Significance
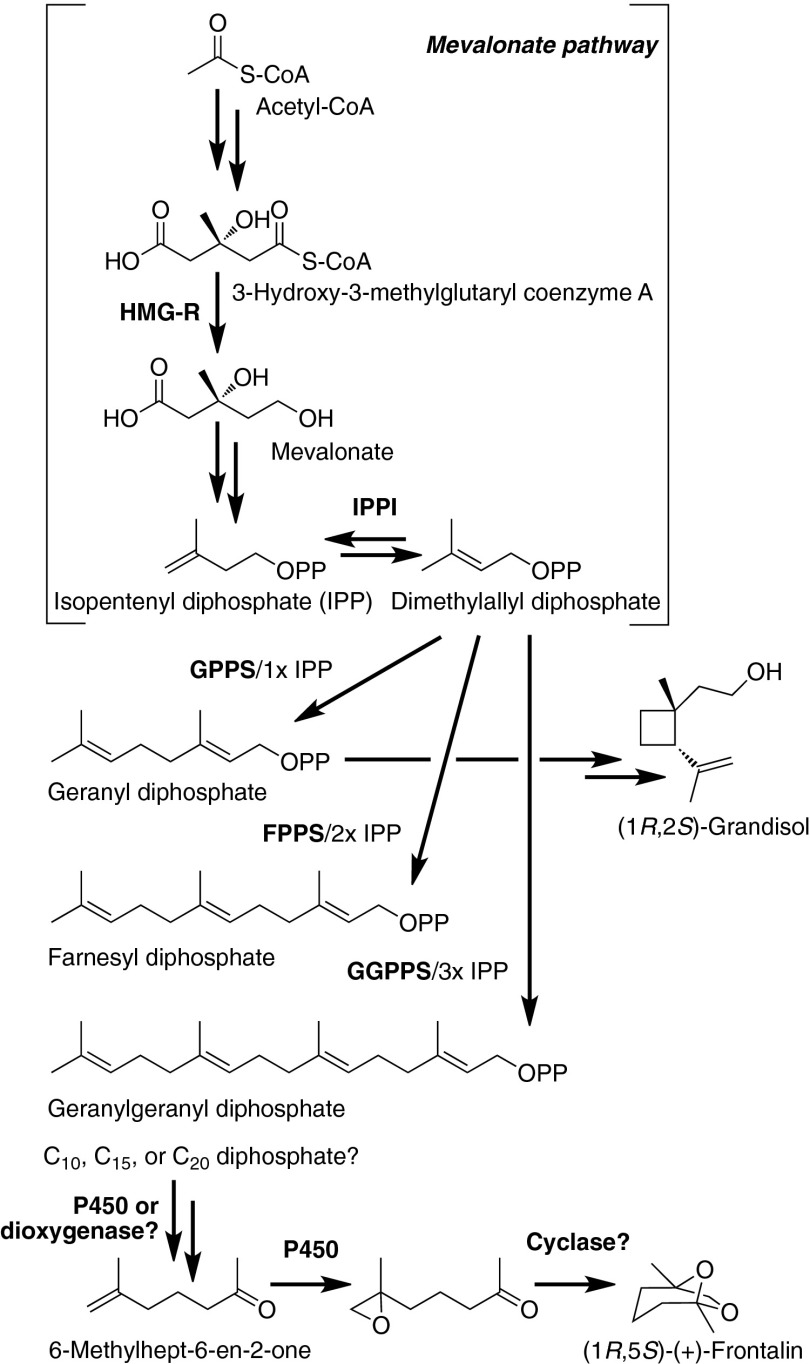
A long-standing question in pheromone biosynthesis is the origin of the mevalonate pathway-derived precursor to frontalin, a key pheromone to the successful mass attack of conifer hosts by Dendroctonus bark beetles. Using a combination of genome and transcriptome analysis, functional characterization of expressed proteins, RNA interference, and transcript and metabolite analysis, we provide evidence that frontalin in the mountain pine beetle (Dendroctonus ponderosae Hopkins) originates from the 20-carbon geranylgeranyl diphosphate rather than from 10-carbon geranyl diphosphate or 15-carbon farnesyl diphosphate. This result opens the way to study the later steps in frontalin biosynthesis.
Keywords: bark beetle, midgut, semiochemistry
Abstract
The mountain pine beetle (Dendroctonus ponderosae Hopkins) is the most destructive pest of western North American pine forests. Adult males produce frontalin, an eight-carbon antiaggregation pheromone, via the mevalonate pathway, as part of several pheromones that initiate and modulate the mass attack of host trees. Frontalin acts as a pheromone, attractant, or kairomone in most Dendroctonus species, other insects, and even elephants. 6-Methylhept-6-en-2-one, a frontalin precursor, is hypothesized to originate from 10-carbon geranyl diphosphate (GPP), 15-carbon farnesyl diphosphate (FPP), or 20-carbon geranylgeranyl diphosphate (GGPP) via a dioxygenase- or cytochrome P450-mediated carbon–carbon bond cleavage. To investigate the role of isoprenyl diphosphate synthases in pheromone biosynthesis, we characterized a bifunctional GPP/FPP synthase and a GGPP synthase in the mountain pine beetle. The ratio of GPP to FPP produced by the GPP/FPP synthase was highly dependent on the ratio of the substrates isopentenyl diphosphate and dimethylallyl diphosphate used in the assay. Transcript levels in various tissues and life stages suggested that GGPP rather than GPP or FPP is used as a precursor to frontalin. Reduction of transcript levels by RNA interference of the isoprenyl diphosphate synthases identified GGPP synthase as having the largest effect on frontalin production, suggesting that frontalin is derived from a 20-carbon isoprenoid precursor rather than from the 10- or 15-carbon precursors.
The mountain pine beetle (Dendroctonus ponderosae Hopkins; MPB) is the most destructive insect pest of western North American pine forests (1). Its strategy to overcome the chemical defenses of their host trees (2) is to mass attack individual trees using several different pheromones that initiate and modulate beetle aggregation behavior. (+)-Frontalin, (1R,5R)-1,5-dimethyl-6,8-dioxabicyclo[3.2.1]octane (Fig. 1), is an antiaggregation pheromone known to be synthesized de novo via the mevalonate pathway in male midgut tissue (3, 4). Frontalin acts as a pheromone, attractant, or kairomone in most other Dendroctonus species, other insects (5), and even elephants (6, 7) and has been studied in bark beetles for more than 40 y (8). Its biosynthetic precursor, 6-methylhept-6-en-2-one (6MHO), found in both insects and elephants (6, 9), is hypothesized to originate from cleavage of geranyl diphosphate (GPP), farnesyl diphosphate (FPP), or geranylgeranyl diphosphate (GGPP) by a dioxygenase or cytochrome P450 (10). These isoprenyl diphosphates are biosynthesized from the five-carbon precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) by the isoprenyl diphosphate synthases (IDSs) GPP synthase (GPPS), FPP synthase (FPPS), and GGPP synthase (GGPPS). However, it is not known which of these isoprenyl diphosphates is used as a precursor in frontalin biosynthesis.
