Abstract
Aminomorphinans are a relatively young class of opioid drugs among which substances of high in vitro efficacy and favorable in vivo action are found. We report the synthesis and pharmacological evaluation of novel 6β-acylaminomorphinans. 6β-Morphinamine and 6β-codeinamine were stereoselectively synthesized by Mitsunobu reaction. The aminomorphinans were subsequently acylated with diversely substituted cinnamic acids. In vitro binding studies on cinnamoyl morphinamines showed moderate affinity for all opiate receptors with some selectivity for mu opioid receptors, while cinnamoyl codeinamines only showed affinity for mu opioid receptors. In vivo analgesia studies showed significant analgesic activity of 6β-cinnamoylmorphinamine mediated by mu and delta receptors. The lead compound was found to be roughly equipotent to morphine (ED50 3.13 ± 1.09 mg/kg) but devoid of the dangerous side-effect respiratory depression, a major issue associated with traditional opioid therapy.
Keywords: aminomorphinan, MOR/DOR agonist, opioid, analgesia, respiratory depression, cinnamoyl morphinamine
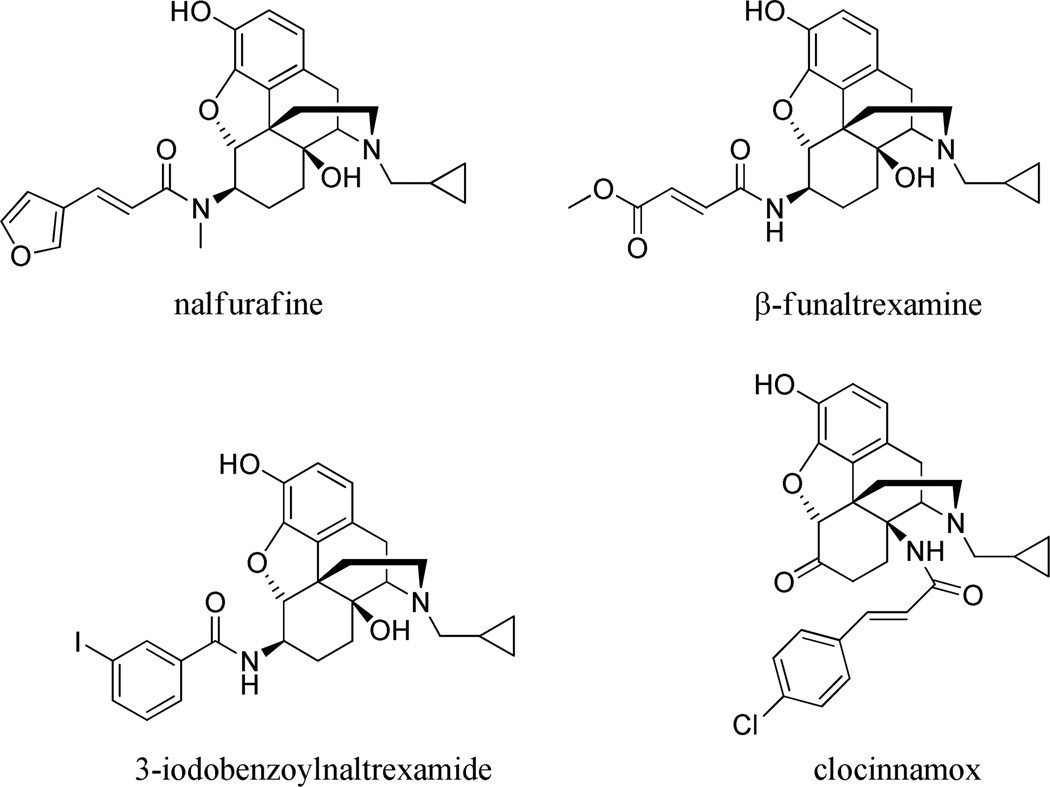
Morphine, the principal drug of the opioid family is among the most important agents used for the treatment of severe pain. [1] Clinically relevant effects, including various dangerous adverse effects are predominantly mediated by mu-opioid receptors (MOR-1). The structural features of the morphine skeleton have long been the basis of successful drug development and the development of novel morphine-like drugs with improved efficacy, receptor selectivity and side-effects. Aminomorphinans and their derivatives containing a nitrogen atom instead of oxygen in position C-6 of the morphinan ring system have been used in the development of a number of new opioids. Naloxamine and naltrexamine were the first two representatives of this family followed by an array of compounds generated by acylation and