Abstract
Paracrine signals, essential for the proper survival and functioning of tissues, may be mimicked by delivery of therapeutic proteins within engineered tissue constructs. Conventional delivery methods are of limited duration and are unresponsive to the local environment. We developed a system for sustained and regulated delivery of paracrine signals by encapsulating living cells of one type in alginate beads and co-suspending these cell-loaded particles along with unencapsulated cells of a second type within a 3D protein gel. This system was applied to vascular tissue engineering by placing human placental microvascular pericytes (PCs) in the particulate alginate phase and human umbilical vein endothelial cells (HUVECs) in the protein gel phase. Particle characteristics were optimized to keep the encapsulated PCs viable for at least two weeks. Encapsulated PCs were bioactive in vitro, secreting hepatocyte growth factor, an angiogenic protein, and responding to externally applied HUVEC-derived signals. Encapsulated PCs influenced HUVEC behavior in the surrounding gel by enhancing the formation of vessel-like structures than empty alginate bead controls. In vivo, encapsulated PCs modulated the process of vascular self-assembly by HUVECs in 3D gels following implantation into immunodeficient mice. We conclude that alginate encapsulated cells can provide functional paracrine signals within engineered tissues.
Keywords: protein delivery, cell encapsulation, pericyte, co-culture, alginate, vascular tissue engineering
1. INTRODUCTION
Engineering complex, 3D tissues is an ambitious task; achieving this goal means that one must take into consideration many levels of physiology – from molecular interactions among cells of the graft to integration into the host organism. The right cells must be incorporated and kept alive during preparation and implantation. Further, these cells must retain their intrinsic functions – pancreatic beta cells must sense glucose levels and secrete the appropriate amount of insulin, hepatocytes must produce albumin and metabolize carbohydrates, and endothelial cells must form lumenized, non-thrombogenic tubes that anastomose with host vasculature. The local environment may dictate if these functions are preserved. The scaffold is often the first consideration – the composition, topography and mechanical properties of the supporting biomaterials or extracellular matrix provide essential cues that guide cellular growth and function (1–3). Importantly, protein growth factors and other signals are key contributors that influence cell survival and behavior within the chosen scaffold. This local, paracrine interaction among different cell types can be exploited to improve function within an engineered tissue. Many paracrine signals are proteins and may be mimicked by systems that deliver active proteins to cells within 3D scaffolds. Such protein delivery systems include free incorporation into the scaffold, immobilization within the scaffold, coupling of the protein to the scaffold with an intermediate, matrix-binding proteins, fusion proteins and micro-/nanoparticles (please refer to (4) for a comprehensive review of these methods as they apply to inducing microvascular networks). Two major shortcomings of the protein delivery approach are (a) that only a limited amount of one or two specific proteins can incorporated into a delivery system; and (b) the proteins are delivered with an unregulated release profile – typically a burst of protein is initially released followed by a slower release over a finite period of time. An ideal delivery system would provide a controlled delivery of the desired protein(s) for a specified duration, and release would be responsive to signals from the local environment. Living cells naturally provide this kind of delivery: Cells can secrete many different proteins over a long period of time and the local chemical environment can modulate the release profile of these proteins.
To engineer a controllable delivery system, cells can be encapsulated to isolate them from the surrounding cells. Alginate has been widely investigated for this purpose. Alginate is an inert polysaccharide derived from seaweed and brown algae that can be ionically crosslinked with divalent cations – a gentle process that does not damage the suspended cells (5, 6). Importantly, the resulting hydrogel created by ionically crosslinking is porous enough for the diffusion of soluble protein factors (5, 6). These microcapsules have been widely studied with various goals in mind, including the delivery of immune-isolated pancreatic islets (7, 8), and of mesenchymal stem cells for osteogenesis (9) and therapeutic revascularization (10).
We are interested in applying this technology in the field of vascular tissue engineering, specifically in the formation of microvascular network within 3D protein gels, an approach that has potential applications in many types of tissue engineering. The cell-to-capillary distance in most tissues is just 200 μm; therefore, any engineered tissue exceeding this dimension requires a mature and stable vascular network. The formation of a functional microvascular network is one of the largest challenges in the effort to design a complex tissue. For that reason, successes to date in tissue engineering have been largely limited to tissues that are either normally avascular or thin-walled such as articular cartilage (11), large-caliber blood vessels (12–14), tracheas (15, 16) and bladders (17). Many attempts to induce the formation of such a network have been made, with varying success including the delivery of various proangiogenic proteins, transplantation of vascular cells and macroporous hydrogels with conduits for vascular growth (4).
A promising system for the creation of microvascular networks for engineered tissues is the transplantation of human endothelial cells (ECs) within protein gels. ECs spontaneously self-assemble into tubule networks in vitro and form functional vascular beds after implantation into immunodeficient mice (18–20). Delivery of angiogenic proteins from within the protein gel, such as vascular endothelial growth factor (VEGF) and monocyte chemotactic protein -1 (MCP-1), has been shown to improve vessel formation in this system, though protein delivery was limited to a period of about one week (21). An alternative method is to co-deliver a second, supporting cell type. Pericytes (PCs), the mural cell in most microvessels, are known to contribute significantly to the formation and maturation of capillary networks (22). PCs may act to stabilize mature microvessels in a process involving cell contact and secreted proteins such as angiopoietin-1 (Ang-1) and transforming growth factor-beta1 (TGF-β1) (22) but also may secrete several proangiogenic factors, including vascular VEGF and hepatocyte growth factor (HGF) (Chang et al., submitted) (23, 24) that can promote new vessel formation. PCs cannot be directly incorporated into protein gels without additional support because they can contract the protein matrix until it is too dense to permit vascularization (18). We sought a method to harness the proangiogenic paracrine properties of this cell type.
