Significance
Nitrogen is essential for all organisms and limits primary production in the ocean. It is mainly lost from low-oxygen environments by the activity of microorganisms that convert fixed nitrogen to N2 gas. The isotopic composition of nitrogen species can be used to assess nitrogen sinks in the environment, but its use in biogeochemical studies can be fully exploited only if the isotope discrimination that is associated with the respective nitrogen-converting pathways is known. This study reveals the wide range of nitrogen isotope effects of anaerobic oxidation of ammonium (anammox), a major player in marine fixed nitrogen loss, reconciling experimental data with nitrogen isotope signatures observed in the ocean.
Abstract
Nitrogen (N) isotope ratios (15N/14N) provide integrative constraints on the N inventory of the modern ocean. Anaerobic ammonium oxidation (anammox), which converts ammonium and nitrite to dinitrogen gas (N2) and nitrate, is an important fixed N sink in marine ecosystems. We studied the so far unknown N isotope effects of anammox in batch culture experiments. Anammox preferentially removes 14N from the ammonium pool with an isotope effect of +23.5‰ to +29.1‰, depending on factors controlling reversibility. The N isotope effects during the conversion of nitrite to N2 and nitrate are (i) inverse kinetic N isotope fractionation associated with the oxidation of nitrite to nitrate (−31.1 ± 3.9‰), (ii) normal kinetic N isotope fractionation during the reduction of nitrite to N2 (+16.0 ± 4.5‰), and (iii) an equilibrium N isotope effect between nitrate and nitrite (−60.5 ± 1.0‰), induced when anammox is exposed to environmental stress, leading to the superposition of N isotope exchange effects upon kinetic N isotope fractionation. Our findings indicate that anammox may be responsible for the unresolved large N isotope offsets between nitrate and nitrite in oceanic oxygen minimum zones. Irrespective of the extent of N isotope exchange between nitrate and nitrite, N removed from the combined nitrite and nitrate (NOx) pool is depleted in 15N relative to NOx. This net N isotope effect by anammox is superimposed on the N isotope fractionation by the co-occurring reduction of nitrate to nitrite in suboxic waters, possibly enhancing the overall N isotope effect for N loss from oxygen minimum zones.
The nitrogen (N) isotope effect associated with N loss pathways allows the isotopic tracing of N transformations in ocean waters and provides critical constraints on the global marine N budget (1–5). Culture studies have shown that heterotrophic denitrification exhibits a large N isotope effect (ε)* of up to +30‰ (6–9). For decades, heterotrophic denitrification was the only known N loss pathway in the ocean (10). Consequently, strong enrichment of 15N in residual nitrite and nitrate (NOx) from oxygen-deficient waters, as for example in the Eastern Tropical North Pacific and the Arabian Sea, was fully attributed to water column denitrification with an N isotope effect around +25‰ (2, 11). Recent studies have highlighted the significance of anaerobic ammonium oxidation (anammox) for regional N fluxes (12, 13), with possible ramifications regarding the global N balance (14, 15). However, N isotope effects associated with the anammox metabolism were unknown and therefore their potential impacts on the distribution of oceanic N isotopes could not be addressed. This lack of knowledge severely hampers the N-isotope–based assessment of the relative importance of water-column N loss compared with sedimentary N loss, the most poorly constrained flux in the marine combined nitrogen budget (16).
Results
We used the only available highly enriched (>98%) culture of anammox single cells (Kuenenia stuttgartiensis) (17, 18) to investigate N isotope ratio changes in ammonium, nitrite, nitrate, and NOx during the anammox reaction in four sampling campaigns (C1–C4).
The anaerobic oxidation of ammonium with nitrite to N2 followed the previously observed stoichiometry (19): 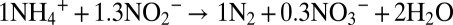 ; i.e., per 1 mol ammonium oxidized, 1.3 mol nitrite is reduced, and 1.0 mol N2 gas is produced along with ∼0.3 mol nitrate (Fig. 1A, Figs. S1 and S2, and Table S1). The equation as written is unbalanced with regard to the transfer of electrons. The excess production of nitrate is balanced by the reduction of inorganic carbon during production of biomass (19). For example, if the excess nitrate is exactly 0.3 mol, addition of 0.15 CO2 as a reactant, with 0.15 CH2O as product, and correction of 2 H2O to 1.85 H2O provides a balance.