alkylation of the C-6 amino moiety, especially that of β-naltrexamine (β-NTA). [2] This group of compounds includes the novel kappa-opioid receptor (KOR-1) agonist nalfurafine (TRK-820) (Remitch® in Japan) used as a treatment for hemodialysis-related uremic pruritus [3,4] and β-funaltrexamine (β-FNA), a covalent MOR-1 antagonist affinity label with partial KOR-1 agonist activity has been widely used to investigate opioid receptor mechanisms. [5,6]
More recently iodobenzoylnaltrexamide (IBNtxA), a very potent analgesic with a novel pharmacological profile devoid of most side-effects characteristic of traditional opioid agonists due to its activity at a recently identified target (6TM/E11 sites) has been reported. [7,8] The naloxone analog, IBNalA, is more selective for these sites and has a similar side-effect profile. [9] The equatorial β-acylamino moiety is a common structural feature of these molecules. The C-6 amino group has been acylated by a diverse range of substituted aliphatic and aromatic carboxylic acids, including furanylacrylic acid (nalfurafine), 3-iodobenzoic acid (IBNtxA and IBNalA) and methylfumaric acid (β-FNA).
The cinnamoyl amides of β-NTA possess KOR-1 agonist and MOR-1 antagonist activity, similar to β-FNA. [10–12] C-14 cinnamoyl amides (e.g. clocinnamox) and esters of various morphinans have received significant attention for their varying MOR-1 activity (Figure 1). [13–15] 6-arylamido morphinans have been proposed as analogues of morphine-6-O-glucuronide. [16] Similarly, aryl naloxamides have been considered as alcohol cessation agents [17,18] and as potential MOR-1 antagonists. [19–22] N-Naphthoyl-naltrexamide (NNTA) reportedly targets MOR-KOR opioid dimers. [23]
Figure 1. Structures of pharmacologically important aminomorphinans.