Here we report the development of a transplantable, dual-cell system in which each cell type is confined to a separate gel phase: one cell type is encapsulated in a dispersed alginate phase and the other is suspended within a continuous protein gel phase. This system exploits paracrine communication between two cell types to enhance survival and remodeling of cells in the continuous phase of an engineered tissue. Additionally, as an in vitro model containing only two cell types, this system allows for identification of salient factors in cell-cell communication, in 3D. We found that soluble proteins were able to diffuse both into and out of the alginate capsules, but the encapsulated cells were prevented from crossing into the protein gel phase. We have tested this system with two cell types relevant to vascularization of engineered tissues: human umbilical vein endothelial cells (HUVECs) – freely suspended in an outer, protein gel phase - and human placental microvascular pericytes (PCs) - in the alginate phase. The EC/PC interaction is ideally dissected in this system because cell behavior is dependent on both paracrine effects, which are retained, and cell-cell contact, which is prevented. Here, we describe the development of methods for encapsulation, evaluation of viability and bioactivity of our encapsulated cells in in vitro and in vivo models of vascularization.
2. MATERIALS AND METHODS
2.1. Materials, cells and animals
Sterile alginate was purchased from NovaMatrix (Sandvika, Norway), calcium chloride was from J.T. Baker (Center Valley, PA, USA), and M199 media was from Gibco (Grand Island, NY, USA). HEPES, sodium hydroxide solution (1 N), sodium bicarbonate solution (7.5%) were all from Sigma (St. Louis, MO, USA). Collagen (rat tail, type I) was from BD (Franklin, NJ, USA) and fibronectin (human plasma) was obtained from Millipore (Billerica, MA, USA). The DuoSet ELISA kit for HGF was purchased from R&D Systems (Minneapolis, MN, USA). Endothelial cell growth supplement, calcein AM, ethidium homodimer and Hoechst 33342 were purchased from Invitrogen (Carlsbad, CA, USA). Isoflurane was purchased from (Bethlehem, PA, USA) and buprenorphine from McKesson (San Francisco, CA, USA). Antibodies against smooth muscle alpha-actin (SMA) and human class 1 major histocompatibility complex (human-MHC) were purchased from Abcam (Cambridge, MA, USA), and appropriate secondary antibodies (tagged with AlexaFluor 555 and AlexaFluor 488) were purchased from Life Technologies (Grand Island, NY, USA). Slides were mounted with Vectasheild with DAPI (Vector Laboratories, Burlingame, CA, USA).
HUVECs and human placental PCs were isolated, under protocols approved by the Yale Human Investigations Committee, from de-identified discarded tissues and cultured as described previously (18, 25). Cells were used between passages 1 and 5. PCs consistently express NG2, Thy-1, SMA, calponin, and PDGFR-β, but lack EC markers (CD31 and CD34), smooth muscle cell markers (CD45 and CD14) and leukocyte markers (CD45 and CD14) (18). As previously described, HUVECs were retrovirally transduced with the caspase-resistant D34A mutant form of Bcl-2 to generate Bcl-2-HUVECs (20) for in vitro gel experiments. Untransduced HUVEC were used for conditioned media and in vivo experiments. HUVECs and PCs were serially cultured in Medium 199 (M199) supplemented with 20% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2mM L-glutamine and HUVEC media was additionally supplemented with 50 μg/ml endothelial cell growth supplement.
Female, 6–8 week old C.B-17 SCID/Bg mice (Taconic Farms, Hudson, NY, USA) were utilized for all in vivo experiments. All experiments were carried out by trained personnel under protocols approved by the Yale Human Investigations Committee and the Yale Institutional Animal Care and Use Committees and conform to the Guide for the Care and Use of Laboratory Animals.
2.2. PC encapsulation
PCs were evenly suspended in a 2% w/v alginate (dissolved in DPBS), at a concentration of 8 or 16 million PCs per milliliter of alginate, unless otherwise specified. The alginate/cell suspension was dropped from a one-milliliter syringe with a 30G needle into a crosslinking solution of 100 mM calcium chloride buffered with 25 mM HEPES. An air jet was employed to prevent agglomeration of the viscous alginate solution at the needle tip – airflow rate was 3 LPM, except where otherwise stated (Figure 1). Particles were crosslinked in the calcium chloride solution for 5 minutes, and then filtered with a 100 μm cell sieve to remove single cells and small acellular satellite particles. Particles were suspended in media until evaluated or included in an experiment (up to 1 day).
Figure 1.
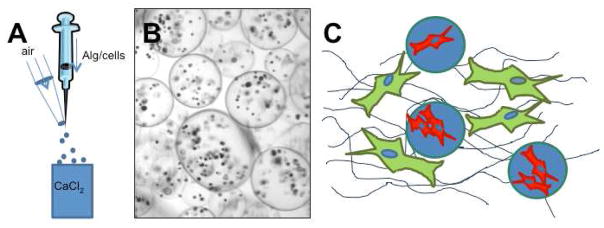
Particle encapsulation and protein gel method. (A) PCs were evenly suspended in an aqueous alginate solution. The alginate/cell suspension was dropped from a one-milliliter syringe into a crosslinking solution of calcium chloride. An air jet was employed to prevent agglomeration of the viscous alginate solution at the needle tip. (B) Resulting particles were spherical with many cells in each particle. (C) A dual-cell 3D protein gel was created by forming two phases: The outer, protein gel phase contained suspended ECs (Bcl-2-EC for in vitro experiments and untransduced EC for in vivo experiments), and the inner, alginate phase contained PCs. This allowed for diffusion of soluble protein factors while preventing cell-cell contact between the two cell types.