; i.e., per 1 mol ammonium oxidized, 1.3 mol nitrite is reduced, and 1.0 mol N2 gas is produced along with ∼0.3 mol nitrate (Fig. 1A, Figs. S1 and S2, and Table S1). The equation as written is unbalanced with regard to the transfer of electrons. The excess production of nitrate is balanced by the reduction of inorganic carbon during production of biomass (19). For example, if the excess nitrate is exactly 0.3 mol, addition of 0.15 CO2 as a reactant, with 0.15 CH2O as product, and correction of 2 H2O to 1.85 H2O provides a balance.
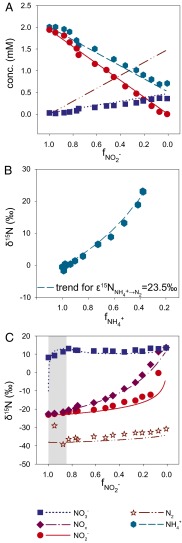
Fig. 1.
Anammox stoichiometry and natural abundance isotope fractionation. (A) Consumption of ammonium and nitrite and production of nitrate agree with anammox stoichiometry (Exp. C3_a). (B) Ammonium N isotope fractionation follows closed-system Rayleigh kinetics (Exp. C3_b). (C) The NOx N isotope fractionation is influenced by isotope exchange between nitrite and nitrate (gray area), which is superimposed on kinetic isotope effects (Exp. C3_a). The x-axis labels 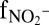 and
and 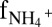 refer to the fraction of remaining nitrite and ammonium, respectively. Symbols represent data, lines are modeled trends, and the concentration and isotope composition of N2 was calculated from NOx.
refer to the fraction of remaining nitrite and ammonium, respectively. Symbols represent data, lines are modeled trends, and the concentration and isotope composition of N2 was calculated from NOx.
Biologically driven variations in N isotope ratios (i.e., 15N:14N ratios) are often controlled by normal kinetic isotope effects that are associated with breakage of chemical bonds during transformation from one N compound to another, for example the N-O bond breakage during the reduction of nitrate to nitrite (20). In these reactions, substrate molecules containing the lighter atoms (i.e., 14N) are typically consumed at slightly higher rates than those containing the heavier atoms (i.e., 15N). During the anammox reaction in batch incubations, the 15N:14N ratio and thus the isotope composition (δ15N) [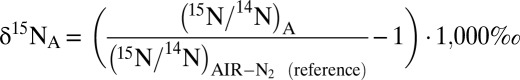 ] of ammonium (
] of ammonium (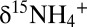 ) and NOx (δ15NOx) increased (Fig. 1 B and C), indicating preferential conversion of
) and NOx (δ15NOx) increased (Fig. 1 B and C), indicating preferential conversion of 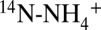 and 14N-NOx to N2. Assuming Rayleigh distillation dynamics in a closed system, we determined a kinetic N isotope effect for the conversion of ammonium to N2 (
and 14N-NOx to N2. Assuming Rayleigh distillation dynamics in a closed system, we determined a kinetic N isotope effect for the conversion of ammonium to N2 (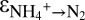 ) of +23.5 ± 0.6‰ for sampling campaign C1 and +29.1 ± 0.7‰ for C3 (Fig. S3). These values fall within the range of the N isotope effects associated with bacterial nitrification (14–38‰) (7, 8, 21, 22). The difference in
) of +23.5 ± 0.6‰ for sampling campaign C1 and +29.1 ± 0.7‰ for C3 (Fig. S3). These values fall within the range of the N isotope effects associated with bacterial nitrification (14–38‰) (7, 8, 21, 22). The difference in 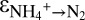 between the campaigns is attributable to factors controlling the reversibility of anammox (Discussion).
between the campaigns is attributable to factors controlling the reversibility of anammox (Discussion).