Fig. 1.
Proposed biosynthetic pathway of frontalin in MPB. Grandisol in the WPB is proposed to be biosynthesized from geranyl diphosphate. P450, cytochrome P450.
A few IDSs have been characterized in Coleoptera, including an FPPS from the cotton boll weevil (Anthonomus grandis) (11), a GPPS/FPPS from the horseradish leaf beetle (Phaedon cochleariae) (12), and a unique GPPS from the pine engraver beetle (Ips pini) that also produces myrcene (13, 14). To assess their importance in pheromone biosynthesis, we identified and functionally characterized two IDSs in MPB. Based on three different lines of evidence, gene-expression patterns of the IDSs, enzyme activity of recombinant proteins, and the frontalin content of dsRNA-treated beetles, we show that frontalin originates from GGPP rather than the shorter isoprenyl diphosphates.
Results
Cloning and Functional Characterization of IDSs.
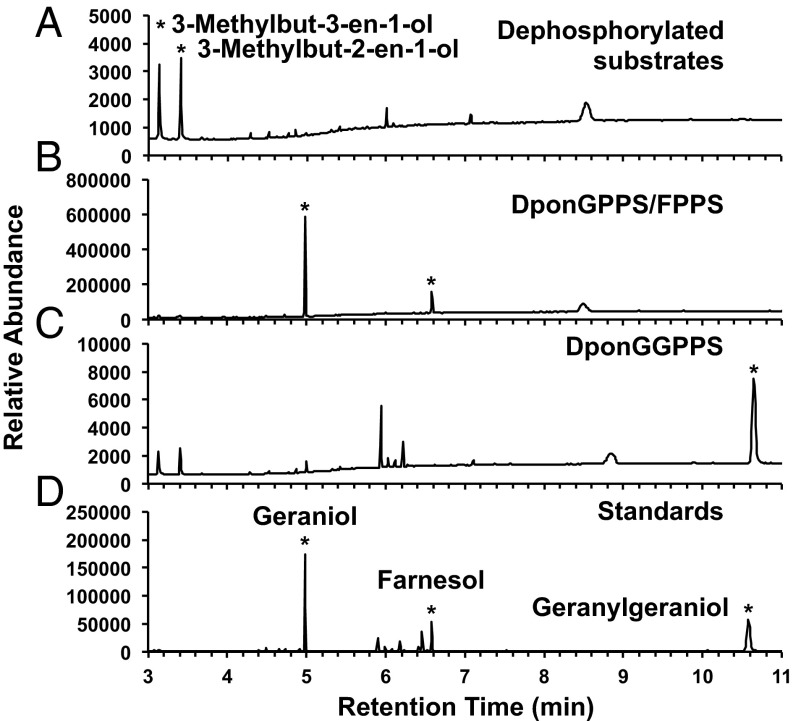
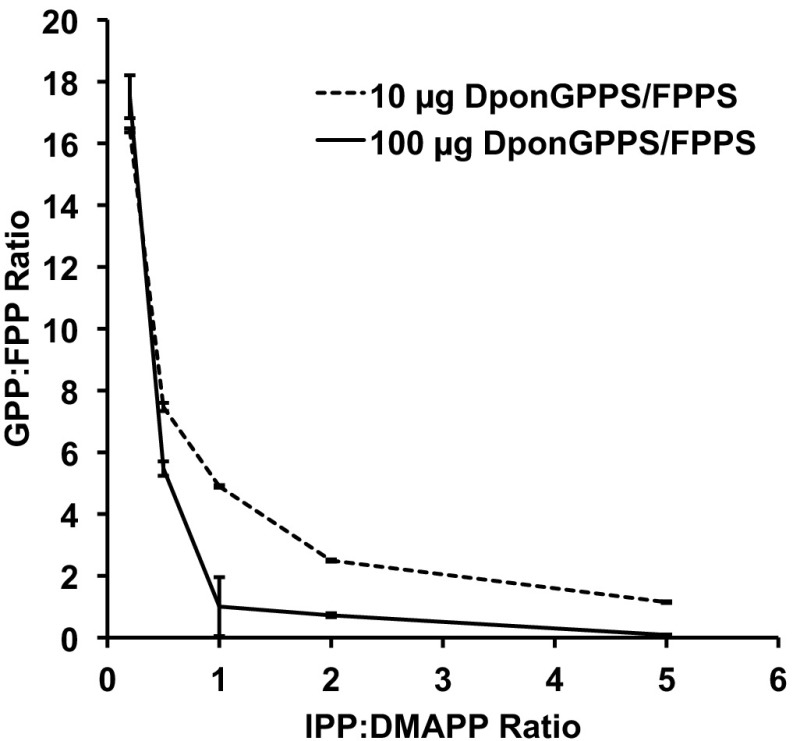
We identified two putative full-length MPB IDS cDNA clones, DPO044_J12 and DPO061_E16. The complete cDNA sequence of DPO044_J12 is 1,601 bp long, includes 82 bp and 229 bp of 5′ and 3′ UTRs, respectively, and encodes a predicted 429-aa protein with a mass of 49.4 kDa and a pI of 9.00. The complete cDNA sequence of DPO061_E16 is 1,033 bp long, includes 73 bp and 66 bp of 5′ and 3′ UTRs, respectively, and encodes a predicted 297-aa protein with a mass of 34.3 kDa and a pI of 5.97. Both proteins had the two aspartate-rich motifs (Fig. S1) characteristic of IDSs (15). Heterologous expression and in vitro assays with IPP and DMAPP as substrates produced both GPP and FPP for DPO044_J12 and only GGPP for DPO061_E16 (Fig. 2). We thus designated the clones as DponGPPS/FPPS and DponGGPPS, respectively. When assayed with a range of IPP:DMAPP substrate ratios between 5:1 and 1:5, DponGGPPS produced GGPP with only a trace of GPP (Fig. S2). However, the ratio of GPP to FPP produced by DponGPPS/FPPS was strongly dependent on the IPP:DMAPP ratio, with more FPP being produced at higher IPP:DMAPP ratios (Fig. 3). DponGPPS/FPPS readily used IPP and GPP as substrates to make FPP, and DponGGPPS readily used IPP and GPP or FPP as substrates to make GGPP. For comparative purposes, we also identified and functionally characterized PstrGGPPS from the white pine weevil [Pissodes strobi (Peck); WPW] (Fig. S3).