2.3. Microparticle characterization
The total number of particles and their concentration was evaluated using a gridded slide (Electron Microscopy Sciences) and bright field light microscopy (Olympus 1×51 Inverted Microscope). Particle diameter was evaluated from bright field microscopic images of 150 particles in each batch and quantified using NIH Image J software.
To evaluate viability and determine the number of cells per microparticle, encapsulated PCs were incubated in 0.5 μg/ml calcein AM, 3.33 μg/ml ethidium homodimer and 5 μg/ml Hoechst 33342 for one hour. A viable cell was defined as a cell double stained with calcein and Hoechst; dead cells were those that stained with ethidium homodimer and Hoechst, except on day 1. Encapsulated PCs on day 1 consistently double stained with calcein and ethidium homodimer, so images are of cells stained with calcein and Hoechst only. This was likely due to a slight disruption of the cell membranes during encapsulation. This was resolved at later time points, however, so images of particles at later time points are of cells stained with calcein, ethidium homodimer and Hoechst. As a control, particles were also treated with 0.1% saponin for 15 minutes after staining with calcein and Hoechst. A Leica TCS SP5 Spectral Confocal Microscope was used for confocal fluorescence imaging; Z-stacks of entire particles were taken with 5 μm slices, and viable cells were counted in a rotation of image 3D projection.
2.4. Conditioned media and HGF measurement
The amount of HGF released by encapsulated PCs was measured and compared to unencapsulated PCs, blank particles (in numbers corresponding to particles containing PCs) and medium alone. Additionally, PCs were stimulated with EC conditioned medium, and the altered HGF concentration was measured. Conditioned medium (M199, 1% serum) was first collected after a 24-hour incubation with 1×106 HUVECs per milliliter of media. This conditioned medium, or fresh medium (M199, 1% serum) was then added to 0.5×106 PCs per milliliter of media (unencapsulated and encapsulated), and controls containing only blank particles, or no PCs or particles for an additional 24 hours. The medium was collected after this second incubation, and HGF concentration was measured by ELISA. The DuoSet ELISA kit for HGF was purchased from R&D Systems (Minneapolis, MN, USA) and used according to the manufacturer’s directions.
2.5. Protein gel preparation
Bcl-2-HUVEC were evenly suspended in a three-dimensional protein gel (3.0×106 Bcl-2-HUVEC/ml gel) - with blank alginate microparticles or with PCs encapsulated in alginate microparticles. The final PC concentration was 1.5×106 cells per milliliter of gel, and corresponding numbers of blank particles were added to control gels. Cells and microparticles were added to a collagen/fibronectin gel with a final concentration of 1.5 mg collagen/ml and 0.1 mg fibronectin/ml. Gels were made in ice-cold M199 buffered with 25 mM HEPES and 1.5 mg/ml NaHCO3. The pH was adjusted to 7.4 with 1 M NaOH. Three hundred microliters of the cell/microparticle/gel suspension was then cast in a 48-well plate and was allowed to polymerize by incubation for 20 minutes at 37°C in a tissue culture incubator with 5% CO2 – humidified air. Five hundred microliters of complete M199 was added to the tops of the gels and media was replaced daily. Vascular self-assembly was allowed to proceed for 7 days.
2.6. In vitro protein gel characterization
At the specified experimental endpoint, gels were fixed in formalin and embedded in paraffin for histological analysis. Hematoxylin and eosin (H&E) staining of mid-gel sections was used to evaluate formation of vascular structures (H&E staining performed by Yale Research Histology). Vascular self-assembly was first evaluated by counting three different cellular structures: multi-cell cords, visible lumens, and tubes. Multi-cell cords were defined as elongated, continuous structures with two or more cells. Visible lumens were void spaces surrounded by one or more cells – these structures excluded collagen and appeared white in H&E sections. Tubes were lumens that were surrounded by two or more cells, and represent the cross-section of a hollow vessel-like structure. Further characterization included the evaluation of average multi-cell cord length and average lumen diameter, both done with NIH Image J. All structures in 4–5 high-powered fields, enough to account for ~100% of the gel area, were evaluated for each sample.
The cellular components of the vascular structures were characterized via sections stained with human-specific CD31 (human-CD31) for HUVECs and with SMA for PCs (performed by Yale Research Histology).
2.7. In vivo implantation of protein gels and analysis
Protein gels containing untransduced HUVECs and either blank particles or encapsulated PCs were implanted subcutaneously in the abdominal wall of 6–8 week old female C.B-17 SCID/Bg mice as described previously (25). Particles for these experiments were made with an initial cell concentration of 8 million PCs/ml alginate. A final concentration of 3 million HUVECs/ml gel and 375,000 PCs/ml gel was used. Per Veterinary Clinical Services standard operating procedure, mice were anesthetized with inhaled 30% isoflurane via nose cone, and buprenorphine was administered post-operatively to minimize discomfort (0.05 μg/g body weight). Gels were retrieved 2 weeks after implantation, fixed in formalin, embedded in paraffin and stained with H&E (Yale Research Histology). Vessels were identified in mid-graft sections as structures having distinct lumens that contained visible erythrocytes. The lumen diameter of all structures in 4–8 high-powered fields (enough to account for 100% of the gel area) were measured for each sample and analyzed from light microscopic images using NIH Image J software.