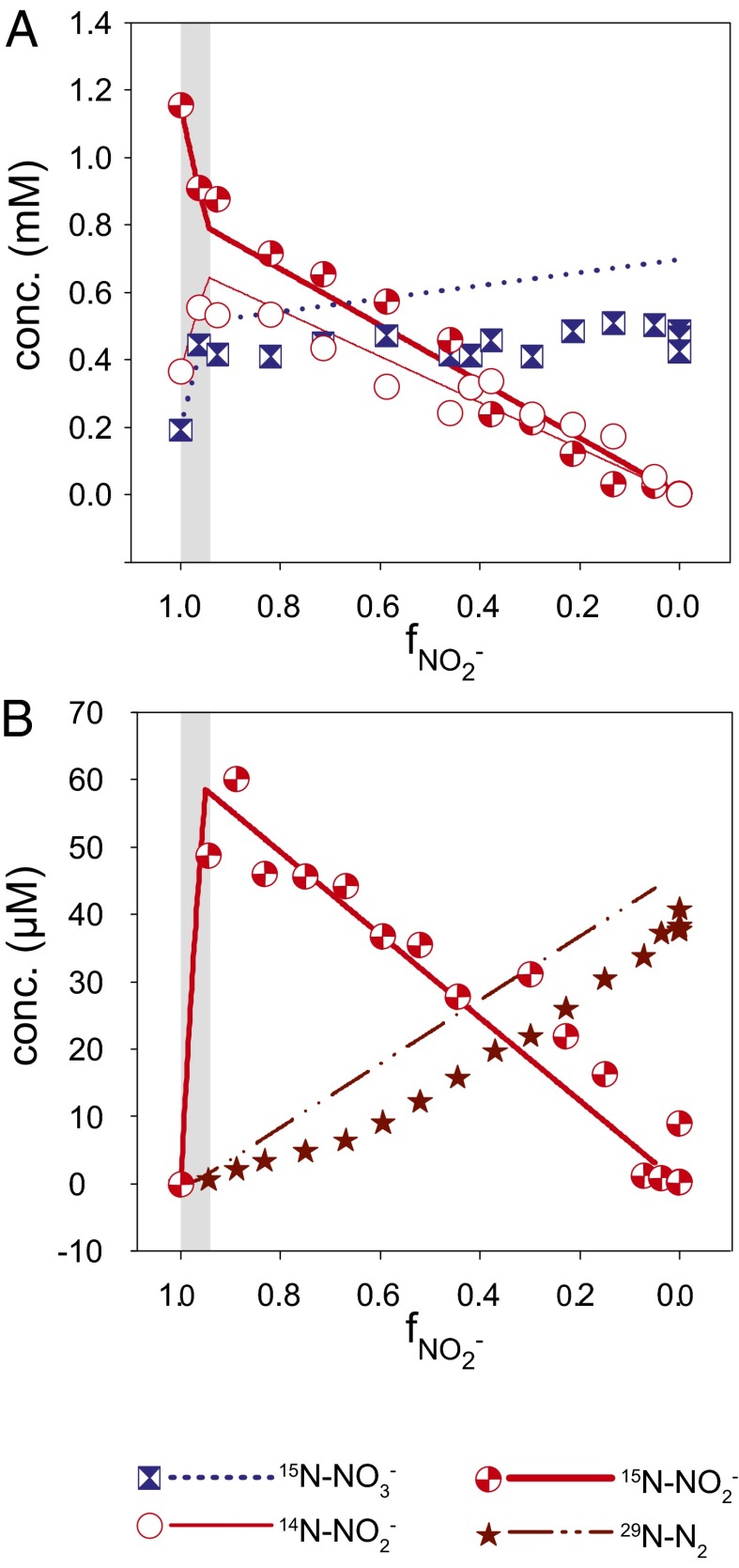
Incubation experiments with 15N-labeled nitrate and nitrite revealed that anammox mediates N isotope exchange between nitrate and nitrite during the initiation of the experiments (Fig. 2). In this early phase, the gross N isotope exchange flux between nitrate and nitrite was approximately six to eight times larger than the nitrite consumption rate, whereas during later stages, transfer of 15N isotope label from nitrate to nitrite pool was negligible (Fig. S4). Based on the observation that anammox can catalyze N isotope exchange between nitrite and nitrate, a process that does not occur spontaneously in aqueous solutions but has to be biologically mediated, it must be expected that their respective changes in N isotope composition disobey typical Rayleigh distillation kinetics during the course of the experiments. Rapid isotope exchange between chemical species induces an equilibrium isotope fractionation that leads to enrichment of the heavy isotopes in the more stable component (23, 24). The equilibrium N isotope effect for the nitrite/nitrate couple in aqueous solutions (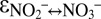 ) is expected to be in the range of −56.6‰ to −81‰, based on calculations from the molecular vibration frequencies for nitrite and nitrate (25–27).
