caregiver issues. Our HF COPE intervention is indicated in the model via dotted lines.
Figure 1.
Stress Process Model
Thus, our intervention might lead to the following benefits: a) decreases in stressors, b) subsequent improvement in patient QOL, c) improved caregiver appraisals of burden and confidence, and d) improved caregiver knowledge. Improvements in any of these domains have the ability to improve caregiver QOL. Also, we explored whether this intervention might have an effect (through improvements in symptom management and decreases in caregiver anxiety) in decreasing emergency room visits and hospitalizations which also can have a negative impact on patient and caregiver QOL.
Methods
A two group mixed methods comparative experimental design with repeated measures was used with a target sample of 60 patient/caregiver dyads, 30 in each of the two groups, Treatment and Control. The controls received usual care only, and the treatment group received usual care plus the COPE educational intervention.
Setting and Sample
Lifepath Hospice and Palliative Care
Lifepath Hospice is a not-for-profit hospice with a census that averages approximately 2,000 patients per day, and it admits nearly 10,000 patients/year. Approximately 62% of patients (6,190/year) receive homecare via a family caregiver, and approximately 13% of patients were dying from heart disease at the time of the study, providing a potential 800 patients per year for the study. The mean length of stay (LOS) for HF patients in this hospice in the year preceding the study was 120 days, but the median LOS was 30 days.
Sample
All eligible consenting patient/caregiver dyads were included in the study.
Inclusion Criteria
Patients were adults with heart disease that was expected to be the cause of death, and an identified family caregiver who provided at least four hours of care per day, and both had to consent to participate, and have at least a sixth grade education, be able to read and understand English, and achieve a minimum score of 8 on the Short Portable Mental Status Exam14.
Exclusion criteria
Patients were excluded if they had Palliative Performance Scale (PPS)15 scores less than 30, to help insure that patients could reliably report their own symptoms through all three data collection periods. The PPS ranges from a low of 0 (dead) in ten point increments to 100 (functioning normally). Because the study focused primarily on management of four common problems, dyspnea, edema, pain, and constipation, patients were excluded if they did not have two of these four problems. Caregivers were excluded if they had mental status scores <8. About 2% of caregivers screened for the earlier Cancer COPE study were excluded due to poor mental status (McMillan et al., 2006).
Instruments
All measures used were matched to the conceptual model and are described here.
Caregiver Stressors
Memorial Symptom Assessment Scale-HF (MSAS-HF)
The MSAS-HF was developed and studied for use with patients with HF6, based on the existing MSAS designed for patients with cancer8,16–18. It has 32 items, including some added specifically for HF patients such as difficulty breathing when lying flat, and chest pain. Participants rate the frequency, severity and distress of the 32 symptoms over the past 7 days on 1 to 4 scales with four being the most frequent, severe, or distressing. Prevalence total is the sum of the number of symptoms present. Global distress is the sum of the distress items18. The MSAS-HF include the target symptoms for this study, dyspnea, pain, edema, and constipation.
Validity and reliability data for the original tool have been strong when the tool was used with persons receiving active cancer therapy8,16–18. Factor analysis confirmed the subscales. Reliability coefficients when the MSAS-HF was used with HF patients indicated strong internal consistency for the Total Prevalence, Psychological and Physical Subscales (alpha=.83–.92) with an alpha of .73 for the subscale containing the new HF symptoms18.
Profile of Mood States (POMS)
The depression subscale from the Profile of Mood States was used for both patients and caregivers. The POMS is a well-used tool with strong psychometric characteristics. The Depression subscale has 15 items and can be used as a stand-alone measure. This subscale asks the patient to respond on a five-point rating scale. Alpha coefficient as assessment of reliability was .95 for depression19–20.
Self Care in Heart Failure Index (SCHFI)
Two subscales of the SCHFI were used to determine the patient’s level of self care related to HF. 1). The Self-care Maintenance subscale has 10 items indicating frequency of self-care behaviors such as ‘weigh daily’ or ‘eat a low salt diet’ ranging from “never or rarely” to “always or daily.” 2). The “Self-care Confidence” subscale includes 6 items addressing the patient’s confidence in recognizing, treating, and evaluating changes in their HF status. Each subscale is standardized to a high value of 100, and scores >70 indicate adequate self-care on each subscale21. The SCHFI has been demonstrated to be a valid and reliable measure of HF self-care sensitive to the often subtle behavioral changes in HF patients22. Construct validity of the full scale and each of the subscales was demonstrated using confirmatory factor analysis and known groups technique21. Testing also indicated adequate reliability of total scale (alpha = 0.76) and Self-Care Management (alpha = 0.82). The Self-Care Maintenance subscale demonstrated a lower than desired alpha (alpha 0.56); however the result was anticipated because the items reflect behaviors known to vary in individuals.