Cells of human origin were identified by two color immunofluorescence staining with antibody to human-MHC (1:250) and mural cells were identified with anti-SMA (1:100). Secondary antibodies Alexa Fluor 555 goat anti-mouse or Alexa Fluor 488 goat anti-rabbit were used at 2 mg/ml. Slides were mounted in Vectasheild with DAPI.
2.8. Statistics
Data are represented as mean±standard deviation. Statistical significance was determined using an unpaired, two-tailed T-test with a 95% confidence interval. Statistical calculations were performed using GraphPad Prisim software (GraphPad Software, San Diego, CA, USA).
3. RESULTS
3.1. PC encapsulation
PCs were successfully encapsulated in 2% w/v alginate microparticles. An air jet was utilized to prevent agglomeration of the viscous alginate/cell suspension, as depicted in Figure 1a. Following crosslinking, the resulting PC-containing microparticles were spherical (Figure 1b). These encapsulated cells could then be incorporated into a system relevant to tissue engineering: the 3D protein gel. Encapsulated PCs were included, along with HUVECs in collagen/fibronectin gels (Figure 1c). This effectively places the PCs in a separate phase within the protein gel, preventing HUVEC-PC contact. Cells were viable after encapsulation, as measured by calcein staining.
3.2. Regulation of particle size
Particle size was regulated with the rate of airflow. Airflow rate was varied from 1 LPM to 5 LPM, and particle diameter was measured for 150 particles per batch; 3 separate particle batches were evaluated for each condition. An airflow rate of 1 LPM resulted in very large milliparticles with an average diameter of 1084±223 μm. Increasing the airflow rate resulted in smaller particles with a plateau at 3 LPM with an average particle diameter of 214±39 μm (Figure 2a, b). Additionally, at higher airflow rates (4 LPM and 5 LPM), resulting particles became less uniform and fewer particles appeared spherical (Figure 2a). An airflow rate of 3 LPM was chosen for all following experiments since this is the value at which diameter plateaued, and resulting particles were consistently spherical.
Figure 2.
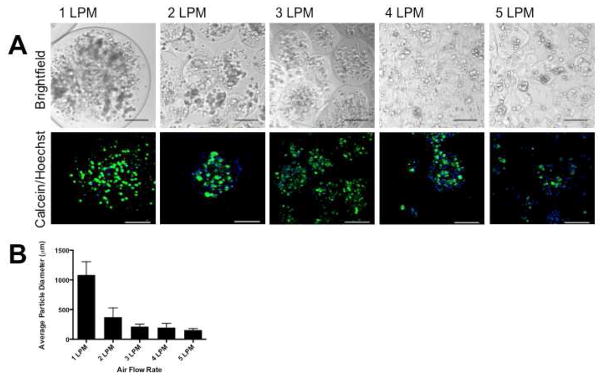
Regulation of particle diameter. By varying airflow rate, particle diameter was tuned. Airflow rate was varied from 1 to 5 LPM, with a constant initial cell number of 8 million PC/ml alginate. Brightfield images and confocal projections (different fields) of the resulting particles are shown in (A). Particle diameter decreased with increasing airflow rates, and particle size plateaued at 3 LPM. Additionally, particles became less spherical and less uniform at 4 and 5 LPM. An average diameter of 214±39 μm was achieved at 3 LPM, and this was used for all subsequent experiments (B). Scale bars = 200 μm.
3.3. Regulation of cell number
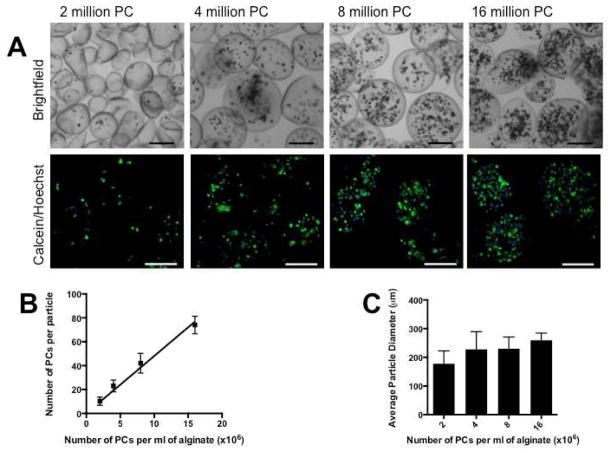
The number of cells per particle was regulated by varying the initial cell concentration within the aqueous alginate. Airflow rate was kept constant at 3 LPM, as particle size plateaued at this value. Initial cell concentrations were varied from 2 million to 16 million PCs/ml alginate, and 3 separate particle batches were evaluated for each condition. It was found that an initial cell number of 2 million PCs/ml alginate yielded particles with 10±3 PCs/particle and particles had an average diameter of 178±45 μm. Four million PCs/ml alginate resulted in 23±5 PCs/particle and an average particle diameter of 228±62 μm; 8 million PCs/ml alginate: 42±8 PCs/particle and 230±41 μm average diameter, 16 million PCs/ml alginate: 74±7 PCs/particle and 260±25 μm average diameter (Figure 3). Within this range, the number of PCs per particle increased linearly with initial cell concentrations (Figure 3b). Particle diameter did not change appreciable between preparations, with the exception of particles made with the smallest initial cell concentration of 2 million PCs/ml alginate (Figure 3c).
Figure 3.
Regulation of cell number. The number of viable PCs per particle can be tuned by altering the initial cell concentration during particle fabrication. Initial cell concentrations were varied from 2 million to 16 million PC per ml of alginate, with a constant airflow rate of 3 LPM. Brightfield images and confocal projections of the resulting particles are shown in (A). The number of viable PC per particle was quantified for each condition and, within this range, there was a linear increase in the number of PCs per particle with increasing initial cell concentrations (B). With the exception of the 2 million PC/ml alginate experimental group, particle size was constant (C). Scale bars = 200 μm.