) is expected to be in the range of −56.6‰ to −81‰, based on calculations from the molecular vibration frequencies for nitrite and nitrate (25–27).
Fig. 2.
Isotope label experiments. (A) In the initial stage (gray area) of an anammox experiment (C3_d) with 15N-labeled nitrite, 15N from the nitrite pool exchanged with 14N from the nitrate pool (rapid decrease in 15N-NO2 and rapid increase in 14N-NO2− and 15N-NO3−). (B) In the initial stage (gray area) of an anammox experiment (C3_c) with 15N-labeled nitrate, 15N-NO2− increases, followed by a transfer of 15N from nitrite to the N2 pool (increase in 29N-N2). The x-axis label 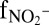 refers to the fraction of remaining nitrite. Symbols represent data, and lines are modeled trends.
refers to the fraction of remaining nitrite. Symbols represent data, and lines are modeled trends.
Indeed, our natural abundance isotope experiments provide evidence for N isotope exchange between nitrate and nitrite, with marked N isotopic offsets between nitrate and nitrite. However, we observed discrepancies between sampling campaigns, despite apparently identical experimental conditions. The results from campaigns C2 and C3 revealed variable 15N enrichment in the nitrate pool at the beginning of the anammox experiments, indicating a variable degree of N isotope exchange between nitrate and nitrite (Fig. 1C, Fig. S5, and Table S2). After this initial stage, isotope exchange between nitrate and nitrite apparently ceased, and the N isotope composition of nitrate and nitrite displayed a pattern that was more consistent with known isotope reservoir effects; that is, both nitrite and nitrate (although less so) became progressively enriched in 15N. Thus, the natural abundance stable N isotope trends confirmed the observations made during the 15N isotope labeling experiments, where N isotope exchange between nitrate and nitrite ceased shortly after the initiation of the experiment. Using a numerical model that considers the relative fluxes (for both 15N and 14N isotopologues) between the nitrite and nitrate pools (exchange and unidirectional net flux) and between nitrite and N2 (unidirectional net flux), we assessed the N isotope fractionation associated with these fluxes (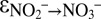 ,
, 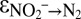 , and
, and 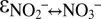 ; SI Text). We observed very good model fits to the measured data from sampling campaigns C2 and C3 for
; SI Text). We observed very good model fits to the measured data from sampling campaigns C2 and C3 for 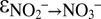 ∼ −30‰,
∼ −30‰, 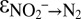 ∼ +15‰, and
∼ +15‰, and 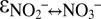 ∼ −60‰ (Fig. 1C and Fig. S5). During the main course of the experiments, i.e., after N isotope exchange between nitrite and nitrate became negligible, the values for the kinetic N isotope effects could be determined from measured data, using a Rayleigh approach. With this approach (SI Text and Fig. S6), we obtained results (
∼ −60‰ (Fig. 1C and Fig. S5). During the main course of the experiments, i.e., after N isotope exchange between nitrite and nitrate became negligible, the values for the kinetic N isotope effects could be determined from measured data, using a Rayleigh approach. With this approach (SI Text and Fig. S6), we obtained results (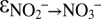 = −31.1 ± 3.9‰ and
= −31.1 ± 3.9‰ and 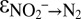 = +16.0 ± 4.5‰; Table S3) similar to the model fitting, indicating that the model-based estimates for the kinetic nitrite N isotope fractionation during anammox are robust.
= +16.0 ± 4.5‰; Table S3) similar to the model fitting, indicating that the model-based estimates for the kinetic nitrite N isotope fractionation during anammox are robust.