Fig. 2.
In vitro assays of recombinant IDS activities. DB-WAX GC/MS traces of the dephosphorylated IDS assay products when the Ni-affinity–purified recombinant protein was incubated with 50 µM DMAPP and 50 µM IPP. (A) Substrates DMAPP and IPP after dephosphorylation. (B) Dephosphorylated assay products of DponGPPS/FPPS. (C) Dephosphorylated assay products of DponGGPPS. (D) Standards of the potential dephosphorylated products. Total ion current traces are shown for substrates, DponGPPS/FPPS, and standards; single-ion monitoring of m/z 69, 81, 86, 93, and 204 were summed for DponGGPPS trace. Asterisks indicate product peaks in B and C and the labeled standards in A and D.
Fig. 3.
Sensitivity of product outcome to IPP:DMAPP substrate ratios in DponGPPS/FPPS. Product GPP:FPP ratios differed when different IPP:DMAPP ratios were incubated with DponGPPS/FPPS. The IPP and DMAPP concentrations were varied 50:10, 25:50, 50:50, 50:25, and 50:10 µM and were incubated with two quantities of Ni-affinity–purified recombinant DponGPPS/FPPS. Total ion current was used to determine product ratios. Error bars indicate the range between duplicate assays.
Protein Quaternary Structure.
Protein cross-linking experiments revealed that DponGPPS/FPPS is a monomer, whereas DponGGPPS forms multimers of up to five units, and PstrGGPPS forms multimers of up to eight units (Fig. S4). For comparison, the human FPPS, the cotton boll weevil FPPS, and the horseradish leaf beetle GPPS/FPPS are dimers (11, 12, 16); human GGPPS is an octamer (17); and bovine GGPPS is a multimer of four to five units (18).
Sequence Relatedness with Other Insect IDSs.
DponGPPS/FPPS protein was 99% identical to a putative FPPS from Dendroctonus jeffreyi (13) and shared 75%, 70%, 62%, 62%, 50%, and 49% amino acid identity with the FPPSs from A. grandis (11), I. pini (13), Tribolium castaneum (GenBank accession no. XP_972537), P. cochleariae (12), Choristoneura fumiferana (19), and Drosophila melanogaster (GenBank accession no. CAA08919), respectively (Fig. S1). DponGGPPS shared 68%, 65%, and 56% amino acid identity with the GGPPSs from T. castaneum (GenBank accession no. XP_971444), C. fumiferana (20), and D. melanogaster (21), respectively. DponGPPS/FPPS and DponGGPPS shared 28% and 16% amino acid identity, respectively, with the GPPS/myrcene synthase (IpinGPPS/MS) from I. pini (13). We found no evidence for an ortholog of this unique I. pini gene (14) in the genome or transcriptome of MPB. PstrGGPPS shared 77% amino acid identity with DponGGPPS.
Orthologs of the two MPB IDS genes also were found in a southern pine beetle (Dendroctonus frontalis; SPB) transcriptome assembly. Two allelic SPB sequences [National Center for Biotechnology Information (NCBI) Transcriptome Shotgun Assembly (TSA) accession nos. GAFI01015128 and GAFI01015578] share 94% amino acid identity with DponGPPS/FPPS, and two other allelic SPB sequences (NCBI TSA accession nos. GAFI01012895 and GAFI01012995) share 90–93% amino acid identity with DponGGPPS. Although we have not characterized these sequences functionally, we designate them DfroGPPS/FPPS-like and DfroGGPPS-like, respectively (Fig. S1). The phylogeny of the insect IDSs showed two separate clades for the GGPPSs and for the FPPSs and GPPS/FPPSs (Fig. S5). IpinGPPS/MS appears separate, but there is poor bootstrap support for its exclusion from the other clades.
Gene Structures of DponGPPS/FPPS and DponGGPPS.
Gene structures of DponGPPS/FPPS and DponGGPPS were determined from both the male and female draft MPB genome assemblies (22) and were consistent between the two assemblies. Only one locus for each gene could be found in either assembly. DponGPPS/FPPS (represented by GenBank accession no. YQE_11313) was located in a 993,900-bp male genomic scaffold (GenBank accession no. KB741247) and in a 772,667-bp female genomic scaffold (GenBank accession no. KB632281). DponGGPPS (represented by GenBank accession no. YQE_09494, a partially incorrect gene model from automated annotation) was located in a 428,599-bp male genomic scaffold (GenBank accession no. KB741092) and in a 445,202-bp female genomic scaffold (GenBank accession no. KB632050). The transcribed portion of DponGPPS/FPPS spanned 6,205 bp with seven introns, whereas the transcribed portion of DponGGPPS spanned 1,709 bp with six introns (Fig. S6).
Transcript Levels of DponGPPS/FPPS and DponGGPPS.
Quantitative RT-PCR (qRT-PCR) analysis of DponGPPS/FPPS and DponGGPPS showed the highest levels for both transcripts in the midguts of males that had fed for 24 h while paired with females as compared with the other developmental stages (whole larvae and pupae, and the midguts of teneral adults, unfed adults, and females that had fed alone for 24 h or had fed for an additional 24 h after being paired with males) (Fig. 4 A and D). The tissue distribution in male adults showed the highest levels of both transcripts in anterior midguts, and the expression in anterior midguts was inducible by feeding (Fig. 4 B and E). The expression of DponGGPPS appeared more midgut specific than DponGPPS/FPPS. A feeding time course revealed that DponGPPS/FPPS transcript levels in the midgut varied with feeding and increased in females (Fig. 4C). However, DponGGPPS transcript levels in the midgut increased strongly with feeding in males (Fig. 4F) and were higher than in females.
Fig. 4.
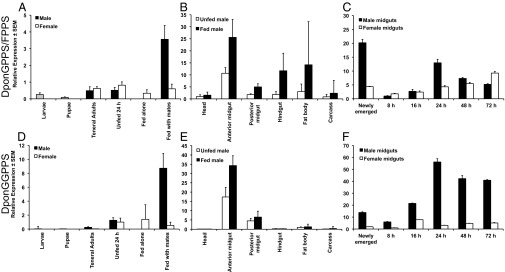
Transcript levels of DponGPPS/FPPS and DponGGPPS. Transcript levels determined by qRT-PCR in whole larvae and pupae and in the midguts of teneral adults, unfed adults, females that had fed alone for 24 h or had fed an additional 24 h after being paired with males, and males that had fed for 24 h with a female (n = 3) (A and D); in adult male tissues (n = 4) (B and E); and in midguts of adults fed for various times (n = 3) (C and F). Bars for larvae and pupae are shown in gray because their sex was not determined. The data in D and E have been reported previously (25).