3.4. Encapsulated PCs remain viable over time
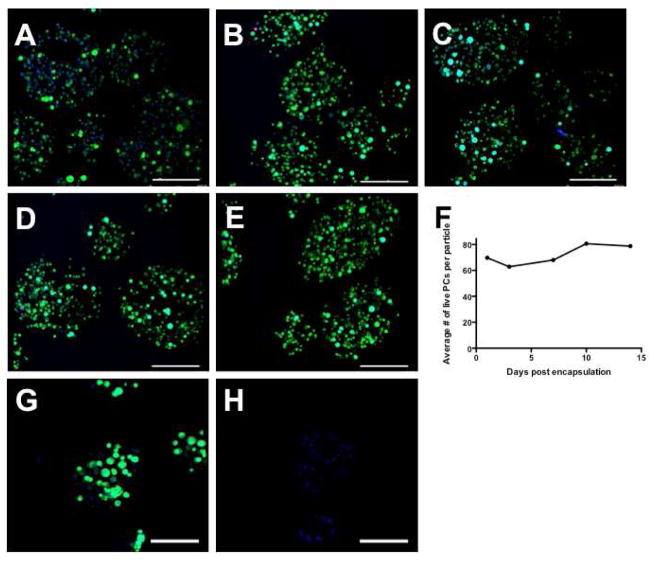
Particles used in this experiment were made with an initial cell concentration of 8 or 16 million PCs/ml alginate PC viability was evaluated with calcein, ethidium homodimer and Hoechst dyes. Viable cells were double stained, displaying a blue nucleus and a green cytoplasm; dead cells were those that stained with ethidium homodimer and Hoechst, except on day 1. Encapsulated PCs on day 1 consistently double stained with calcein and ethidium homodimer (the latter not shown), likely due to transient disruptions of the cell membranes during encapsulation. At later time points, however, cells regained the capacity to exclude ethidium homodimer. The number of live cells per particle (initial concentration of 16 million PCs/ml alginate) was evaluated at several time points over 14 days, and significant numbers of PCs remained alive during this time. No PCs escaped from the particles through day 14. Representative confocal projections of particles at the designated time points are shown in Figure 4a–e, quantification is shown in Figure 4f. Encapsulated PCs (initial concentration of 8 million PCs/ml alginate) were treated with 0.1% saponin after dying with calcein and Hoechst to provide a control (G, H). Live cells are shown in Figure 4g and cells treated with saponin are shown in Figure 4h, a control to demonstrate that alginate does not retain calcein after cell death.
Figure 4.
Encapsulated PCs (encapsulated with an initial concentration of 16 million PCs/ml alginate) remain viable for up to 14 days in vitro, as evaluated with calcein (green), ethidium homodimer (red) and Hoechst (blue). Confocal projections are shown in A (calcein, Hoechst) and B–E (calcein, ethidium homodimer and Hoechst). (A) Day 1; (B) Day 3; (C) Day 7; (D) Day 10; (E) Day 14. Quantification of viable cell number per particle is shown in (F). Encapsulated PCs (initial concentration of 8 million PCs/ml alginate) were treated with 0.1% saponin after dying with calcein and Hoechst to provide a control (G, H). (G) Day 1; (H) Day 1, treated with 0.1% saponin, showing alginate does not retain calcein after cell death. Scale bars = 200 μm.
3.5. Encapsulated PCs deliver a relevant angiogenic growth factor
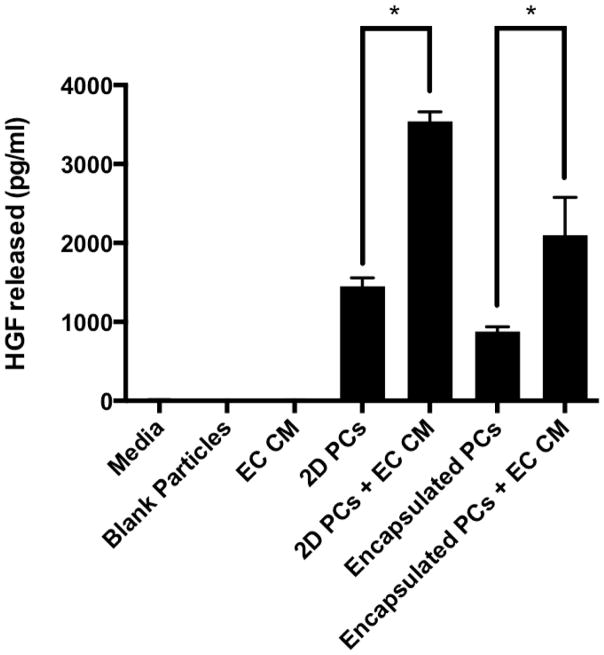
Encapsulated PCs were found to secrete HGF in amounts comparable to unencapsulated PC in a 24-hour period (Figure 5). Additionally, stimulation of both encapsulated and unencapsulated PCs with conditioned media from HUVEC resulted in a 2.4 fold increase in HGF release. The control groups: media alone and blank particles, contained no detectable HGF. The data shown were from PCs encapsulated with an initial concentration of 16 million PC/ml alginate. Similar results were observed with an initial concentration of 8 million PC/ml (data not shown).
Figure 5.