To the best of our knowledge, C-6 acylamines of morphine and its congeners carrying Δ7–8 double bond and a methyl group on N-17 have not been reported. Herein, we present the synthesis of novel C-6 acylamines of morphine and codeine analogues acylated by a series of cinnamic acids. The synthesized compounds were evaluated for their binding affinities to mu (MOR-1), kappa1 (KOR-1) and delta (DOR-1) opioid receptors and analgesia. Based on in vivo results, 6β-cinnamoylmorphinamine was subjected to more detailed studies.
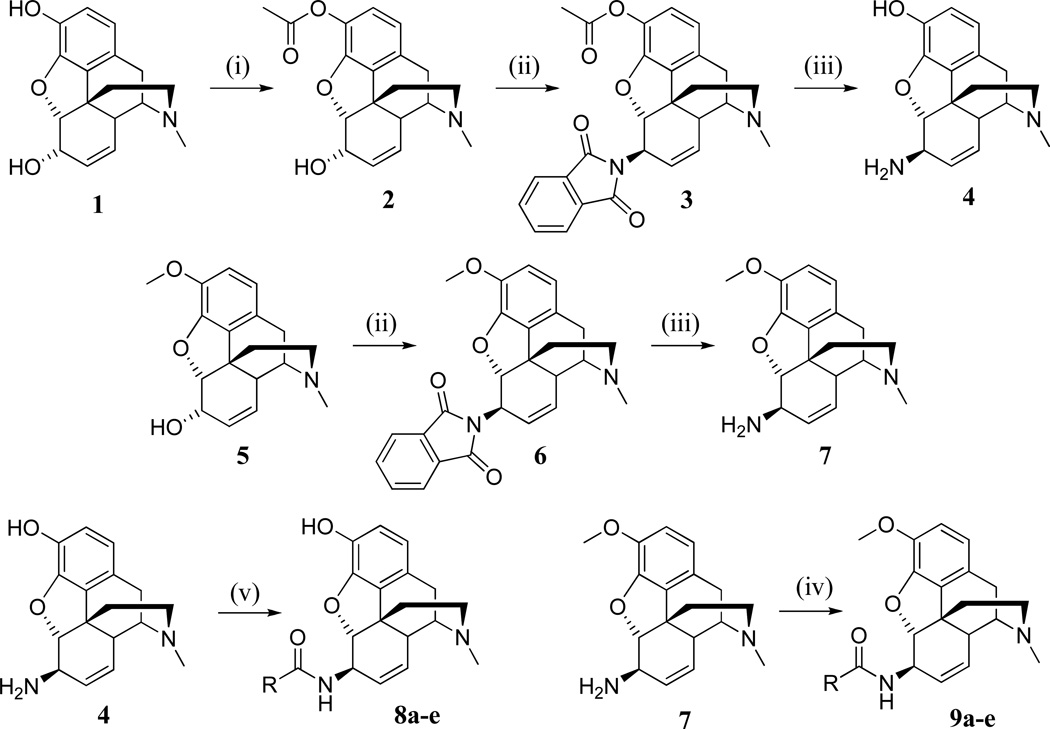
The 6β-amino derivatives of morphine and codeine were synthesized using the Mitsunobureaction. [24] Morphine (1) was selectively acetylated in the C-3 position and subsequently treated with phthalimide and diisopropyl azodicarboxylate (DIAD) in the presence of Ph3P. Codeine (5) was reacted directly under the same conditions. The 6β-phthalimidoyl intermediates (3,6) were isolated as HCl salts and then treated with hydrazine hydrate in ethanol to yield the desired 6β-amino derivatives (4, 7). For amidation reactions, the appropriate carboxylic acids were treated with thionyl chloride and reacted with the 6β-amino compounds in dichloromethane in the presence of Et3N. In case of 6β-morphinamine (4) formation of 3-O-esters could also be detected; the amidation reaction mixture was evaporated to dryness and dissolved in methanolic aqueous sodium carbonate solution and left to stand overnight to ensure cleavage of C-3 phenolic esters. The crude products were purified by column chromatography using chloroform-methanol 9:1 isocratic eluent and crystallized from hexane (Scheme 1, Figure 2).
Scheme 1. Synthesis of 6β-cinnamoyl morphinamines and codeinamines.
(i) acetic anhydride, NaHCO3, H2O, r.t., 1 h. (ii) phthalimide, DIAD, Ph3P, benzene, r.t., 2h. (iii) hydrazine hydrate, EtOH. (iv) 1.1 eq. acyl chloride, Et3N, CH2Cl2, r.t., 2 h. (v) 1.1 eq. acyl chloride, Et3N, CH2Cl2, r.t., 2 h. then Na2CO3, H2O, MeOH, r.t., 12h.
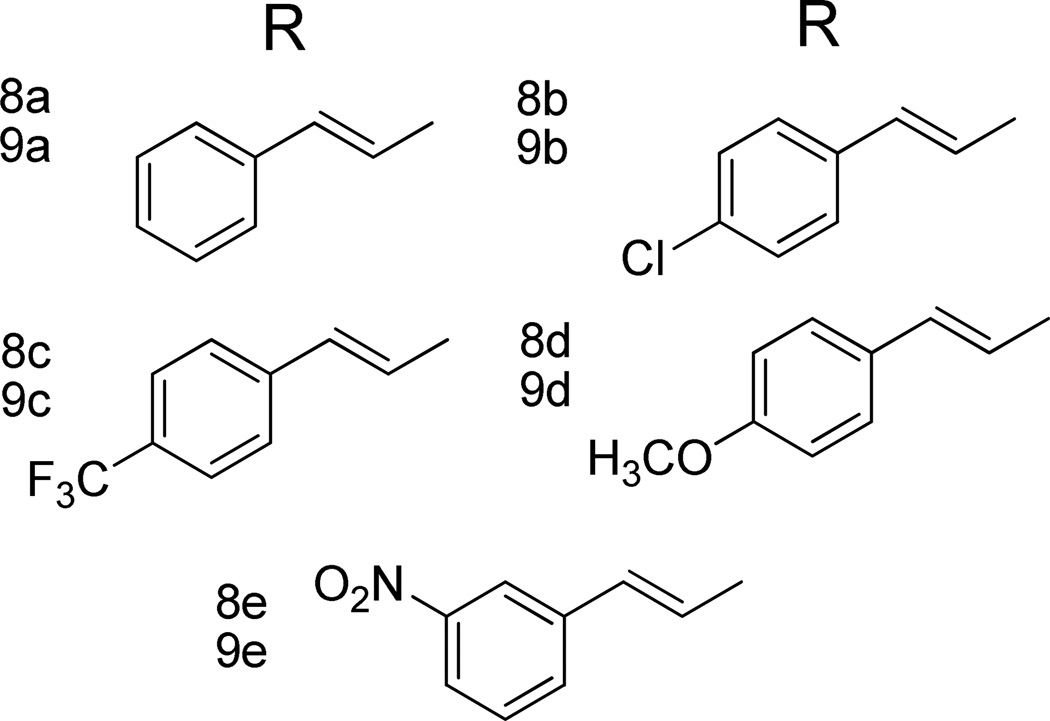
Figure 2. Acyl side chains of the synthesized 6β-cinnamoyl morphinamines and codeinamines.