Related Caregiver Factors
Hospice Quality of Life Index
The Hospice QOL Index (HQLI) is a 28-item self-report tool that includes three aspects of overall QOL: Psychophysiological Well-being; Functional well-being; and Social/spiritual Well-being. Total scores may range from a low of zero to a high of 28023. Evidence of validity was provided by the ability of the HQLI to differentiate between hospice patients and apparently healthy controls (p=.00). Factor analysis confirmed the structure of the HQLI. HQLI scores correlated at the expected level (r=.26; p=.00) with functional status scores. Validity in HF patients in hospice care also has been demonstrated; HQLI scores correlated at the hypothesized level (r=−.41; p=025) with MSAS-HF scores24. Reliability of the HQLI with these patients was strong (alpha=.71–.78). Total scale scores were used to assess patient QOL.
Caregiver Demands Scale
Caregiver Demands Scale (CDS) has 46 items assessing burden and mastery specific to caregiving tasks including assistance with meals, intimate care, treatments, and supervision of the patient. For each item, caregivers rated both how stressful the task was, and their confidence in their ability to manage their stress related to this task on a scale of 0 to 58.
Perceived Illness-related Stressors in Caregivers
The Memorial Symptom Assessment Scale-HF18 was completed by caregivers based on how much distress they were experiencing as a result of patient symptoms. For each symptom the caregiver endorses as occurring in the patient, he or she completed an additional rating describing how stressful this symptom was for the caregiver. Thus, the MSAS-HF functioned as a measure of caregiver appraisal of the perceived stress caused by symptoms experienced by the patient, an approach that has been used in previous work8,25.
Caregiver Knowledge Test
A knowledge test was developed for this study that was used as a pre-and post-test of caregiver knowledge about caregiving and symptom management. To insure validity of the exam, it was built on a test blueprint derived from the content in the Homecare Guide26. It was subjected to analysis by expert reviewers and changed based on reviewer input. Caregivers were tested at baseline and again at the end of their study participation. A significant improvement in test scores from pre- to post-test would have provided further evidence of the validity of the test as well as evidence that the caregivers have gained knowledge from the intervention in spite of the enormous pressure they are under as a result of caregiving. Test-retest reliability from time 2 to time 3 was strong (r=.80, p=.000).
Caregiver Outcomes
Caregiver Quality of Life Index
The Caregiver Quality of Life Index (CQOL) was used to assess family caregiver QOL; it yields a single QOL score27–28. It has 35 items using 5-point summated rating scales. The validity of the CQOL for hospice-HF caregivers was demonstrated by its correlation at the hypothesized levels with another QOL assessment for caregivers (r=.61; p=.000) and a measure of physical and mental health, the SF-12 (r=.46; p=.009). Reliability of the CQOL was high (alpha = .83)24.
Caregiver Anxiety and Depression
The anxiety and depression subscales from the Profile of Mood States both were used for caregivers. The Depression subscale is described above. The Anxiety subscale has 9 items, and both ask the caregiver to respond on a five-point rating scale. Alpha coefficients as assessments of reliability were .95 for depression and .92 for anxiety19–20.
Emergency Room Visits and Hospitalizations
Emergency room visits and hospitalizations are viewed as detrimental to the QOL of both patient and caregiver. An increase in knowledge and ability to manage patient symptoms by the caregiver might help decrease anxiety and thus help to decrease number of ER visits and hospitalizations and both of these were recorded as number of events. This data was collected from the patient record at the hospice after the dyad had completed the study.
Demographic Instruments
Patients
Standard demographic data was collected on patients to allow description of the sample, and included: age; gender; education level; marital status; and length of time since original HF diagnosis.
Caregivers
Demographic variables assessed via self-report in a semi-structured interview included: age; race; gender; education; marital status; and income.
Procedures
The proposal was approved by the Hospice Bioethics Committee and the university Institutional Review Board. The hospice routinely provided some individualized education and support that included teaching both caregivers and patients about how to manage symptoms. The intervention that was provided was in addition to the usual care provided by the hospice.
Control Group
Both the patients and caregivers in the usual care control group participated in the data collection process. Data were collected from caregivers and from the patients by the blinded RA-Home Health Aide (HHA) data collector. Data were collected for both groups at three time points: baseline (admission to the study), the beginning of Week 4 (days 23 to 25 after study admission) and the beginning of Week 5 (days 28 to 30).
Treatment Group
Caregivers in the treatment group received usual care plus the HF COPE intervention. Patients and caregivers participated in the data collection process.
HF-COPE Intervention
This problem-based coping intervention derives from the conceptual and research literature on problem solving training and therapy. The Family COPE model adapts these concepts to address the specific needs of families caring for persons at home12. The model has four components. The Family COPE Program teaches and supports caregiver problem-solving in three ways. First, written information that is organized to facilitate problem-solving is presented in the Home Care Guide for Advanced Heart Disease26, a reference for caregivers that was developed based on the original by Bucher and colleagues12 and given to each caregiver at the first intervention visit. Patient problems are described with suggestions for management included in this book developed for easy reference by caregivers. Second, the RA-intervention nurse reviewed the use of HF COPE problem solving principles in caring for someone with advanced heart disease. Third, two calls from the intervention nurse were made after each of the intervention visits. During these calls, the intervention nurses 1). asked about current problems regarding the targeted symptoms, 2). offered support in solving the problems, and 3). answered questions as needed. The intervention nurses were trained by the investigators prior to beginning the study and were monitored during the project.