For campaign C1, there is evidence that N isotope exchange between nitrate and nitrite persisted throughout the experiments and/or continued after sampling. Unlike for C2 and C3, we observed a continuous decrease in the δ15N of nitrite, from −35‰ to −55‰, with a nearly constant offset of 60‰ relative to the δ15N of produced nitrate (Fig. S5). These trends were consistent with a scenario where equilibrium N isotope exchange maintained the N isotope mass balance of NOx by keeping nitrate enriched in 15N relative to nitrite (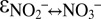 = −60.5 ± 1‰; SI Text) to the effect that the N isotope composition of the shrinking nitrite pool was pushed toward lower δ15NO2− values.
= −60.5 ± 1‰; SI Text) to the effect that the N isotope composition of the shrinking nitrite pool was pushed toward lower δ15NO2− values.
Although N isotope exchange between nitrate and nitrite was mediated by anammox bacteria, it is not clear whether this exchange was the direct result of the activity of intact cells or whether there was a contribution from the activity of the extracellular enzymes. Therefore, we tested whether N isotope exchange between nitrite and nitrate could be induced during sample preparation. Different sampling techniques (poisoning with mercuric chloride, filtration of samples, and French press) with subsequent exposure of the treated sample to a medium containing 15N nitrate and nonlabeled nitrite revealed that cell-free samples derived from anammox cultures mediate N isotope exchange between nitrate and nitrite, with lowest exchange for intact cells that were poisoned with mercuric chloride (5%) and highest exchange (44%) for samples that were treated with a French press (C4; SI Text and Table S4). This shows that in N isotope studies, care must be taken in experimental procedures to minimize lysis of cells, which could cause sampling artifacts and highlights the necessity of using time series in labeling experiments taking transient N isotope effects into account.
Discussion
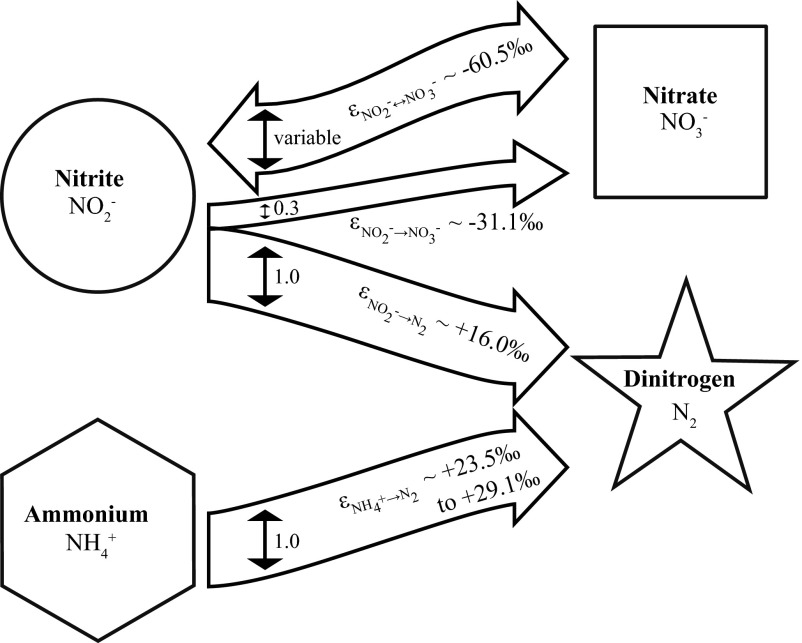
Anammox induces several N isotope effects, which include preferential removal of 14N from the ammonium and nitrite pools during the formation of N2, as well as the preferential removal of 15N from the nitrite pool during oxidation to nitrate. These N isotope effects can overlap with an N isotope equilibrium effect between the nitrite and nitrate pools (Fig. 3). In the following, we discuss these isotope effects in more detail and highlight the consequences for N isotope studies of natural environments.
Fig. 3.
N fluxes (size indicated by vertical arrows) and isotope fractionation during anammox. Anammox converts ammonium and nitrite to nitrate and dinitrogen according to the approximate stoichiometry 1.3 NO2− + 1 NH4+ → 1 N2 + 0.3 NO3− + 2 H2O and exchanges N isotopes between nitrite and nitrate to a variable degree. The isotope effects (ε) that correspond to these N fluxes determined in this study are indicated.