The compounds showed moderate to high affinity for MOR-1 with lower affinities for KOR-1 and DOR-1 (Table 1). Not surprisingly, affinities of codeinamine-derived amides (9a–e) were significantly lower than those of the amides of morphinamine (8a–e). Derivatives 8a–e had very similar subnanomolar affinitites for MOR-1 irrespective of the aryl ring substituent of the acylamino moiety, while KOR-1 binding was approximately 10-fold lower, with highest KOR-1/MOR-1 Ki ratio for 8c (55.4) and the lowest for 8e (6.8). DOR-1 receptor affinity was roughly two magnitudes lower than MOR-1 affinity. DOR-1/MOR-1 Ki ratio for 8a was 102.6. Cinnamoyl morphinamines in our series lacked the marked KOR-1 affinity seen with the previously reported acylated aminomorphinans. [10]
Table 1.
Receptor binding and in vivo analgesia data of selected 6β-acylaminomorphinansa
| Compound | Affinity (Ki, nM) | Analgesia (ED50, mg/kg, s.c.) | ||
|---|---|---|---|---|
| MOR-1 | KOR-1 | DOR-1 | ||
| 8a | 0.10 ± 0.02 | 2.90 ± 0.66 | 10.26 ± 6.76 | 3.13 ± 1.09 |
| 8b | 0.15 ± 0.03 | 1.97 ± 0.01 | 9.38 ± 1.53 | >10 |
| 8c | 0.19 ± 0.09 | 10.52 ± 0.90 | 14.52 ± 6.82 | >10 |
| 8d | 0.74 ± 0.12 | 5.43 ± 1.38 | 15.26 ± 1.74 | >10 |
| 8e | 0.12 ± 0.006 | 0.81 ± 0.09 | 5.15 ± 0.75 | >10 |
| 9a | 4.73 ± 1.64 | >100 | >100 | >10 |
| 9b | 3.81 ± 0.15 | >100 | >100 | >10 |
| 9c | 5.44 ± 0.93 | >100 | >100 | >10 |
| 9d | 25.16 ± 13.02 | >100 | >100 | >10 |
| 9e | 3.74 ± 0.83 | >100 | >100 | >10 |
| morphine | 4.60 ± 1.81b | 4.96 ± 0.96c | ||
Competition studies were performed with the indicated compounds against 125I-BNtxA (0.1 nM) in membranes from CHO cells stably expressing the indicated cloned mouse opioid receptors. Ki values were calculated from the IC50 values [26] and represent the means ± SEM of at least three independent replications. 125IBNtxA KD values for MOR-1, KOR-1, DOR-1 sites were 0.11, 0.03 and 0.24, respectively.