Intervention Visits
During the first visit (45 minutes), the nurse-interventionist reviewed with the caregiver the steps in the problem solving process using one of the patient’s problems (pain, dyspnea, edema, or constipation) as a model. The patient problem was chosen by the caregiver as the one that the caregiver believed was most severe or had the highest priority for management. At the end of the first visit, the nurse assigned the caregiver a second one of the targeted symptom modules to review and use before the next scheduled visit. The caregiver was instructed to use the guide with the newly learned COPE techniques to work through individual caregiving issues. At the second and third visits, the nurse-interventionist reviewed the homework assignments with the caregiver and reinforced appropriate problem solving behaviors. Near the end of the session, the caregiver was asked three (“What would you do if…?”) questions to ascertain whether the content was understood. Before leaving, the nurse-interventionist emphasized the importance of continuing to assess these problems, as well as the importance of communicating with the hospice staff nurses.
Data Analysis
To evaluate the impact of the COPE intervention on changes in caregiver burden, QOL, depression and anxiety, caregiver knowledge, patient QOL, and dyadic ER visits and hospitalizations, a series of random effects models, with intervention group as the between subjects factor, was computed on scores from the three measurement points. Although this method provides the same basic information as traditional repeated measures analysis, the chief advantage of this method of data analysis29–30 is the ability to include persons for whom complete data is not available. In this analysis, the presence of a time by intervention group interaction would indicate differences in the rate of change over the follow-up interval. Considerations of sample size are less relevant here because the study was designed to generate information about effect sizes, rather than to provide definitive statistical results.
Results
Sample
No significant differences were found between the groups on the demographic variables, so the two groups are combined in demographic tables. Patients had a mean age of 79.6 (SD=11.5), an average of about 12 years of education (SD=2.3) and an average of 10 years since their diagnosis of HF. The majority of patients were white (85%), male (65%), and slightly less than half (47.5%) were currently married. Caregivers were slightly younger than patients (mean=63.3 years; SD=13.4) and had only slightly more years of education (mean=12.9; SD=2.0). Caregivers were predominantly white (85%), female (70%) and currently married (65%). The largest number of caregivers were spouses (42.5%), followed by adult children (40%). Only five of the caregivers were working; four of these were working full time (Table 1).
Table 1.
Means and Standard Deviations of Patient and Caregiver Age and Years of Education, and Frequency and Percent of Patients and Caregivers by Ethnicity, Marital Status, and Religious Affiliation (N=40 dyads)
| Variable | Patient | Caregiver | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 79.6 | 11.5 | 63.3 | 13.4 |
| Years of Education | 12.0 | 2.3 | 12.9 | 2.0 |
| Years since diagnosis (range <1–32) | 10.1 | 8.9 | ||
|
|
||||
| Frequency | Percent | Frequency | Percent | |
|
|
||||
| Gender | ||||
| Female | 14 | 35 | 28 | 70 |
| Male | 26 | 65 | 12 | 30 |
| Ethnicity | ||||
| White, non-Hispanic | 34 | 85 | 34 | 85 |
| African American | 3 | 7.5 | 3 | 7.5 |
| Hispanic | 1 | 2.5 | 1 | 2.5 |
| Asian/Pacific Islander | 1 | 2.5 | 1 | 2.5 |
| Other | 1 | 2.5 | 1 | 2.5 |
| Marital Status | ||||
| Currently married | 19 | 47.5 | 26 | 65 |
| Divorced | 7 | 17.5 | 6 | 15 |
| Widowed | 14 | 35 | 4 | 10 |
| Separated | 0 | 0 | 1 | 2.5 |
| Never married | 0 | 0 | 1 | 2.5 |
| Caregiver working | - | - | 5 | 12.5 |
Caregivers
Using random effects modeling, no time by group effects were found. Thus, descriptive data are presented.
Caregiver Distress from Patient Symptoms
Both Treatment and Control groups reported distress from patient symptoms at baseline, with caregivers in the control reporting slightly higher scores, but the difference between groups was not statistically significant (Table 2). Distress in both groups decreased only 4 to 6 points from baseline to Week 5 on a 0 to 128 scale.
Table 2.