Isotope Effects Related to the Conversion of Ammonium and Nitrite to N2.
Three redox reactions are responsible for the conversion of N from nitrite and ammonium to N2, the reduction of nitrite to nitric oxide (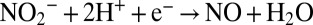 ), the formation of hydrazine from ammonium and nitric oxide (
), the formation of hydrazine from ammonium and nitric oxide (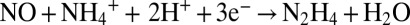 ), and the oxidation of hydrazine to N2 (N2H4 → N2 + 4H+ + 4e−), resulting in the overall reaction
), and the oxidation of hydrazine to N2 (N2H4 → N2 + 4H+ + 4e−), resulting in the overall reaction 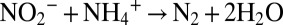 (18, 28, 29). Hydrazine synthesis, which involves a three-electron reduction, is likely the rate-limiting step in this reaction sequence (18, 29), which implies that no N isotope effect related to the consumption of hydrazine is expressed. In our experiments, the breaking of the two N-O bonds of nitrite yielded a smaller isotope effect (
(18, 28, 29). Hydrazine synthesis, which involves a three-electron reduction, is likely the rate-limiting step in this reaction sequence (18, 29), which implies that no N isotope effect related to the consumption of hydrazine is expressed. In our experiments, the breaking of the two N-O bonds of nitrite yielded a smaller isotope effect (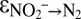 = +16.0 ± 4.5‰) than the breaking of the four N-H bonds of ammonium (
= +16.0 ± 4.5‰) than the breaking of the four N-H bonds of ammonium (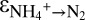 of +23.5 ± 0.6‰ for C3 and +29.1 ± 0.7‰ for C1). The different values for
of +23.5 ± 0.6‰ for C3 and +29.1 ± 0.7‰ for C1). The different values for 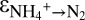 for different sampling campaigns suggest that the expression of the enzyme-level N isotope effect of ammonium oxidation could vary as a function of the reversibility of the metabolic reaction steps in the conversion of ammonium to N2. A higher reversibility, i.e., a larger
for different sampling campaigns suggest that the expression of the enzyme-level N isotope effect of ammonium oxidation could vary as a function of the reversibility of the metabolic reaction steps in the conversion of ammonium to N2. A higher reversibility, i.e., a larger 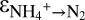 in C1 than in C3, implies that hydrazine formation was more rate limiting in C1, which could be the case if nitric oxide supply limited hydrazine formation in C1.
in C1 than in C3, implies that hydrazine formation was more rate limiting in C1, which could be the case if nitric oxide supply limited hydrazine formation in C1.
The N isotope fractionation during the conversion of ammonium to N2 by anammox falls in the range of N isotope effects reported for bacterial nitrification (14.2–38.2‰) (7, 8, 21, 22). In the environment, these N isotope effects are expressed only when ammonium is not limiting, like in sediments and in the close vicinity of sinking aggregates (30–32).
Isotope Effects Between Nitrite and Nitrate.
In biological processes, true inverse kinetic isotope effects are extremely rare. Often, apparent inverse isotope effects can be explained as a result of a “hidden” equilibrium isotope effect (27, 33). Our observation of an inverse kinetic isotope effect for the oxidation of nitrite to nitrate by anammox is interesting because K. stuttgartiensis possesses enzymes that are highly similar to the ones of nitrite-oxidizing bacteria, where they function in the oxidation of nitrite (28, 29, 34). Nitrite-oxidizing bacteria have been shown to express inverse kinetic N isotope effects, which can be attributed to an inverse kinetic effect and reversibility of the nitrite oxidation at the enzyme level (27, 35). However, the 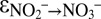 for anammox determined from C2 and C3 (−31.1 ± 3.9‰) exceeds the range of N isotope effects thus far observed for nitrite-oxidizing bacteria (between −9.1‰ and −20.6‰) (31). Increased reversibility of enzymatic pathways and efflux of substrates from the cells lead to a stronger expression of enzyme-level kinetic isotope effects (e.g., 20, 36). Hence, the more pronounced inverse N isotope fractionation in our anammox experiments compared with previous work on nitrite-oxidizing bacteria (35) suggests that some enzymatic reaction steps involved in the oxidation of nitrite to nitrate by anammox are more reversible than nitrite oxidation by aerobic nitrite oxidizers. This finding is corroborated by the fact that anammox is capable of reducing nitrate (37), which shows that anammox can reverse nitrate production. Moreover, reversibility of an entire multistep biochemical pathway can lead to isotope exchange between reactant and product and, in an extreme scenario, to equilibrium isotope fractionation (38).