Role of IDSs in Frontalin Production in Vivo.
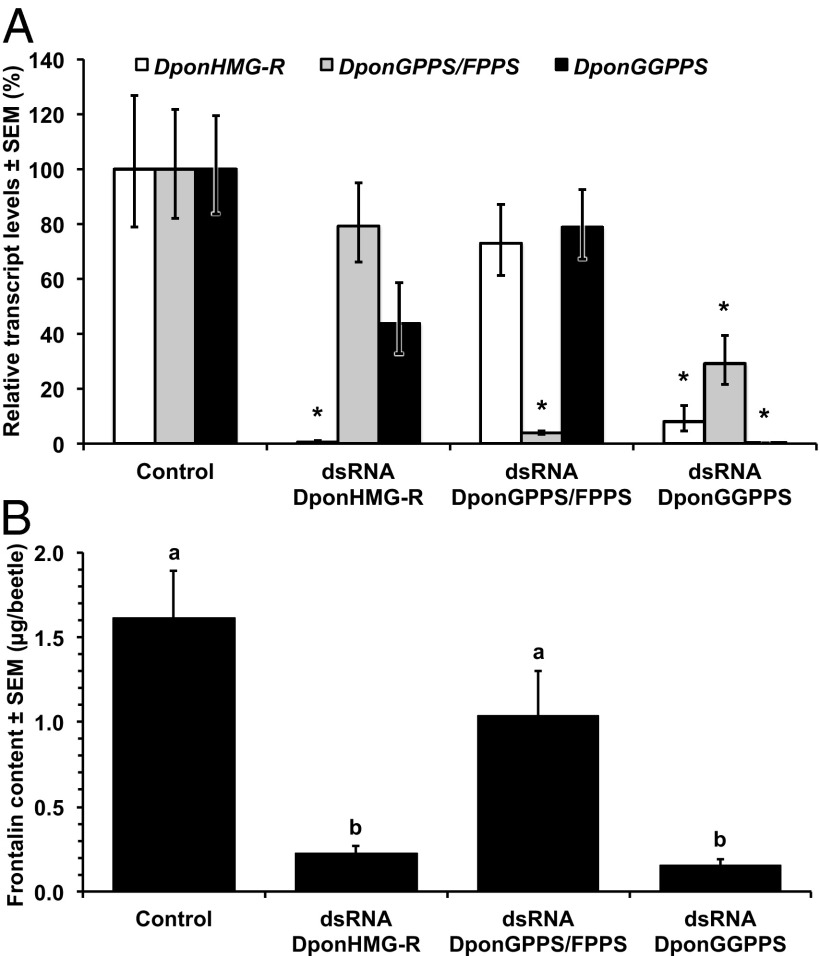
We successfully used RNAi to investigate IDS gene function in MPB. Beetle survival after dsRNA injection was acceptable, with 56–83% of the beetles surviving for the full 19 d of the experiment, compared with 87% of the noninjected controls (Fig. S7). Fig. 5 shows the differences in transcript levels and frontalin content of dsRNA-injected beetles. Injection of dsRNA significantly reduced transcript levels (Fig. 5A) and was largely gene specific. The transcript levels of hydroxy-3-methylglutaryl-CoA reductase (HMG-R) in D. ponderosae (DponHMG-R) were significantly different between treatments (one-way ANOVA F3,34 = 26.5, P < 0.00001): DponHMG-R transcript levels of beetles injected with dsRNA DponHMG-R were only 0.6% of the levels in control beetles. Transcript levels of DponGPPS/FPPS were significantly different between treatments (one-way ANOVA F3,35 = 44.1, P < 0.00001): DponGPPS/FPPS transcript levels in beetles injected with dsRNA DponGPPS/FPPS were only 3.9% of the levels in control beetles. Transcript levels of DponGGPPS were significantly different between treatments (one-way ANOVA F3,36 = 108, P < 0.00001): DponGGPPS transcript levels in beetles injected with dsRNA DponGGPPS were only 0.2% of the levels in control beetles. Surprisingly, DponHMG-R transcript levels were reduced to 8.0% and DponGPPS/FPPS levels were reduced to 29.2% in beetles injected with dsRNA DponGGPPS.
Fig. 5.
RNAi of frontalin biosynthesis. (A) Transcript levels for DponHMG-R, DponGPPS/FPPS, and DponGGPPS in juvenile hormone-treated male adults injected with dsRNA for DponHMG-R, DponGPPS/FPPS, and DponGGPPS. Asterisks indicate treatments that were significantly different from the control (P < 0.05, by two-tailed t test with Holm–Bonferroni correction on the ∆∆CT values, after significant one-way ANOVA tests; n = 10). (B) Frontalin levels in juvenile hormone-treated male adults injected with dsRNA for DponHMG-R, DponGPPS/FPPS, and DponGGPPS (one-way ANOVA: F3,44 = 12.1383, P < 0.00001, n = 12). Treatments with the same letter are not significantly different (P < 0.05, by two-tailed t test with Holm–Bonferroni correction).
Frontalin content differed significantly between treatment groups (one-way ANOVA F3,44 = 12.1383, P < 0.00001) (Fig. 5B). As would be expected for one of the most highly regulated and central genes in the mevalonate pathway (23), injection of DponHMG-R dsRNA significantly reduced frontalin content to 14% of the level in control beetles. DponGGPPS dsRNA also reduced frontalin content significantly, to 9% of the level in control beetles, a level not significantly different from the results obtained with DponHMG-R dsRNA. Injection of DponGPPS/FPPS dsRNA did not reduce frontalin content significantly. For comparison, exo-brevicomin content, a male-produced pheromone not biosynthesized via the mevalonate pathway, was unaffected by any of the dsRNA injections (one-way ANOVA F3,44 = 0.913; not significant).