Caregiver Outcome Means and Standard Deviations by Time
| Variable | Intervention Group | Control Group | ||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| MSAS Distress from Symptoms | ||||||
| CG Distress Baseline | 19 | 21.3 | 14.5 | 21 | 28.9 | 21.5 |
| CG Distress Week 4 | 14 | 16.3 | 14.9 | 14 | 21.7 | 23.0 |
| CG Distress Week 5 | 13 | 16.8 | 17.4 | 13 | 22.5 | 27.2 |
| Caregiver Burden Scale | ||||||
| CDS Stress Baseline | 19 | 1.9 | .64 | 21 | 1.8 | .76 |
| CDS Stress Week 4 | 19 | 1.6 | .67 | 21 | 1.5 | .81 |
| CDS Stress Week 5 | 19 | 1.6 | .76 | 21 | 1.3 | .55 |
| CDS Confidence Baseline | 19 | 3.1 | 1.2 | 21 | 3.2 | 1.2 |
| CDS Confidence Week 4 | 19 | 2.6 | 1.5 | 21 | 2.6 | 1.5 |
| CDS Confidence Week 5 | 19 | 2.4 | 1.4 | 21 | 2.4 | 1.4 |
| POMS | ||||||
| Depression Baseline | 19 | 8.4 | 7.2 | 21 | 8.0 | 7.3 |
| Depression Week 4 | 14 | 8.9 | 7.3 | 15 | 8.6 | 9.6 |
| Depression Week 5 | 14 | 9.9 | 9.7 | 14 | 8.0 | 8. |
| Anxiety Baseline | 19 | 8.8 | 7.2 | 21 | 8.0 | 7.3 |
| Anxiety Week 4 | 14 | 8.9 | 7.3 | 15 | 8.7 | 9.6 |
| Anxiety Week 5 | 14 | 9.9 | 9.7 | 14 | 8.0 | 8.7 |
| CQOL | ||||||
| Quality of Life Baseline | 19 | 43.2 | 20.5 | 21 | 46.0 | 23.0 |
| Quality of Life Week 4 | 14 | 40.8 | 19.4 | 15 | 44.3 | 26.1 |
| Quality of Life Week 5 | 14 | 40.2 | 1834 | 14 | 40.2 | 24.2 |
| Caregiver Knowledge | ||||||
| Knowledge Baseline | 19 | 4.2 | 1.8 | 21 | 4.3 | 1.7 |
| Knowledge Week 4 | 14 | 5.0 | 2.4 | 15 | 4.0 | 1.9 |
| Knowledge Week 5 | 14 | 4.8 | 2.3 | 14 | 4.2 | 1.9 |
Caregiver Stress from Caregiving
The groups reported similar scores at baseline and both decreased slightly over the course of the study (Table 2). Although mean scores were low, there was a large variability in the scores; scores over time in both groups ranged from a low of 1 to a high of 3.7 on a 0–5 scale.
Caregiver Confidence about Caregiving
Caregiver confidence scores were very similar at Baseline, and Weeks 4 and 5, and scores decreased slightly for both groups over the course of the study. No significant differences were found (Table 2). Caregivers reported the full range of scores from 1 to 5.
Caregiver Depression
Scores on the POMS Depression subscale could vary from 0 to 60, and the caregivers in the two groups had similarly low scores at baseline that decreased somewhat in the Treatment group and increased slightly in the Control group at Week 4; however, this difference was not significant (Table 2).
Caregiver Anxiety
Anxiety scores could range from 0 to 36, and caregivers in both groups had scores ranging from 8 to 10 (2). Anxiety went up a little more in the control group from Baseline to post-intervention (Time 2) but the difference was not significantly or clinically significant.
Caregiver QOL
Scores on this measure could range from 0 to 140. Caregivers in both groups reported fairly low scores, ranging from 40 to 46 (Table 2) with no significant differences.
Caregiver Knowledge
No significant differences were found between the two groups on their knowledge at any of the three data collection points. Although the intervention group increased their knowledge scores from baseline to the first measure after the intervention, the increase was less than 10%, and decreased again at Time 3 (Table 2).
Patients
Symptoms
All of the patients in the study had at least two of the targeted symptoms. The target symptoms reported by more than half of the sample were shortness of breath (65%) and pain other than chest pain (52.5%). Between 30 and 48% of patients reported one or more of the following target symptoms: Swelling of arms and/or legs, chest pain, breathless at night, and constipation. There also were reports of symptoms that were not targets of this project. Most commonly reported were dry mouth (72.5%) and fatigue (70%).
Patient Self-care Maintenance
On this subscale of the SCHFI, patient scores were remarkably similar for both groups across time (Table 3). Mean scores (0–100) ranged from 58.8 to 62.3.
Table 3.