Encapsulated PCs release a proangiogenic factor, HGF, in amounts comparable to unencapsulated PCs (2D PCs), as measured by ELISA in conditioned media. Incubation of PCs, encapsulated or unencapsulated, in endothelial cell conditioned media (EC CM) stimulates the release of significantly increased amounts of HGF. HGF was not detected in media, media with blank particles or in endothelial cell conditioned media.
3.6. Encapsulated PCs influence the formation of vascular structures in vitro
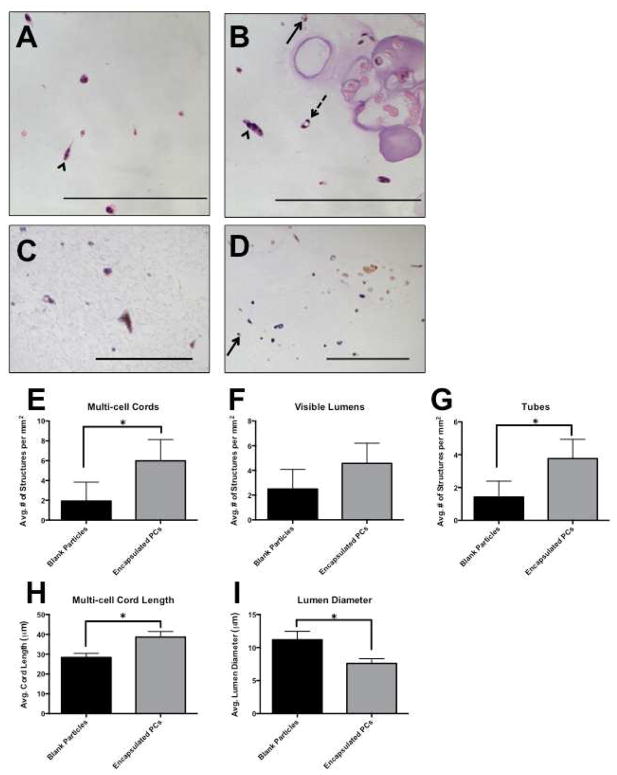
Encapsulated PCs or blank particles were suspended in Bcl-2-HUVEC containing collagen/fibronectin gels, so that the final concentration of cells was 3.0×106 Bcl-2-HUVEC and 1.5×106 PCs per ml of gel. Particles were made with an initial cell concentration of 16 million PCs/ml alginate. The protein gels were incubated for 7 days and media was replaced daily. At the desired time point, gels were fixed, embedded and sectioned. H&E staining revealed cellular structures and vessel-like structures were quantified. Vascular self-assembly was evaluated by counting three different cellular structures: multi-cell cords, visible lumens, and tubes (Figure 6a, b), as defined in the methods section of this paper.
Figure 6.
Alginate-encapsulated PCs were suspended with Bcl-2-EC in protein gels to create a dual-cell, two phase 3D construct. After 7 days, gels were fixed and sectioned, and vascular structures were counted. We define cords, lumens, and tubes as separate structures: Multi-cell cords contained more than one cell, stretched out, but no lumens (arrowhead); visible lumens were all empty spaces surrounded by one or more cells (arrow, solid line), and tubes were lumens that were surrounded by two or more cells (arrow, dashed line). Encapsulated PC are outlined in grey. H&E: (A) Bcl-2-EC with blank particles. (B) Bcl-2-EC with encapsulated PC. Human CD31 staining confirmed that structures in the gel phase consisted of Bcl-2-EC only (C), and staining for SMA confirmed that PCs did not escape the particulate phase (D). Experimental groups that contained encapsulated PCs formed more multi-cell cords (E), visible lumens (F) and tubes (G). Additionally, paracrine factors released from encapsulated PCs stimulated longer multi-cell cords (H) and limited the diameter of lumens (I), when compared to gels with blank particles. Scale bars = 200 μm.
The inclusion of encapsulated PCs in the protein gels resulted in the formation of increased numbers of multi-cell cords and tubes than gels that contained blank particles (Figure 6e, g). This finding was accompanied by an apparent increase in the number of visible lumens, although this difference did not reach statistical significance (Figure 6f). Further, gels with encapsulated PCs displayed statistically significant differences in average multi-cell cord length – greater with PCs – and average lumen diameter – less with PCs (Figure 6h, i). These findings were consistent over several experiments made with PCs encapsulated with initial concentrations of 8 and 16 million PCs/ml alginate. Further evaluation was performed by staining with human-CD31 and αSMA. PCs stained positively for αSMA, but not human-CD31, and were found residing only in the alginate particle space. All cellular structures in the protein gel phase stained positively for human-CD31, and were negative for SMA indicating they were formed by Bcl-2-HUVEC. This confirmed that the PCs did not escape the particle phase for the duration of the experiment (Figure 6c, d).