Discussion
Frontalin, the antiaggregation pheromone of male MPB (24) and a pheromone of most Dendroctonus species, was first identified more than 40 y ago in the alimentary canal of male SPB (8). Its precursor later was identified in MPB as 6MHO (9). Additional studies implicated the mevalonate pathway in frontalin biosynthesis (3) and identified the anterior midgut as the site of its biosynthesis (4). A dual-labeling experiment with [1-14C]- and [1-3H]-isopentenol has eliminated the possibility that frontalin originates from direct elongation of IPP via the fatty acid synthesis pathway (3). The precursor to 6MHO has yet to be determined, although GPP was proposed to be the substrate of a dioxygenase/P450 to produce 6MHO (10). To gain further insight into this question, we identified two IDSs in MPB, which we characterized as DponGPPS/FPPS and DponGGPPS. We also identified and functionally characterized a GGPPS from P. strobi (Peck) (PstrGGPPS) and identified GPPS/FPPS-like and GGPPS-like transcripts from SPB (DfroGPPS/FPPS-like and DfroGGPPS-like). Unlike IpinGPPS/MS, which produces myrcene in addition to GPP (13), we saw no significant pentane-extractable products from any of the IDS tested without prior alkaline phosphatase treatment. In addition, an ortholog to the I. pini GPPS/MS (13) could not be found in the MPB genome (22) or in the MPB, SPB, or WPW transcriptome assemblies (NCBI TSA accession no. GAEO00000000) (25, 26), reinforcing the unique role that IpinGPPS/MS plays in pheromone biosynthesis in I. pini (13, 14).
For DponGPPS/FPPS, the GPP/FPP product ratio was strongly dependent on the IPP:DMAPP substrate ratio, implying that the expression and activity of isopentenyl diphosphate isomerase (IPPI) could have a large impact on the product outcome for this IDS in vivo (Fig. 1). In I. pini, the IPPI transcript is up-regulated by both juvenile hormone III and feeding in adults for pheromone biosynthesis (27, 28). Dual-function GPPS/FPPS enzymes have been reported previously in other insects (12, 29, 30). In the horseradish leaf beetle, P. cochleariae, the GPP/FPP product ratio is affected by the divalent metal cofactor used in the assay (12). Using site-directed mutagenesis, a previous study has shown that two residues (indicated in bold) preceding the first aspartate-rich motif of the IDS from the aphid Acyrthosiphon pisum (ApisGPPS/FPPS, YQLVLDDIMD, at positions 194–203 of the alignment in Fig. S1) determines the GPP:FPP ratio for a specific IPP:DMAPP ratio (30). Mutating the Q residue to F in ApisGPPS/FPPS results in the GPP:FPP ratio switching from 2:1 to 1:9. In the case of DponGPPS/FPPS, the corresponding residues are YFLILDDIMD, and the GPP:FPP ratio at a corresponding IPP:DMAPP ratio was ∼7:1 (Fig. 3). These differences highlight how divalent metal cofactors and single amino acid changes can affect product outcomes and how orthologous proteins in distantly related species may not share the same product ratios.
Real-time PCR analyses of the transcript levels of the MPB genes showed that both IDSs were induced by feeding, with most of the transcript induction and accumulation occurring in the male anterior midgut, the site of frontalin biosynthesis in males (3, 4). Although both transcripts showed induction with feeding, DponGGPPS had the most male-specific, anterior midgut-specific, and feeding-inducible expression. The expression patterns of DponGPPS/FPPS and DponGGPPS suggested that the precursor to 6MHO is most likely to be GGPP produced by DponGGPPS. This result was surprising, because the eight-carbon 6MHO could be produced from a 10-carbon GPP precursor or a 15-carbon FPP precursor rather than from the 20-carbon GGPP. However, cases of carbon loss or chain shortening have been described in lepidopteran (31) and honey bee pheromone biosynthesis (32).
To test further our hypothesis that DponGGPPS provides the isoprenyl precursor for frontalin biosynthesis, we successfully used RNAi to reduce the transcript levels of DponHMG-R, DponGPPS/FPPS, and DponGGPPS significantly. Reduction of the DponHMG-R and DponGGPPS transcripts significantly decreased male frontalin content, but reduction of DponGPPS/FPPS transcript did not. Surprisingly, injection of the DponGGPPS dsRNA also significantly reduced the transcript levels of both DponHMG-R and DponGPPS/FPPS. Off-target analysis found fewer than 10 consecutive complementary base pairs and matches no higher than 14 of 15 bp, and 12 of 13 bp between dsRNA DponGGPPS and the DponHMG-R and DponGPPS/FPPS transcripts, respectively. These data suggested that dsRNA DponGGPPS was not targeting these transcripts directly but instead that feedback mechanisms may exist to modulate the expression levels of genes earlier in the mevalonate pathway. Despite the multiple effects of dsRNA DponGGPPS injection on DponGGPPS, DponGPPS/FPPS, and DponHMG-R transcript levels, the combined results from our RNAi analysis allowed us to draw specific conclusions concerning the role of DponGPPS/FPPS and DponGGPPS in frontalin biosynthesis. Given that (i) frontalin originates from GPP, FPP, or GGPP; (ii) dsRNA of DponGPPS/FPPS had no effect on frontalin levels; (iii) dsRNA of DponGGPPS had a major effect on reducing frontalin levels; and (iv) no additional IDS genes were found in the MPB genome, we conclude that DponGGPPS and not DponGPPS/FPPS contributes the isoprenyl diphosphate precursor for frontalin biosynthesis in MPB.
The gene structures of DponGPPS/FPPS and DponGGPPS contain seven and six introns, respectively, which are two more than and equal to, respectively, the orthologous genes in Drosophila melanogaster (21). Two intron splice sites were conserved between DponGPPS/FPPS and DmelFPPS, and four of the intron splice sites were conserved between DponGGPPS and DmelGGPPS (Fig. S6). Comparing DponGPPS/FPPS and DmelFPPS with DponGGPPS and DmelGGPPS, several intron splice sites were near each other, but no splice sites were exactly conserved between the two gene types, confirming the phylogeny shown in Fig. S5, which suggests that these genes diversified before species diversification. Only one copy of each MPB gene was found in the draft genome, whereas more than one FPPS are found in some Lepidoptera (33). In MPB, the only juvenile hormone biosynthesized is juvenile hormone III, whereas some Lepidoptera also have juvenile hormone 0, juvenile hormone I, or juvenile hormone II (34). These other types of juvenile hormone contain one or more ethyl branches rather than the methyl branches of juvenile hormone III, perhaps necessitating the additional IDSs in Lepidoptera to accommodate the larger substrates.
Conclusions
Using a combination of genome and transcriptome analysis, functional characterization of expressed proteins, RNAi, and transcript and metabolite analysis, we have provided evidence supporting the hypothesis that the pheromone frontalin originates from the 20-carbon GGPP synthesized by DponGGPPS rather than from the bifunctional DponGPPS/FPPS. This finding answers a long-standing question about the origin of frontalin via the mevalonate pathway and opens the way for studying the latter steps in the biosynthesis of frontalin, a pheromone that is key to the successful mass attack of conifer hosts by Dendroctonus beetles.