Patient Outcomes: Descriptive Data for Symptom Distress, Depressive Symptoms, Quality of Life, SCHFI Scores and ER Visits with and Without Hospital Days
| Variable | Treatment | Control | ||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| MSAS | ||||||
| Symptom Distress Baseline | 18 | 17.1 | 12.3 | 21 | 25.1 | 19.1 |
| Symptom Distress Week 4 | 14 | 17.4 | 14.5 | 15 | 21.3 | 19.2 |
| Symptom Distress Time Week 5 | 13 | 14.0 | 14.9 | 14 | 19.6 | 14.6 |
| POMS | ||||||
| Depression Baseline | 19 | 6.6 | 7.3 | 21 | 7.7 | 10.7 |
| Depression Week 4 | 14 | 5.0 | 5.4 | 15 | 10.3 | 17.3 |
| Depression Week 5 | 14 | 5.9 | 5.8 | 14 | 7.5 | 15.2 |
| Hospice Quality of Life Index | ||||||
| QOL Baseline | 19 | 200.5 | 36.6 | 21 | 210.7 | 31.4 |
| QOL Week 4 | 14 | 189.8 | 67.4 | 15 | 214.9 | 32.1 |
| QOL Week 5 | 14 | 195.2 | 65.7 | 14 | 210.6 | 32.4 |
| SCHFI Scores | ||||||
| Self care Maintenance Baseline | 17 | 59.1 | 17.1 | 21 | 60.7 | 10.6 |
| Week 4 | 14 | 59.8 | 18.1 | 15 | 59.2 | 10.5 |
| Week 5 | 13 | 58.8 | 19.4 | 14 | 61.4 | 14.6 |
| Self care Confidence Baseline | 17 | 62.3 | 19.8 | 21 | 62.3 | 15.8 |
| Week 4 | 14 | 61.3 | 21.6 | 15 | 59.2 | 14.4 |
| Week 5 | 13 | 59.9 | 20.2 | 14 | 62.2 | 14.6 |
| ER visits Hospital Visits | N | Frequency | N | Frequency | ||
| ER without hospitalization | 17 | 1 | 21 | 0 | ||
| Number of Hospital days without ER Visit | 17 | 6 | 21 | 0 | ||
| ER with hospitalization | 19 | 1 | 21 | 3 | ||
| Number of Hospital days with ER Visit | 19 | 0 | 21 | 53 | ||
Patient Self-care Confidence
Patient mean scores on the Confidence subscale of the SCHIFI were also remarkably similar in the two groups, with no change over time (Table 3). Standardized mean scores ranged from 59.2 to 62.3.
Patient Symptom Distress
Scores on the MSAS Global Distress subscale could vary from 0 to 128, depending on the number of symptoms reported by the patient and the distress the endorsed symptom was causing. Scores were relatively low across both groups. No significant differences were found over time (Table 3).
Patient Depression
Depression mean scores, which could range from 0 to 60 were relatively low (<10) in both groups and not significantly different at any time point (Table 3).
Patient QOL
HQLI mean scores could range from 0 to 280. Patient mean scores were relatively high (>189), and there were no significant differences between the groups over time (Table 3).
ER Visits and Hospital Days
No differences were found between groups on hospitalization or in ER visits with or without hospitalization. There was a difference in the number of hospital days following ER visits in the Control group compared to the Treatment group at 3 months following the intervention (Table 3). However, the large number of days accounted for in the Control group was a result of one patient in that group having a long hospital stay.
Discussion
This pilot study was designed to test the feasibility and acceptability of an intervention for caregivers of hospice patients with HF. Acceptability data were qualitative and are presented elsewhere31. Our group had successfully implemented this intervention with caregivers of hospice patients with cancer, and believed that when modified, it would work as well for caregivers of hospice patients with HF. As it turned out, what we actually tested was our ability to recruit and retain these dyads in an intervention study. We learned that accrual and retention of the targeted dyads in the study was extremely difficult in spite of their length of stay (LOS) in hospice that is longer than the LOS for patients with cancer8; our recruitment and retention issues are described elsewhere32. We also found that the COPE intervention did not show significant effects in improving patient or caregiver variables, although it should be noted that the power for these analyses was low due to the small sample. In spite of our problems, the study revealed some important information about hospice patients with HF and their family caregivers. Because of the qualitative aspect of the project that is published elsewhere31, we were able to confirm that caregivers had been managing HF symptoms for a very long time, and that our intervention near the end of the disease trajectory was not effective; caregivers already felt competent to manage the care of these patients and did not feel the need for the intervention. This helps to explain the complete lack of change in any variable as a result of the intervention.
Our results have proven useful to other scholars in this area, because when these results were communicated verbally to other HF investigators in other parts of the country, they saw the need to move the study of the COPE-HF intervention into settings where HF patients are newly diagnosed. Thus, a very positive, albeit unexpected, outcome of the study was achieved (see for example, Bakitis33).
Caregiver Outcomes
The primary target of the intervention was the family caregiver; however, no statistically significant improvement in any outcomes could be seen at either Week 4 or Week 5. One might suggest that no significant differences could be found primarily because the sample was so small. But in this data, the treatment and control groups seemed to be remarkably similar, not different, at the end of the study.
Caregiver Burden
Caregiver burden was assessed with two instruments. The Caregiver Burden scale yielded two subscale scores, stress and confidence. The MSAS-HF yielded one score on total distress caregivers reported they felt as a result of patient symptoms. Interestingly, stress from caregiving and distress from symptoms both went down slightly from baseline to Week 4 in both groups although the decrease was not significant and thus, these results should be viewed with caution. However, confidence, a variable that might have increased as a result of either the intervention or time in hospice, was not different. Patients in this study were newly admitted to hospice care even though they might have had a HF diagnosis for years. Thus, it might be expected that stress and distress from symptoms might decrease and confidence might increase as a result of support given to patients and caregivers by the hospice team. However, although the stress and distress did increase, and confidence decreased, the changes were not significant and thus may have occurred randomly. It may be that although the caregivers were receiving support from the hospice, the patients were five weeks closer to death at the final data point, causing stress for caregivers that could not be completely addressed by hospice staff. And confidence might have declined because the caregiver felt ill-prepared to care for a patient so near to death. However, more study is needed about caregiver burden in this population in studies with larger samples.