3.7. Encapsulated PCs limit vessel diameter in in vivo gel implants
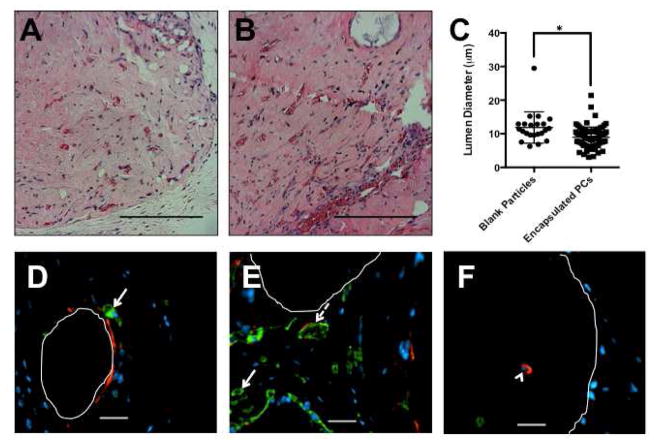
To assess whether encapsulated PCs modulate the process of vascular self-assembly and remodeling in vivo, protein gels that contained suspended untransduced HUVEC and either blank particles or encapsulated PCs were implanted subcutaneously. Gels from these initial studies were harvested 2 weeks after implantation and vessels were identified in mid-graft sections as structures having distinct lumens that contained visible erythrocytes. The average lumen diameter was statistically smaller in gels containing encapsulated PCs compared to gels containing blank particles (Figure 7a–c). This corresponded to the smaller lumen diameter observed in in vitro gels containing encapsulated PCs. Samples were further analyzed by staining for human-MHC (green) and SMA (red). Vessels in gels containing blank particles (Figure 7d) and gels containing encapsulated PCs (Figure 7e) were lined with human-MHC+ cells (arrow, solid line) – the implanted HUVEC cells. Many vessels were invested with SMA positive/human MHC negative cells (arrow, dashed line) – invading mouse mural cells. Human pericytes were found only in the particle space (Figure 7f); all stained positive for human MHC, and most stained positive for SMA (arrowhead). Many of the particles and the encapsulated PCs were lost during processing of the sections, but some remained in place (particle space outlined in white). These data show that paracrine signals from encapsulated PCs affect vessel formation by transplanted ECs.
Figure 7.
Protein gels containing untransduced ECs and either blank particles (A) or encapsulated PCs (B) were implanted subcutaneously and evaluated after 2 weeks with H&E. Vessels formed in the gels with encapsulated PCs were smaller in diameter than those formed in the gels with blank particles (C). Sections were stained for human-MHC (green) and SMA (red). Vessels in gels containing blank particles (D) and gels containing encapsulated PCs (E) were lined with human-MHC+ cells (arrow, solid line) – the implanted HUVECs. Many vessels were invested with SMA-positive/human MHC negative cells (arrow, dashed line) – invading mouse mural cells. Human pericytes were found only in the particle space (F); all stained positive for human MHC, and most stained positive for SMA (arrowhead). Many of the particles and the encapsulated PCs were lost during processing of the sections, but some remained in place (particle space outlined in white). These data show that paracrine signals from encapsulated PCs affect vessel formation by transplanted ECs. Scale bars = 200 μm.
4. DISCUSSION
Local interactions between different cell types are essential for the proper survival and functioning of all tissues. These interactions can be dependent on contact or on soluble factors that act on cell surface receptors. The paracrine delivery of molecular signals has been extensively investigated for supporting cell survival and influencing cell behavior within engineered tissue constructs. Conventional protein delivery methods fall short of the necessary release profile and adaptability necessary for long-term support of engineered tissues. We have developed a system for delivery of these paracrine signals. By encapsulating PCs, we are able to delivery important supporting growth factors to transplanted endothelial cells for the vascularization of engineered tissues. We can control the size of the resulting alginate particles, as well as the number of cells encapsulated within each particle. The encapsulated PCs remain viable for at least two weeks, and secrete HGF in amounts comparable to unencapsulated PCs. Further, when incorporated into the complete, dual-cell system, encapsulated PCs are bioactive. In vitro, they stimulate the formation of more multi-cell cords, lumens and tubes in Bcl-2-HUVEC containing protein gels. Additionally, multi-cell cords are longer, and lumen diameter is restricted. This influence is also observed upon subcutaneous implantation. Vessels that form within the protein gel display smaller lumen when encapsulated pericytes are present.
Importantly, we have demonstrated that our encapsulated PCs are still functioning in a desirable manner. They produce a relevant paracrine signal, the angiogenic growth factor, HGF, and this protein is permitted to diffuse through the encapsulating alginate. While the absolute concentration of HGF secreted by PCs in 24 hours varied from isolation to isolation – the concentration ranged from ~500 pg/ml to 2000 pg/ml - the concentration was always similar in each groups within one experiment. Each isolation was from a single donor, and variation between individuals is likely the cause of these differences. Further, encapsulated PCs adapt these signals based on protein messages from the surrounding environment. We tested this by stimulating PCs, encapsulated or unencapsulated, with HUVEC conditioned media – not only can proteins exit the alginate phase, but they can also enter this phase and reach the contained cells.
HGF is likely not the only molecule released by encapsulated PCs and influencing EC behavior within the protein gels, and this is an advantage of our system. We have not encapsulated one or two specific proteins, but a potential source of many different protein signals. While we have yet to identify additional paracrine signals involved in this interaction, we hypothesize that other molecules that induce or regulate angiogenesis, such as VEGF, Ang-1, and TGF-β1 may be involved (22).
Here we describe a dual-cell system, consisting of a continuous gel phase with suspended HUVECs, and a particulate alginate phase containing PCs (the alginate particles). Our results indicate that paracrine signals from PCs stimulate the formation of more and longer multi-cell cords, and more lumens and tubes. Further, encapsulated PCs appear to lead to important functional consequences in the developing vascular network: paracrine signals from the entrapped PCs lead to smaller lumen diameters in vessels formed in vitro and in vivo. In native in vivo microvessels, PCs reside in in the basement membrane and directly contact EC tubes. Previous studies, in which EC-PC contact was permitted, showed that the presence of PCs lead to smaller vessel diameter (26–28). These earlier studies hypothesize that this limitation of lumen diameter requires EC-PC contact, and was due to a physical restriction. Our studies, on the other hand, suggest that paracrine signals contribute to this control of vessel diameter. We aim to utilize this unique system that separates paracrine from contact dependent effects to further investigate these observations.