Materials and Methods
Identification, Cloning, Expression, and Purification of IDSs.
Putative full-length IDS cDNA clones DPO044_J12 and DPO061_E16 were identified from transcriptome assemblies of MPB (NCBI TSA accession nos. GAFW00000000 and GAFX00000000) (25, 26) by BLASTx and tBLASTn comparisons with known insect IDSs and were fully sequenced. Sequences were confirmed and their gene structures were determined by BLASTn searches of the male MPB draft genome assembly (22) and alignment with exonerate (35). Orthologs to the putative MPB IDSs were identified in the transcriptome assembly of the SPB (Dendroctonus frontalis, NCBI BioProject: PRJNA187208; NCBI TSA accession nos. GAFI01015577 and GAFI01012895) by tBLASTn. For functional and phylogenetic comparisons, an ortholog of DPO061_E16 in WPW [Pissodes strobi (Peck)] was identified and functionally characterized (SI Materials and Methods). A maximum-likelihood phylogeny of insect IDSs was created with FastTree 2.1.7 (36) from a protein alignment prepared by MUSCLE (37) in CLC Main Workbench 6.8.4 (CLC Bio). Sequences were deposted in GenBank [accession nos. AFI45068 (DponGPPS/FPPS, DPO044_J12); AFI45070 (DponGGPPS, DPO061_E16); and KC464331 (PstrGGPPS, PST046_L05)]. The full ORFs of the putative IDSs were cloned into the pET28b(+) vector (Novagen). The recombinant C-terminal 6xHis-tagged enzymes were Ni-affinity purified (SI Materials and Methods).
IDS Assay and Product Analysis.
IDS activity was tested in a 500-µL reaction volume containing 50 mM HEPES buffer (pH 7.2), 5 mM MgCl2, 10% (vol/vol) glycerol, 5 mM fresh DTT, and 50:0, 50:10, 50:25, 50:50, 25:50, 10:50, or 0:50 µM of IPP:DMAPP substrates (Sigma) and 10 or 100 µg of purified enzyme. Assays were incubated for 1 h at 30 °C and then were treated with 10 U of calf-alkaline phosphatase (Invitrogen) for 12 h at 37 °C to hydrolyze the diphosphate substrates and products. The alcohol products were extracted with pentane and analyzed by GC/MS. Assay products were analyzed on an Agilent DB-WAX column (polyethylene glycol, 30 m long, 250 µm i.d., 0.25-µm film) at 1 mL⋅min−1 He on an Agilent 6890N GC, 7683B series autosampler and a 5975 Inert XL MS Detector at 70 eV. The GC temperature program was 40 °C for 3 min, 10 °C⋅min−1 to 240 °C, hold 15 min, pulsed splitless injector held at 240 °C. Compounds were identified by comparison with authentic geraniol, farnesol, and geranylgeraniol (Sigma), and 3-methylbut-2-en-1-ol and 3-methylbut-3-en-1-ol, the dephosphorylated substrates.
Protein Cross-Linking to Determine Quaternary Structure.
Protein cross-linking was accomplished with 5 µg and 10 µg dimethyl suberimidate (Pierce Biotechnology) and 4 µg of purified protein in 20 µL 0.2 M triethanolamine⋅HCl at pH 8.0 according to manufacturer’s instructions. After incubation at room temperature for 30 or 60 min, the reaction was quenched by the addition of 20 µL SDS/PAGE sample buffer, and the product was analyzed by SDS/PAGE.
Analysis of DponGPPS/FPPS and DponGGPPS Transcript Levels.
Transcript levels of DponGPPS/FPPS and DponGGPPS in beetles collected from infested lodgepole pine from the Whittell Forest and Wildlife Area (Little Valley, NV, approximately N 39°16′30″ W 119°52′41″) were analyzed by qRT-PCR relative to the endogenous reference gene beta-tubulin (DponbTubulin; GenBank accession no. AEE63092) using the ∆∆CT method (38). qRT-PCR primers to amplify ∼100-bp amplicons were designed with Primer Express 2.0 (Applied Biosystems) at the Nevada Genomics Center (Table S1) and were examined for primer–dimer and hairpin formation using Vector NTI 9.0 (Invitrogen) and for nonspecific amplification by visual inspection of their melting curves; their amplification efficiencies were calculated using relative standard curves. Total RNA was isolated using the RNeasy Plant Mini Kit (Qiagen), followed by RNase-free DNase digestion (Qiagen) and was purified further with the MasterPure RNA Purification Kit (Epicentre). First-strand cDNA was prepared from 500 ng RNA for each sample using random primers (Invitrogen) and SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer’s protocol.
Development.
First-strand cDNA templates were prepared from the whole bodies of unsexed MPB larvae and pupae and from the midguts and associated fat bodies of teneral adults, unfed adults, and lodgepole pine phloem-fed adults of both sexes. Because adult males normally do not feed in the absence of a female, females were introduced into a pine bolt and allowed to feed for 24 h before a male was placed at the entrance of each female’s gallery. Both male and female then were allowed to feed for an additional 24 h. Beetles were dissected with the aid of a stereomicroscope while pinned underwater. There were three biological replicates per treatment, each with tissue pooled from 20 insects, except for the pupal samples, which had six insects pooled per replicate.
Tissue distribution.
First-strand cDNA templates were prepared from the anterior midguts, posterior midguts, hindguts, heads, fat bodies, and carcasses of adult male MPB that had fed for 24 h on lodgepole pine phloem with females. Four biological replicates were measured, each containing tissue from five to eight beetles.
Time course.
First-strand cDNA templates were prepared from the anterior midguts of male and female MPB that had fed on lodgepole pine phloem for various times. Females were allowed feed for 24 h before a male was introduced into her gallery. The beetles then were allowed to feed for an additional 8, 16, 24, 48, or 72 h. Three biological replicates were measured, each containing tissue from 20 beetles.
RNAi.