Depression and Anxiety
Although caregivers reported some depressive symptoms and evidence of anxiety, the mean scores are relatively low and are not different between groups or over time. Thus, the intervention had no effect, and it appears that the four weeks of hospice care also was not an influence on these scores (Table 2). Further research is needed about the issue of caregiver anxiety and depression because of the central role caregivers play in hospice care.
QOL
The relatively low scores on QOL may indicate that the burden, anxiety and depression reported by these caregivers might be additive taking a toll on caregiver QOL in spite of the intervention and the support from hospice staff. Thus, this group of caregivers appeared to need support from the hospice staff.
Knowledge
Caregivers receiving the intervention were taught how to manage symptoms, how to report problems, and how to get help, but knowledge scores did not improve. One explanation could be that the interventionist was in some way ineffective. While this is very possible, it seems unlikely given that the interventionist was trained in the method and was an experienced and motivated hospice nurse. Based on the qualitative results of the study31, it appears that the caregivers did not feel they needed the intervention because of their prior experience caring for these patients with long-standing HF; perhaps they just did not attend to the intervention or use the manual as we had asked. Another explanation could be the test which might have had problems with validity.
Patient Outcomes
A secondary target of the intervention was the patient. That is, if the caregiver could be taught how to manage symptoms, the patients’ symptom severity and symptom distress might improve, thereby improving overall QOL. In the clinical trial of the COPE intervention with hospice patients with cancer, symptom distress was significantly better in the treatment group after the intervention was completed34. However, we had no such finding in this study. We found no improvement in any patient outcomes at Weeks 4 or 5.
Self Care
In spite of the lack of difference in the two treatment groups, some interesting findings about hospice patients with HF have emerged from these data. Patient self-care as measured by the SCHFI was found to be less than adequate (Table 3). Riegel and colleagues21 described standardized scores below 70 as an indicator of inadequate self care; all mean scores for both groups at all time points were less than 70, with the highest mean for either group at any time point being 62.9. These scores are lower than scores in an earlier U.S. sample of 439 HF patients recruited from both inpatient and outpatient settings21. It might be expected that HF patients who are judged to be nearing the end of life might engage in fewer self-care behaviors as family caregivers and hospice staff members do more for the patients, so the scores lower than 70 are not surprising. However, these mean scores are not very much lower than those found by Riegel and colleagues in 2009. Thus, it appears that patients either continue to be able to provide at least some of their own self-care, or perceive that they do.
Symptoms
Of the five targeted symptoms, the most commonly reported was shortness of breath. This was found to be the second most common symptom reported in a group of patients with HF in earlier studies18,24. Dyspnea and shortness of breath are known to be common in patients with HF, so this was an expected finding. Second most commonly reported in this group of patients (52.5%) was pain other than chest pain, while chest pain was reported by only 45%. Zambroski and colleagues18 had similar findings with HF clinic patients. The higher percentage of patients having pain other than chest pain is probably related to the fact that these patients were very elderly and probably had musculoskeletal pain from arthritis. Swelling of arms and legs was the third most frequently endorsed of our targeted symptoms (47.5%). This is similar to findings from earlier results in this population and in HF clinic patients18,24.
Although fatigue is common in HF patients, we did not target it. Research has been conducted that demonstrates that cancer-related fatigue can be improved with exercise and other approaches35,36. However, none of this research was conducted with hospice patients. And it is likely that HF patients in hospice care might be even less able to exercise than cancer patients receiving therapy, the groups with whom these fatigue interventions have been shown to be effective.
Although dry mouth was found to be the most commonly reported symptom in this group of patients, it was found in only 25% of HF patients in an earlier study24. Dry mouth also occurs commonly in hospice patients with cancer37, but no intervention research was found focusing on dry mouth for patients with either diagnosis. Further exploration of this common problem is needed to determine the common sources and the most appropriate interventions.
Symptom Distress
Patients had mean symptom distress scores that did not differ significantly by group and did not change much over time. These scores seem to be low, but it should be noted that no patient had all the symptoms on the MSAS-HF and reported an average of 12.1 symptoms each. Thus, it is unlikely that these patients would have mean scores higher than 50. Their symptom distress scores ranged from a low of 14.0 to a high of 25.1 (Table 3), indicating that some patients had substantial symptom distress in the symptoms they were experiencing while others did not. Continued focus on this issue is needed, especially the symptoms causing the greatest distress.
Depression
POMS depression scores showed that most of the patients did not have serious problems with depressive symptoms. However, scores indicated that at least some patients had depression scores that needed follow-up by staff. Similar results were found in an earlier study of hospice patients with cancer38. Although these investigators found relatively low mean depression scores, they also reported that 40% of patients met the cut-off for clinical depression. Further study of this issue is needed; depression has a negative impact on quality of life and deserves attention.