for anammox determined from C2 and C3 (−31.1 ± 3.9‰) exceeds the range of N isotope effects thus far observed for nitrite-oxidizing bacteria (between −9.1‰ and −20.6‰) (31). Increased reversibility of enzymatic pathways and efflux of substrates from the cells lead to a stronger expression of enzyme-level kinetic isotope effects (e.g., 20, 36). Hence, the more pronounced inverse N isotope fractionation in our anammox experiments compared with previous work on nitrite-oxidizing bacteria (35) suggests that some enzymatic reaction steps involved in the oxidation of nitrite to nitrate by anammox are more reversible than nitrite oxidation by aerobic nitrite oxidizers. This finding is corroborated by the fact that anammox is capable of reducing nitrate (37), which shows that anammox can reverse nitrate production. Moreover, reversibility of an entire multistep biochemical pathway can lead to isotope exchange between reactant and product and, in an extreme scenario, to equilibrium isotope fractionation (38).
It is evident that N isotope exchange between nitrate and nitrite occurred at least in the initial stage of our experiments C2 and C3 and maybe throughout experiment C1. With an depletion of nitrite in 15N relative to nitrate of −60.5 ± 1‰ (based on C1; SI Text), the equilibrium isotope effect reported here is remarkably close to the most recent estimate for 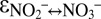 based on calculations from the molecular vibration frequencies for nitrite and nitrate of −56.6‰ (27). Our finding that some N isotope exchange between nitrate and nitrite can also occur in cell-free sample extracts derived from anammox cultures indicates that cell lysis could be a cause for the observed isotope exchange. However, the fact that we did not observe any N isotope exchange for the major part of the experiments during C2 and C3 indicates that our sampling approach was not prone to yield artifacts due to cell lysis. Instead, we attribute the N isotope exchange in C1, C2, and C3 to environmental stress to which the cultures were exposed during the experiment before sampling (SI Text). Such stress could include the exposure to oxygen and centrifugation, which occurred at the initiation of new experimental campaigns. We hypothesize that the bacteria in C1 must have been exposed to additional stress factors that were not identified at the time the experiments were carried out. These factors could include agitation, age of the culture, or recent exponential growth, stress factors that have recently been identified to impact the isotope effect during denitrification (39). In C1, such stress factors may not only have had an impact on the N isotope effects between nitrite and nitrate, but also have affected the N isotope effect of ammonium oxidation. Anammox may couple the oxidation of nitrite to nitrate to the reduction of nitrite to nitric oxide (29), and a sluggish nitrite oxidation (high reversibility) would diminish production of nitric oxide, rendering the ammonium consumption more reversible as well, which could explain the higher
based on calculations from the molecular vibration frequencies for nitrite and nitrate of −56.6‰ (27). Our finding that some N isotope exchange between nitrate and nitrite can also occur in cell-free sample extracts derived from anammox cultures indicates that cell lysis could be a cause for the observed isotope exchange. However, the fact that we did not observe any N isotope exchange for the major part of the experiments during C2 and C3 indicates that our sampling approach was not prone to yield artifacts due to cell lysis. Instead, we attribute the N isotope exchange in C1, C2, and C3 to environmental stress to which the cultures were exposed during the experiment before sampling (SI Text). Such stress could include the exposure to oxygen and centrifugation, which occurred at the initiation of new experimental campaigns. We hypothesize that the bacteria in C1 must have been exposed to additional stress factors that were not identified at the time the experiments were carried out. These factors could include agitation, age of the culture, or recent exponential growth, stress factors that have recently been identified to impact the isotope effect during denitrification (39). In C1, such stress factors may not only have had an impact on the N isotope effects between nitrite and nitrate, but also have affected the N isotope effect of ammonium oxidation. Anammox may couple the oxidation of nitrite to nitrate to the reduction of nitrite to nitric oxide (29), and a sluggish nitrite oxidation (high reversibility) would diminish production of nitric oxide, rendering the ammonium consumption more reversible as well, which could explain the higher 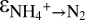 for C1 compared with C3.
for C1 compared with C3.