We prepared dsRNA from the pDNR-Lib clones described above using T7-modified primers (Table S1) and the MEGAscript RNAi Kit (Invitrogen) following the manufacturer’s instructions. As a positive RNAi control, DponHMG-R (GenBank accession no. KF444677) was amplified from cDNA of midgut/fat body from juvenile hormone-treated adult males, cloned into pJet1.2 (Thermo Scientific), and fully sequenced. Cloning primers (Table S1) were designed based on the DponHMG-R sequence derived from transcriptome sequencing (NCBI TSA accession no. GAFX01005425) (26). We minimized off-target effects by dividing each dsRNA sequence in silico into all possible 21mers and ensuring at least two mismatches by BLASTn (-task blastn-short) to all nontarget gene models (22). Teneral adults collected from infested lodgepole pine bolts from the Cheakamus Community Forest, Whistler, BC, Canda (approximately N 50°10′17′′ W 122°52′35′′) were injected with 200 ng of dsRNA (concentration 400 ng⋅µL−1) into the ventral abdomen with a Picospritzer II microinjector (General Valve Corporation). Uninjected beetles were used as a negative control. Each treatment had 24 beetles. After injection, the beetles were kept in the dark for 18 d to mature at room temperature in plastic, flat-bottom, 24-well culture plates with each well containing one beetle, two disks of lodgepole pine phloem, a disk of lodgepole pine phloem with bark, and a sprinkle of dried baker’s yeast between the layers. Moisture in the plate was maintained with a moist Kimwipe (Kimberly-Clark) in the lid of the plate. Beetles then were held together in treatment groups in cylindrical cages under ambient laboratory daylight for 24 h before treatment with 1 µg juvenile hormone III (Sigma) in 0.5 µL acetone on their abdominal venter to induce frontalin biosynthesis. Beetles then were held individually for 16 h in 24-well culture plates with only the moist Kimwipe in the lid. The beetles then were flash frozen in liquid N2 and stored at −80 °C.
Individual beetles were extracted for both metabolite and transcript analysis. For metabolite analysis, 12 beetles from each treatment were individually pulverized with the tip of a glass rod on dry ice in 500 µL of methyl tertiary-butyl ether containing 1 ng⋅µL−1 tridecane as an internal standard. The samples were extracted for 30 min with shaking at 4 °C. This extraction was repeated again with an additional 500 µL of methyl tertiary-butyl ether with internal standard. Extracts were combined and centrifuged at 1,000 × g for 5 min, and then a 100-µL sample was removed for GC/MS analysis. The pulverized samples were returned to −80 °C for subsequent RNA extraction. Metabolite samples were analyzed with an Agilent DB-WAX column (polyethylene glycol, 30 m long, 250 µm i.d., 0.25-µm film) at 1 mL⋅min−1 He on an Agilent 7890A GC, Agilent GC Sampler 80, and a 5975C Inert XL MS Detector at 70 eV. The GC temperature program was as follows: 40 °C for 3 min, 6 °C⋅min−1 to 110 °C, 15 °C⋅min−1 to 240 °C, hold 5 min, pulsed splitless injector held at 250 °C. The MS was run in timed SIM mode such that frontalin and exo-brevicomin and the closely eluting internal standard tridecane had optimized SIM ions of 72, 100, and 142 and 85, 114, and 156, respectively. Absolute quantities of frontalin per beetle were calculated using a standard curve of frontalin against the internal standard.
Total RNA was extracted individually from each of the beetles using the RNeasy Mini Plant Kit (Qiagen) with on-column DNase treatment. The quantity and quality of RNA were examined using the Agilent 2100 Bioanalyzer and the Agilent RNA 6000 Nano Kit. cDNA was synthesized with SuperScript III and random primers. qRT-PCR primers were redesigned (Table S1) to exclude dsRNA sequences and to account for small sequences differences between Nevada and British Columbia MPB populations and made use of intron/exon information available from the draft genome sequence (22). qRT-PCR was completed on a CFX96 Real-Time System with fluorescence detection between cycles using SsoFast EvaGreen Supermix (Bio-Rad).
Supplementary Material
Acknowledgments
We thank Joy M. Richman [University of British Columbia (UBC)] for use of the microinjector and Karen Reid and Angela Chiang (UBC) for laboratory and project-management support. Beetle-infested logs were kindly provided by Peter Ackhurst (Cheakamus Community Forest) and David Ehrhardt (Wedgewoods Estates) with the assistance from Stirling Angus (JCH Forestry Ltd.) and from the US Forest Service and the managers of the Nevada Whittell Forest and Wildlife Area. This work was supported with funds from Genome Canada, Genome British Columbia, and Genome Alberta in support of the Tria 1 and Tria 2 projects (www.thetriaproject.ca) (C.I.K. and J.B.) and the Natural Sciences and Engineering Research Council of Canada (J.B.); Grant P20 RR-016464 from the National Institutes of Health Idea Networks for Biomedical Excellence Program of the National Center for Research Resources; National Science Foundation Grant IOS 0642182, US Department of Agriculture (USDA) National Institute of Food and Agriculture Grant 2009-05200; USDA National Research Initiative Grant 2005-35604-16727; and Hatch Act Formula Fund Grant NEV00339 and McIntyre–Stennis Grant NEV0369 from the Nevada Agriculture Experiment Station. J.B. is a UBC Distinguished University Scholar.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AFI45068, AFI45070, and KC464331).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316498110/-/DCSupplemental.
References
- 1.Safranyik L, Carroll AL. The biology and epidemiology of the mountain pine beetle in lodgepole pine forests. In: Safranyik L, Wilson B, editors. The Mountain Pine Beetle—A Synthesis of Biology, Management, and Impacts on Lodgepole Pine. Victoria, Canada: Natural Resources Canada, Canadian Forest Service; 2006. pp. 3–66. [Google Scholar]
- 2.Keeling CI, Bohlmann J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006;170(4):657–675. doi: 10.1111/j.1469-8137.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- 3.Barkawi LS, Francke W, Blomquist GJ, Seybold SJ. Frontalin: De novo biosynthesis of an aggregation pheromone component by Dendroctonus spp. bark beetles (Coleoptera: Scolytidae) Insect Biochem Mol Biol. 2003;33(8):773–788. doi: 10.1016/s0965-1748(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 4.Hall GM, et al. Male jeffrey pine beetle, Dendroctonus jeffreyi, synthesizes the pheromone component frontalin in anterior midgut tissue. Insect Biochem Mol Biol. 2002;32(11):1525–1532. doi: 10.1016/s0965-1748(02)00073-5. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayed AM. The Pherobase: Database of Insect Pheromones and Semiochemicals. 2012. Available at www.pherobase.com. Accessed August 1, 2013.