QOL
Although we found a wide range of QOL scores, means seemed relatively high in this group of patients (Table 3). This is consistent with earlier hospice research with patients with cancer3.
Sample
The sample was much smaller than anticipated, which had an impact on our ability to complete the analyses. This was a two year NIH-funded project, and we were able to get a one year, no cost extension to increase the sample size. However, this resulted in a final sample of only 40 patient/caregiver dyads (80 subjects total) who completed the study, 19 dyads in the COPE group and 21 in the control group. The sample was predominantly white and non-Hispanic. Reasons for refusal to enter the study and for attrition are being published separately32. The very small sample is a limitation of the study and reveals how difficult and expensive it is to conduct self-report studies with hospice patients with any diagnosis, but particularly with HF. An earlier clinical trial with a cancer focus that was conducted in this same hospice resulted in a sample of 329 patient/caregiver dyads in 4 years8, demonstrating that although their LOS is greater, HF patients in hospice care are even more difficult to accrue and keep in an intervention study such as this. Future studies should focus on accrual of non-white and Hispanic samples into hospice studies. In addition, funding must be sufficient to allow for longer periods of patient accrual so that sample sizes will be adequate for conducting meaningful analyses.
Conclusions
This study showed no significant effect from the COPE-HF intervention on caregiver or patient variables when piloted with this small sample of hospice patients with HF and their family caregivers. COPE was shown to work well with caregivers of hospice patients with cancer8 and hospices around the world began asking for the intervention to use with their patients. Results of this study should give them pause; while COPE worked well in cancer caregivers, its appropriateness for HF patients and caregivers near the end of life is clearly is question. We concluded from this failed trial that this intervention should be tested with heart failure patients and caregivers soon after the patient is diagnosed, not near the end of life. That work is now on-going. Future research still is needed to answer questions about hospice patients with heart failure and their caregivers because so little research has been conducted with this group. Work is needed that includes diverse samples and tests interventions that are designed to control physical and psychological symptoms and improve quality of life of this growing hospice population.
Acknowledgments
The support of the National Institute for Nursing Research is gratefully acknowledged (R21NR011224)
Contributor Information
Susan C. McMillan, Email: smcmilla@health.usf.edu, University of South Florida College of Nursing, Center for Hospice, Palliative Care and End of Life Studies at USF, Tampa, FL 33612.
Brent J. Small, School of Aging Studies, University of South Florida.
William E. Haley, School of Aging Studies, University of South Florida.
Cheryl Zambroski, University of South Florida, College of Nursing.
Harleah G. Buck, Pennsylvania State University.
References
- 1.Rogers VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Turner MB. Heart disease and stroke statistics. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JC, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MNHFSA. Comprehenive Heart Failure Practice Guidelines. Journal of Cardiac Failure. 2010;16(6):194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.McMillan SC. The quality of life of patients with cancer receiving hospice care. Oncology Nursing Forum. 1996;23:1221–1228. [PubMed] [Google Scholar]
- 4.National Hospice and Palliative Care Organization. Hospice Facts and Figures. 2012 www.nhpco.org.
- 5.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandburg KR, Weaver WD. Confirmation of a heart failure epidemic: Findings from the Resource Utilization Among Congestive Heart Failure (REACH) Study. Journal of the American College of Cardiology. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 6.Zambroski CH, Moser DK, Roser LP, Heo S, Chung ML. Patients with Heart Failure who Die in Hospice. American Heart Journal. 2005;149(3):558–564. doi: 10.1016/j.ahj.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Cella D. Measuring quality of life in palliative care. Seminars in Oncology. 1995;22:73–81. [PubMed] [Google Scholar]
- 8.McMillan SC, Small BJ, Weitzner M, Schonwetter R, Tittle M, Moody L, Haley WE. Impact of Coping Skills Intervention with Family Caregivers of Hospice Patients with Cancer: A Randomized Clinical Trial. Cancer. 2006;106(1):214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]
- 9.Laizner AM, Shegda B, Yost LM, Barg FK, McCorkle R. Needs of family caregivers of persons with cancer: A review. Seminars in Oncology Nursing. 1993;9:114–120. doi: 10.1016/s0749-2081(05)80107-x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrell BR, Grant M, Chan J, Ahn C, Ferrell BA. The impact of cancer pain education on family caregivers of elderly patients. Oncology Nursing Forum. 1995;22:1211–1218. [PubMed] [Google Scholar]