5. CONCLUSIONS
We have described a transplantable, dual-cell system in which each cell type is confined to a separate phase: one cell type encapsulated in a dispersed alginate phase and the other suspended within a continuous protein gel phase. We show that this system can exploit paracrine communication between two cell types to enhance survival and cell behavior in the continuous phase of an engineered tissue. As proof of concept we demonstrate that PCs can successfully be encapsulated in alginate, and that these cells remain viable for at least 2 weeks. These encapsulated cells secrete relevant proteins out of the alginate phase into the gel phase in a manner that is responsive to signals originating in the gel phase. Consequently, encapsulated PCs are biologically active in a model of vascularization in an engineered tissue. While we have tested this system using HUVECs and PCs, relevant to the vascularization of engineered tissues, our system can be readily extended to other cell types, serving as a platform to deliver paracrine signals, as well as an in vitro method for studying paracrine interactions. We envision that protein delivery can be further enhanced within this system by overexpressing known proteins of interest in the encapsulated cell under control of an inducible enhancer/promoter, creating a device for long term, regulated protein delivery.
Acknowledgments
We thank Elise Wilcox and Young-Eun Hyun for assistance with some experiments, and Themis R. Kyriakides, Yibing Qyang and Martin S. Kluger for technical advice and helpful discussions. The authors acknowledge funding for this research by the National Institutes of Health (NIH) grant R01-HL085416 (to W.M.S. and J.S.P.), CSTA Grant Number KL2 RR024138 (to W.G.C.) and CTSA Grant Number TL1 RR024137 from the National Center for Advancing Translational Science (NCATS), components of the NIH, and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu T, Houle JD, Xu J, Chan BP, Chew SY. Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng Part A. 2012;18(9–10):1057–66. doi: 10.1089/ten.tea.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nuernberger S, Cyran N, Albrecht C, Redl H, Vecsei V, Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32(4):1032–40. doi: 10.1016/j.biomaterials.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Yang C, Khan M, Liu S, Hedrick JL, Yang YY, et al. Nanostructured PEGbased hydrogels with tunable physical properties for gene delivery to human mesenchymal stem cells. Biomaterials. 2012;33(27):6533–41. doi: 10.1016/j.biomaterials.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Andrejecsk J, Chang W, Pober J, Saltzman WM. Controlled protein delivery in the generation of microvascular networks. Drug Deliv and Transl Res. 2012:1–14. doi: 10.1007/s13346-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt NC, Grover LM. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32(6):733–42. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 6.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 7.Opara EC, Mirmalek-Sani SH, Khanna O, Moya ML, Brey EM. Design of a bioartificial pancreas(+) J Investig Med. 2010;58(7):831–7. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27(32):5603–17. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Xu HH. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials. 2011;32(30):7503–13. doi: 10.1016/j.biomaterials.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landazuri N, Levit RD, Joseph G, Ortega-Legaspi JM, Flores CA, Weiss D, et al. Alginate microencapsulation of human mesenchymal stem cells as a strategy to enhance paracrine-mediated vascular recovery after hindlimb ischaemia. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1680. [DOI] [PubMed] [Google Scholar]
- 11.Iwasa J, Engebretsen L, Shima Y, Ochi M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc. 2009;17(6):561–77. doi: 10.1007/s00167-008-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107(10):4669–74. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson JT, Gilliland T, Maxfield MW, Church S, Naito Y, Shinoka T, et al. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regen Med. 2012;7(3):409–19. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet. 2012;380(9838):230–7. doi: 10.1016/S0140-6736(12)60633-3. [DOI] [PubMed] [Google Scholar]
- 15.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 16.Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozoky B, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2011;378(9808):1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 17.Drewa T, Adamowicz J, Sharma A. Tissue engineering for the oncologic urinary bladder. Nat Rev Urol. 2012;9(10):561–72. doi: 10.1038/nrurol.2012.158. [DOI] [PubMed] [Google Scholar]
- 18.Maier CL, Shepherd BR, Yi T, Pober JS. Explant outgrowth, propagation and characterization of human pericytes. Microcirculation. 2010;17(5):367–80. doi: 10.1111/j.1549-8719.2010.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harding MJ, Lepus CM, Gibson TF, Shepherd BR, Gerber SA, Graham M, et al. An implantable vascularized protein gel construct that supports human fetal hepatoblast survival and infection by hepatitis C virus in mice. PLoS One. 2010;5(4):e9987. doi: 10.1371/journal.pone.0009987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A. 2000;97(16):9191–6. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jay SM, Shepherd BR, Andrejecsk JW, Kyriakides TR, Pober JS, Saltzman WM. Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials. 2010;31(11):3054–62. doi: 10.1016/j.biomaterials.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 23.Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47(3):149–61. [PubMed] [Google Scholar]
- 24.Wang H, Keiser JA. Hepatocyte growth factor enhances MMP activity in human endothelial cells. Biochem Biophys Res Commun. 2000;272(3):900–5. doi: 10.1006/bbrc.2000.2852. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd BR, Jay SM, Saltzman WM, Tellides G, Pober JS. Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Eng Part A. 2009;15(1):165–73. doi: 10.1089/ten.tea.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114(24):5091–101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109(24):9342–7. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters JP, Kluger MS, Graham M, Chang WG, Bradley JR, Pober JS. In vitro Self-Assembly of Human Pericyte-Supported Endothelial Microvessels in Three- Dimensional Coculture: A Simple Model for Interrogating Endothelial-Pericyte Interactions. J Vasc Res. 2013;50(4):324–31. doi: 10.1159/000353303. [DOI] [PMC free article] [PubMed] [Google Scholar]