- 6.Goodwin TE, et al. Insect pheromones and precursors in female African elephant urine. J Chem Ecol. 2006;32(8):1849–1853. doi: 10.1007/s10886-006-9094-z. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen LE, Greenwood DR. Frontalin: A chemical message of musth in Asian elephants (Elephas maximus) Chem Senses. 2003;28(5):433–446. doi: 10.1093/chemse/28.5.433. [DOI] [PubMed] [Google Scholar]
- 8.Kinzer GW, et al. Bark beetle attractants: Identification, synthesis and field bioassay of a new compund isolated from Dendroctonus. Nature. 1969;221(5179):477. [Google Scholar]
- 9.Perez AL, Gries R, Gries G, Oehlschlager AC. Transformation of presumptive precursors to frontalin and exo-brevicomin by bark beetles and west Indian sugarcane weevil (Coleoptera) Bioorg Med Chem. 1996;4(3):445–450. doi: 10.1016/0968-0896(96)00024-7. [DOI] [PubMed] [Google Scholar]
- 10.Blomquist GJ, et al. Pheromone production in bark beetles. Insect Biochem Mol Biol. 2010;40(10):699–712. doi: 10.1016/j.ibmb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Taban AH, Tittiger C, Blomquist GJ, Welch WH. Isolation and characterization of farnesyl diphosphate synthase from the cotton boll weevil, Anthonomus grandis. Arch Insect Biochem Physiol. 2009;71(2):88–104. doi: 10.1002/arch.20302. [DOI] [PubMed] [Google Scholar]
- 12.Frick S, et al. Metal ions control product specificity of isoprenyl diphosphate synthases in the insect terpenoid pathway. Proc Natl Acad Sci USA. 2013;110(11):4194–4199. doi: 10.1073/pnas.1221489110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilg AB, Bearfield JC, Tittiger CR, Welch WH, Blomquist GJ. Isolation and functional expression of an animal geranyl diphosphate synthase and its role in bark beetle pheromone biosynthesis. Proc Natl Acad Sci USA. 2005;102(28):9760–9765. doi: 10.1073/pnas.0503277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilg AB, Tittiger C, Blomquist GJ. Unique animal prenyltransferase with monoterpene synthase activity. Naturwissenschaften. 2009;96(6):731–735. doi: 10.1007/s00114-009-0521-1. [DOI] [PubMed] [Google Scholar]
- 15.Kellogg BA, Poulter CD. Chain elongation in the isoprenoid biosynthetic pathway. Curr Opin Chem Biol. 1997;1(4):570–578. doi: 10.1016/s1367-5931(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh KL, et al. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci USA. 2006;103(20):7829–7834. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyagi Y, Matsumura Y, Sagami H. Human geranylgeranyl diphosphate synthase is an octamer in solution. J Biochem. 2007;142(3):377–381. doi: 10.1093/jb/mvm144. [DOI] [PubMed] [Google Scholar]
- 18.Sagami H, Morita Y, Ogura K. Purification and properties of geranylgeranyl-diphosphate synthase from bovine brain. J Biol Chem. 1994;269(32):20561–20566. [PubMed] [Google Scholar]
- 19.Cusson M, et al. Characterization and tissue-specific expression of two lepidopteran farnesyl diphosphate synthase homologs: Implications for the biosynthesis of ethyl-substituted juvenile hormones. Proteins. 2006;65(3):742–758. doi: 10.1002/prot.21057. [DOI] [PubMed] [Google Scholar]
- 20.Barbar A, et al. Cloning, expression and characterization of an insect geranylgeranyl diphosphate synthase from Choristoneura fumiferana. Insect Biochem Mol Biol. 2013;43(10):947–958. doi: 10.1016/j.ibmb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Lai C, McMahon R, Young C, Mackay TF, Langley CH. quemao, a Drosophila bristle locus, encodes geranylgeranyl pyrophosphate synthase. Genetics. 1998;149(2):1051–1061. doi: 10.1093/genetics/149.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keeling CI, et al. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 2013;14(3):R27. doi: 10.1186/gb-2013-14-3-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friesen JA, Rodwell VW. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004;5(11):248. doi: 10.1186/gb-2004-5-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryker LC, Libbey LM. Frontalin in the male mountain pine beetle. J Chem Ecol. 1982;8(11):1399–1409. doi: 10.1007/BF01403103. [DOI] [PubMed] [Google Scholar]
- 25.Aw T, et al. Functional genomics of mountain pine beetle (Dendroctonus ponderosae) midguts and fat bodies. BMC Genomics. 2010;11(1):215. doi: 10.1186/1471-2164-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keeling CI, et al. Transcriptome and full-length cDNA resources for the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major insect pest of pine forests. Insect Biochem Mol Biol. 2012;42(8):525–536. doi: 10.1016/j.ibmb.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Keeling CI, Bearfield JC, Young S, Blomquist GJ, Tittiger C. Effects of juvenile hormone on gene expression in the pheromone-producing midgut of the pine engraver beetle, Ips pini. Insect Mol Biol. 2006;15(2):207–216. doi: 10.1111/j.1365-2583.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 28.Keeling CI, Blomquist GJ, Tittiger C. Coordinated gene expression for pheromone biosynthesis in the pine engraver beetle, Ips pini (Coleoptera: Scolytidae) Naturwissenschaften. 2004;91(7):324–328. doi: 10.1007/s00114-004-0523-y. [DOI] [PubMed] [Google Scholar]
- 29.Vandermoten S, et al. Characterization of a novel aphid prenyltransferase displaying dual geranyl/farnesyl diphosphate synthase activity. FEBS Lett. 2008;582(13):1928–1934. doi: 10.1016/j.febslet.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Vandermoten S, et al. Structural features conferring dual geranyl/farnesyl diphosphate synthase activity to an aphid prenyltransferase. Insect Biochem Mol Biol. 2009;39(10):707–716. doi: 10.1016/j.ibmb.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Jurenka RA, Roelofs WL. Biosynthesis and endocrine regulation of fatty acid derived sex pheromones in moths. In: Stanley-Samuelson DW, Nelson DR, editors. Insect Lipids: Chemistry, Biochemistry and Biology. Lincoln, NE: Univ of Nebraska Press; 1993. pp. 353–388. [Google Scholar]
- 32.Plettner E, Slessor KN, Winston ML. Biosynthesis of mandibular acids in honey bees (Apis mellifera): De novo synthesis, route of fatty acid hydroxylation and caste selective β-oxidation. Insect Biochem Mol Biol. 1998;28(1):31–42. [Google Scholar]
- 33.Sen SE, et al. Purification, properties and heteromeric association of type-1 and type-2 lepidopteran farnesyl diphosphate synthases. Insect Biochem Mol Biol. 2007;37(8):819–828. doi: 10.1016/j.ibmb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 34. Riddiford LM (1994) Cellular and molecular actions of juvenile hormone. I. General considerations and premetamorphic actions. Adv. Insect Physiol., ed Evans PD (Elsevier, Philadelphia), Vol 24, pp 213–274.
- 35.Slater GS, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005;6:31. doi: 10.1186/1471-2105-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.