- 11.Given CW, Given B, Stommel M, Collins C, King S, Franklin S. The caregiver reaction assessment (CRA) for caregivers of persons with chronic physical and mental impairments. Research in Nursing and Health. 1992;15:271–183. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 12.Bucher JA, Houts PS, Ades T. Family Caregiving. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 13.Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- 14.Pfeiffer E. A Short Portable Mental Status Questionnaire for the assessment of organic brain deficit in elderly patients. Journal of American Geriatrics Society. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 15.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative Performance Scale (PPS): A new tool. Journal of Palliative Care. 1996;12(1):5–11. [PubMed] [Google Scholar]
- 16.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer. 1994;30A(9):1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 17.McMillan SC, Small BJ. Symptom distress and quality of life in hospice patients with cancer. Oncology Nursing Forum. 2002;29(10):1421–1428. doi: 10.1188/02.ONF.1421-1428. [DOI] [PubMed] [Google Scholar]
- 18.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. European Journal of Cardiovascular Nursing. 2005;4:198–206. doi: 10.1016/j.ejcnurse.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Boyle GJ. A cross-validation of the factor structure of the Profile of Mood States: Were the factors correctly identified in the first instance? Psychological Reports. 1987;60:343–354. [Google Scholar]
- 20.Conn VS, Taylor SG, Abele PB. Myocardial infarction survivors: age and gender differences in physical health, psychosocial state and regimen adherence. Journal of Advanced Nursing. 1991;16:1026–34. doi: 10.1111/j.1365-2648.1991.tb03362.x. [DOI] [PubMed] [Google Scholar]
- 21.Riegel B, Lee CS, Dickson VV, Carlson B. An Update on the Self-Care of Heart Failure Index. Journal of Cardiovascular Nursing. 2009;24(6):485–497. doi: 10.1097/JCN.0b013e3181b4baa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004 Aug;10(4):350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 23.McMillan SC, Weitzner M. Quality of life in cancer patients: Use of a revised hospice index. Cancer Practice. 1998;6(5):282–288. doi: 10.1046/j.1523-5394.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- 24.McMillan SC, Dunbar SB, Zhang W. The Prevalence of Symptoms in Hospice Patients with End Stage Heart Disease. Journal of Hospice & Palliative Nursing 2007. 2007;9(3):124–131. [Google Scholar]
- 25.Haley WE, Roth DL, Coleton MI, Ford GR, West CAC, Collins RP, Isobe TL. Appraisal, coping and social support as mediators of well-being in black and white family caregivers of patients with Alzheimer’s disease. Journal of Consulting and Clinical Psychology. 1996;64:121–129. doi: 10.1037//0022-006x.64.1.121. [DOI] [PubMed] [Google Scholar]
- 26.McMillan SC, Buck H, Dunbar SB. The Home Care Guide for Advanced Heart Disease (HF-COPE) 2007. unpublished. [Google Scholar]
- 27.Weitzner MA, McMillan SC, Jacobsen PB. Family caregiver quality of life: Differences between curative and palliative cancer treatment settings. Journal of Pain and Symptom Management. 1999;17(6):418–428. doi: 10.1016/s0885-3924(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 28.Weitzner M, Meyers C, Steinbruecker S, Saleeba A, Sandifer SD. Developing a caregiver quality of life instrument: Preliminary steps. Cancer Practice. 1997;5:25–31. [PubMed] [Google Scholar]
- 29.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2. Cary, N.C: SAS Press; 2006. [Google Scholar]
- 30.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 31.Buck HG, Zambroski CH, Garrison C, McMillan SC. 2012. “Everything They Were Discussing, We Were Already Doing”: Hospice Heart Failure Caregivers Reflect on a Palliative Caregiving Intervention. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zambroski CH, Buck HG, Garrison C, Steele L, McMillan SC. Lessons from the Field: Challenges in Accruing Hospice Heart Failure Patients to Intervention Research. Journal of Cardiovascular Nursing. 2012 doi: 10.1097/JCN.0b013e3182784cc0. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakitas M. Concurrent Heart Failure Palliative Care RCT for Rural Caregivers (5R01NR011871), unfunded. 2012. in revision. [Google Scholar]
- 34.McMillan SC, Small BJ. Using the COPE Intervention for Family Caregivers to Improve Symptoms of Hospice Homecare Patients: A Clinical Trial. Oncology Nursing Forum. 2007;34(2):313–321. doi: 10.1188/07.ONF.313-321. [DOI] [PubMed] [Google Scholar]
- 35.Stone PC, Minton O. Cancer-related fatigue. European Journal of cancer. 2008;44(8):1097–1104. doi: 10.1016/j.ejca.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 36.Carroll JK, Kohli S, Mustian KM, Roscoe JA, Morrow GR. Pharmacologic treatment of cancer-related fatigue. The Oncologist. 2007;12(Supplement 1):43–51. doi: 10.1634/theoncologist.12-S1-43. [DOI] [PubMed] [Google Scholar]
- 37.Stark L, Tofthagen C, Garrison C, McMillan SC. The Symptom Experience of Patients with Cancer. Journal of Hospice and Palliative Care. 2012;14(1):61–70. doi: 10.1097/NJH.0b013e318236de5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera HR, McMillan SC. Predictors of Depression Symptoms in Hospice Caregivers. Journal of Hospice and Palliative Nursing. 2010;12(6):345–357. [Google Scholar]
- 39.McMillan SC, Small BJ, Haley WE. Improving Hospice Outcomes through Systematic Assessment: A Clinical Trial. Cancer Nursing. 2011;34(2):89–97. doi: 10.1097/NCC.0b013e3181f70aee. [DOI] [PMC free article] [PubMed] [Google Scholar]