Values from the literature. [7]
Values from the literature. [25]
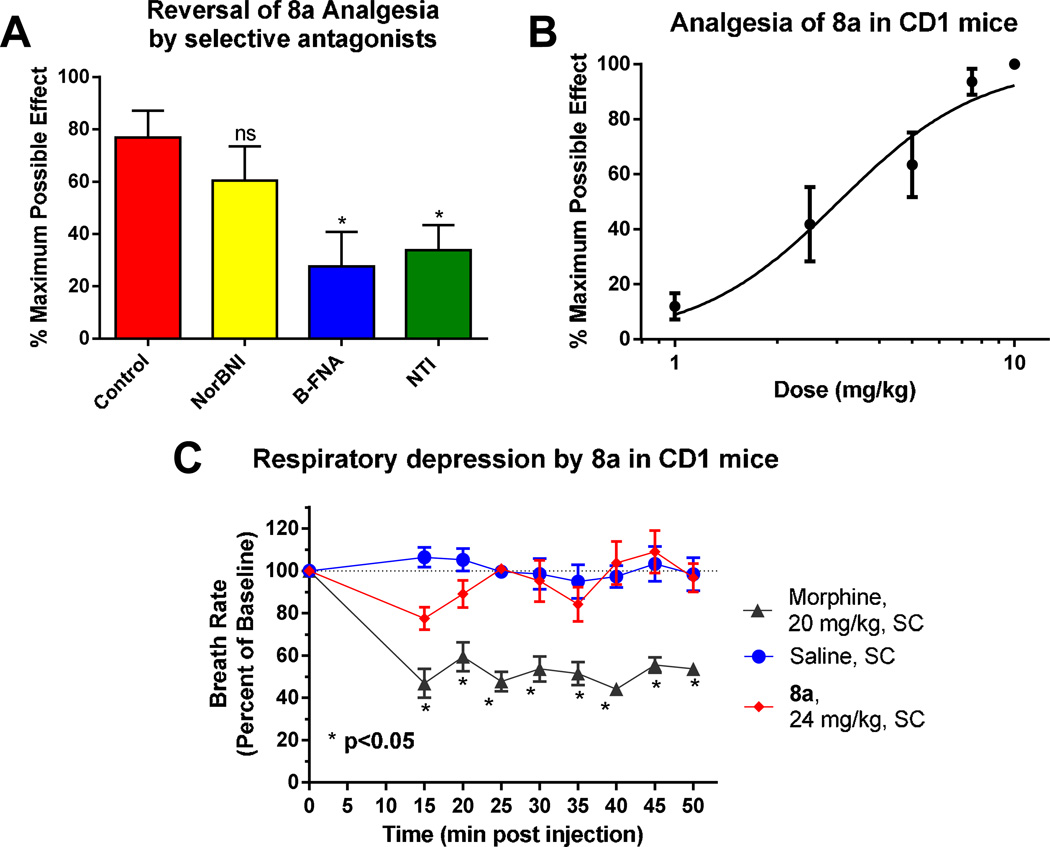
Next, the substances were administered to mice subcutaneously to determine their in vivo analgesic activity (Table 1, Figure 3 A). Somewhat surprisingly only the unsubstituted cinnamoyl morphamine analog 8a (ED50 3.13 ± 1.09 mg/kg) was analgesic with potency similar to that of morphine (4.96 ± 0.97 mg/kg s.c.). [25] Despite similar affinities against MOR-1, KOR-1 and DOR-1 binding, substitution on the aromatic ring of the cinnamoyl acid eliminated the analgesic activity of analogs (8b–e) at the highest dose tested, i.e. 10 mg/kg. Thus, the in vivo active compound 8a was selected as lead compound and further characterized. The analgesic activity of 8a was partially reversed by both the MOR-1 selective antagonist β-FNA and the DOR-1 selective antagonist NTI but, interestingly, was insensitive to the KOR-1 selective antagonist norBNI (Table 1, Figure 3 B).
Figure 3. Pharmacology of 6β-cinnamoylmorphinamine 8a.
(A) Analgesia: Cumulative dose-response curves were carried out on groups of mice (n = 10) with 8a at the indicated doses (s.c.) and analgesia tested 30 min later at peak effect. The ED50 value was 3.13 ± 1.09 mg/kg in CD1 mice by using the radiant heat tail-flick assay. Results were evaluated as %MPE [(observed latency − baseline latency)/(maximal latency − baseline latency)] and shown as the average of each group (n = 10). (B) Sensitivity of 8a to opioid antagonists: Groups of mice (n = 6) received a fixed dose of 8a (5 mg/kg, s.c.) alone or with NorBNI (10 mg/kg, s.c.), β-FNA (40 mg/kg, s.c.), NTI (20 mg/kg, s.c.). β-FNA and NorBNI were given 24h before 8a while NTI was given 15 min before 8a. Tail flick analgesia was measured 30 min after 8a. Similar results were observed in two independent replications. 8a analgesia is insensitive to NorBNI while analgesia is partially antagonized by both β-FNA and NTI (ANOVA followed by Bonferroni multiple comparison test (p < 0.05). (C) Respiratory rate. Animals were randomly assigned to receive saline (n = 3), 8a (24 mg/kg, n = 3), or morphine (20 mg/kg, n = 3). Each animal’s baseline average breath rate was measured every 5 min for 25 min before drug injection, and breath rates after drug injection are expressed as a percent of baseline. 8a did not depress respiratory rate and was not significantly different from saline at any time point, whereas morphine decreased respiratory depression in comparison with both saline and 8a (p < 0.05) as determined by repeated-measures ANOVA followed by Bonferroni multiple-comparison test.
The [35S]GTPγS-binding assay was used to characterize the functional activity of 8a on in vitro opioid transfected cell lines (Table 2). 8a produced near maximum stimulation in all cell lines when compared with the MOR-1 agonist DAMGO, DOR-1 agonist DPDPE and KOR-1 agonist U50,488H suggesting that it acts as a full agonist at all three opioid receptors. 8a showed a 25-fold and 4-fold selective potency for MOR-1 and DOR-1 over the KOR-1 in this assay which partially explains why in spite of high, nanomolar affinity for KOR-1 it does not exert KOR-mediated agonism in vivo, as shown by the in vivo behavior assay in which 8a analgesia could be antagonized by selective MOR-1 and DOR-1 antagonists but not by KOR-1 antagonist. Since in vivo behavior is a better predictor of functional activity, it is safe to say that analgesia of 8a is mediated by agonism at both MOR-1 and DOR-1.
Table 2.
Opioid receptor efficacy of 8aa
| Compound | EC50 (nM) | % stimulation | ||||
|---|---|---|---|---|---|---|
| MOR-1 | KOR-1 | DOR-1 | MOR-1 | KOR-1 | DOR-1 | |
| 8a | 1.38 ± 0.8 | 36.5 ± 19.2 | 9.1 ± 3.0 | 95.3 ± 0.8 | 106.3 ± 8.5 | 101.7 ± 2.8 |
| DPDPE | ndb | ndb | 2.05 ± 0.6 | |||
| DAMGO | 5.6 ± 3 | ndb | ndb | |||
| U50,488H | ndb | 21.7 ± 4.6 | ndb | |||
Efficacy data were obtained using agonist induced stimulation of [35S]GTPγS binding assay. Efficacy is represented as EC50 (nM) and percent maximal stimulation relative to standard agonist DAMGO (MOR-1), DPDPE (DOR-1), or U50,488H (KOR-1) at 100 nM. All values are expressed as the mean ± SEM of three separate assays performed in triplicate.
Not determined
Perhaps the most serious, potentially life-threatening side-effect of opiate therapy is respiratory depression. We tested the effect of 8a on the respiratory rate of CD1 mice and found that at doses approximately four times the ED50 morphine (20 mg/kg) caused the expected marked respiratory depression, while after treatment with even a high, 8-times the ED50 dose of 8a (24 mg/kg) no significant difference was found in respiratory rate compared with saline (Figure 3 C). Significantly reduced risk of respiratory depression has been reported in the literature for MOR-1/DOR-1 mixed opioid agonists, including DPI-3290 and the peptide MMP-2200. [27, 28]
In conclusion we have synthesized a series of novel 6β-cinnamoyl morphinamines carrying various cinnamoyl side chains. Characterization of compounds in vitro and in vivo revealed high affinity for MOR-1 receptors. Analgesic activity of 6β-cinnamoylmorphinamine was found to be comparable to morphine, but without causing respiratory depression, a major side-effect of commonly used opioids. Functional assays and reversal of analgesia by selective antagonists revealed an interesting receptor activity profile. The results suggest that new 6β-acylamino derivatives of morphine and its congeners are promising new opioids worthy of further investigation.
Supplementary Material
We designed and synthesized the 6β-acylamino derivatives of morphine and codeine
The synthesis of 10 novel potentially analgesic compounds is reported
The compounds were tested for opioid receptor binding and in vivo analgesia
6β-Cinnamoylmorphinamine were shown to possess MOR-1/DOR-1 analgesia
6β-Cinnamoylmorphinamine did not cause respiratory depression
Acknowledgements
This work was supported, in part, by research grants from the National Institute on Drug Abuse (DA034106-01) to SM, (DA06241, DA02165, and DA07242) to GWP, a Core Grant from the National Cancer Institute to MSKCC (CA08748) as well as the Technology Development Fund of Memorial Sloan-Kettering Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trescot AM, Datta S, Lee M, Hans H. Opioid pharmacology. Pain Physician. 2008;11:S133–S153. [PubMed] [Google Scholar]
- 2.Jiang JB, Hanson RN, Portoghese PS, Takemori AE. Stereochemical studies on medicinal agents. 23. Synthesis and biological evaluation of 6-amino derivatives of naloxone and naltrexone. J. Med. Chem. 1977;20:1100–1102. doi: 10.1021/jm00218a023. [DOI] [PubMed] [Google Scholar]
- 3.Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol. Dial. Transplant. 2010;25:1251–1257. doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- 4.Phan NQ, Lotts T, Antal A, Bernhard JD, Stander S. Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review. Acta Derm. Venereol. 2012;92:555–560. doi: 10.2340/00015555-1353. [DOI] [PubMed] [Google Scholar]
- 5.Sayre LM, Larson DL, Takemori AE, Portoghese PS. Design and synthesis of naltrexone-derived affinity labels with nonequilibrium opioid agonist and antagonist activities. Evidence for the existence of different μ receptor subtypes in different tissues. J. Med. Chem. 1984;27:1325–1335. doi: 10.1021/jm00376a018. [DOI] [PubMed] [Google Scholar]
- 6.Takemori AE, Portoghese PS. Affinity labels for opioid receptors. Annu. Rev. Pharmacol. Toxicol. 1985;25:193–223. doi: 10.1146/annurev.pa.25.040185.001205. [DOI] [PubMed] [Google Scholar]
- 7.Majumdar S, Burgman M, Haselton N, Grinnell S, Ocampo J, Pasternak AR, Pasternak GW. Generation of novel radiolabeled opiates through site-selective iodination. Bioorg. Med. Chem. Lett. 2011;21:4001–4004. doi: 10.1016/j.bmcl.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar Y-X, Pan J, Pasternak GW. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc. Natl. Acad. Sci. USA. 2011;108:19778–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majumdar S, Subrath J, Le Rouzic V, Polikar L, Burgman M, Nagakura K, Ocampo J, Haselton N, Pasternak AR, Grinnell S, Pan YX, Pasternak GW. Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated μ opioid receptor (MOR-1) splice variants. J. Med. Chem. 2012;55:6352–6362. doi: 10.1021/jm300305c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cami-Kobeci G, Neal AP, Bradbury FA, Purington LC, Aceto MD, Harris LS, Lewis JW, Traynor JR, Husbands SM. Mixed k/μ opioid receptor agonists: The 6β-naltrexamines. J. Med. Chem. 2009;52:1546–1552. doi: 10.1021/jm8015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derrick I, Lewis JW, Moynihan HA, Broadbear J, Woods JH. Potential irreversible ligands for opioid receptors. Cinnamoyl derivatives of beta-naltrexamine. J. Pharm. Pharmacol. 1996;48:192–196. doi: 10.1111/j.2042-7158.1996.tb07121.x. [DOI] [PubMed] [Google Scholar]
- 12.Derrick I, Moynihan HA, Broadbear J, Woods JH, Lewis JW. 6N-cinnamoyl-β-naltrexamine and its p-nitro derivative. High efficacy κ-opioid agonists with weak antagonist actions. Bioorg. Med. Chem. Lett. 1996;6:167–172. [Google Scholar]
- 13.Comer SD, Burke TF, Lewis JW, Woods JH. Clocinnamox: a novel, systemicallyactive, irreversible opioid antagonist. J. Pharmacol. Exp. Ther. 1992;262:1051–1056. [PubMed] [Google Scholar]
- 14.Derrick I, Neilan CL, Andes J, Husbands SM, Woods JH, Traynor JR, Lewis JW. 3-Deoxyclocinnamox: the first high-affinity, nonpeptide mu-opioid antagonist lacking a phenolic hydroxyl group. J. Med. Chem. 2000;43:3348–3350. doi: 10.1021/jm0009641. [DOI] [PubMed] [Google Scholar]
- 15.Moynihan H, Jales AR, Greedy BM, Rennison D, Broadbear JH, Purington L, Traynor JR, Woods JH, Lewis JW, Husbands SM. 14β-O-cinnamoylnaltrexone and related dihydrocodeinones are mu opioid receptor partial agonists with predominant antagonist activity. J. Med. Chem. 2009;52:1553–1557. doi: 10.1021/jm8012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdougall JM, Zhang XD, Polgar WE, Khroyan TV, Toll L, Cashman JR. Synthesis and biological evaluation of some 6-arylamidomorphines as analogues of morphine-6-glucuronide. Bioorg. Med. Chem. 2004;12:5983–5990. doi: 10.1016/j.bmc.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Ghirmai S, Azar MR, Cashman JR. Synthesis and pharmacological evaluation of 6-naltrexamine analogs for alcohol cessation. Bioorg. Med. Chem. 2009;17:6671–6681. doi: 10.1016/j.bmc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghirmai S, Azar MR, Polgar WE, Berzetei-Gurske I, Cashman JR. Synthesis and biological evaluation of alpha- and beta-6-amido derivatives of 17-cyclopropylmethyl-3, 14beta-dihydroxy-4, 5alpha-epoxymorphinan: potential alcohol-cessation agents. J. Med. Chem. 2008;51:1913–1924. doi: 10.1021/jm701060e. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Aschenbach LC, Chen J, Cassidy MP, Stevens DL, Gabra BH, Selley DE, Dewey WL, Westkaemper RB, Zhang Y. Design, synthesis, and biological evaluation of 6α- and 6β-N-heterocyclic substituted naltrexamine derivatives as μ opioid receptor selective Antagonists. J. Med. Chem. 2009;52:1416–1427. doi: 10.1021/jm801272c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Y, Elbegdorj O, Chen J, Akubathini SK, Beletskaya IO, Selley DE, Zhang Y. Structure selectivity relationship studies of 17-cyclopropylmethyl-3, 14β-dihydroxy-4,5α- epoxy-6β-[(4'-pyridyl)carboxamido]morphinan derivatives toward the development of the mu opioid receptor antagonists. Bioorg. Med. Chem. Lett. 2011;21:5625–5629. doi: 10.1016/j.bmcl.2011.06.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan Y, Elbegdorj O, Chen J, Akubathini SK, Zhang F, Stevens DL, Beletskaya IO, Scoggins KL, Zhang Z, Gerk PM, Selley DE, Akbarali HI, Dewey WL, Zhang Y. Design, synthesis, and biological evaluation of 17-cyclopropylmethyl- 3,14beta-dihydroxy-4,5alpha-epoxy-6beta-[(4'-pyridyl)carboxamido]morphinan derivatives as peripheral selective mu opioid receptor Agents. J. Med. Chem. 2012;55:10118–10129. doi: 10.1021/jm301247n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, Stevens DL, Braithwaite A, Scoggins KL, Bilsky EJ, Akbarali HI, Dewey WL, Zhang Y. 6beta-N-heterocyclic substituted naltrexamine derivative NAP as a potential lead to develop peripheral mu opioid receptor selective antagonists. Bioorg. Med. Chem. Lett. 2012;22:4731–4734. doi: 10.1016/j.bmcl.2012.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yekkirala AS, Lunzer MM, McCurdy CR, Powers MD, Kalyuzhny AE, Roerig SC, Portoghese PS. N-naphthoyl-beta-naltrexamine (NNTA), a highly selective and potent activator of mu/kappa-opioid heteromers. Proc. Natl. Acad. Sci. U. S. A. 2011;108:5098–5103. doi: 10.1073/pnas.1016277108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon C, Hosztafi S, Makleit S. Application of the Mitsunobu Reaction for Morphine Compounds. Preparation of 6β-Aminomorphine and Codeine Derivatives. Synth. Commun. 1992;22:913–921. [Google Scholar]
- 25.Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc. Natl. Acad. Sci. USA. 1993;90:5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 27.Gengo PJ, Pettit HO, O'Neill SJ, Su YF, McNutt R, Chang KJ. DPI-3290 [(+)-3- ((alpha-R)-alpha-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)-N-(3- fluorophenyl)-N-methylbenzamide]. II. A mixed opioid agonist with potent antinociceptive activity and limited effects on respiratory function. J. Pharmacol. Exp. Ther. 2003;307:1227–1233. doi: 10.1124/jpet.103.054429. [DOI] [PubMed] [Google Scholar]
- 28.Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ. In vivo characterization of MMP-2200, a mixed delta/mu opioid agonist, in mice. J. Pharmacol. Exp. Ther. 2011;336:767–778. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.