Although N isotope effects associated with the conversion of nitrite into N2 and nitrate by anammox do not strongly differ from the range of N isotope effects reported for nitrite reduction by canonical denitrification (+5‰ to +25‰) (27, 40) and nitrite oxidation (−9‰ to −21‰) (35), respectively, they are unique. Whereas the N isotope effects of nitrite reduction by denitrifiers and nitrite oxidizers are not expressed when nitrite concentrations are limiting, the anammox nitrite isotope effects are likely to be manifested in the environment even under nitrite-depleted conditions because anammox partitions N from nitrite into 15N-enriched nitrate and 15N-depleted N2 pools.
Consequences for N Isotope Studies of Natural Environments.
For N isotope budget considerations, it is crucial to know the N isotope fractionation between the total fixed inorganic N pool (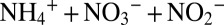 ) and the N2 gas that is lost from marine oxygen-deficient waters and sediments to the atmosphere. Previous field-based assessments of this net N isotope effect yielded values of 20–30‰ (2, 11). These estimates are based on profiles of the N isotope composition of nitrate in oxygen minimum zones (OMZs), where the ratio between nitrate and phosphate concentration is taken as a measure for nitrate consumption coupled to the mineralization of organic matter. However, this approach does not differentiate between the processes responsible for the loss of fixed N (i.e., denitrification and anammox) or between the mechanisms that directly or indirectly affect the N isotope composition of the newly formed N2 gas (i.e., nitrate reduction, nitrite reduction, nitrite oxidation and ammonium oxidation) even though this information is essential for the assessment of how future changes in the extent and structure of OMZs (i.e., expansion and intensification) affect the loss of fixed N from the ocean.
) and the N2 gas that is lost from marine oxygen-deficient waters and sediments to the atmosphere. Previous field-based assessments of this net N isotope effect yielded values of 20–30‰ (2, 11). These estimates are based on profiles of the N isotope composition of nitrate in oxygen minimum zones (OMZs), where the ratio between nitrate and phosphate concentration is taken as a measure for nitrate consumption coupled to the mineralization of organic matter. However, this approach does not differentiate between the processes responsible for the loss of fixed N (i.e., denitrification and anammox) or between the mechanisms that directly or indirectly affect the N isotope composition of the newly formed N2 gas (i.e., nitrate reduction, nitrite reduction, nitrite oxidation and ammonium oxidation) even though this information is essential for the assessment of how future changes in the extent and structure of OMZs (i.e., expansion and intensification) affect the loss of fixed N from the ocean.
Assessing the overall N isotope effect (εDIN→N2) by anammox is intricate, because εDIN→N2 depends not only on the relative pool sizes of ammonium, nitrate, and nitrite, respectively, but also on the N isotope differences between ambient nitrate and nitrite (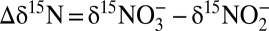 ), which, as this study shows, likely depend on the mode of function of anammox bacteria. Nevertheless, it is possible to draw conclusions with respect to the potential influence of anammox on the overall N isotope effect between total fixed N and N2 gas. For the anammox N isotope effects to be fully expressed in the environment, both ammonium and nitrite have to be in excess, conditions that hardly persist in the ocean. For example, water-column ammonium concentrations in the interior of OMZs are generally at or below detection, indicating that ammonium supply from the degradation of organic matter is limited (32), whereas nitrite can be replete. On the other hand, anammox bacteria living in sediments and associated with sinking aggregates (30–32) may experience limitation with respect to nitrate/nitrite, resulting in a suppressed N isotope effect for benthic NOx conversion to N2 (41, 42). In sediments and aggregates, ammonium is rarely limiting, allowing the N isotope effect of ammonium oxidation to N2 (
), which, as this study shows, likely depend on the mode of function of anammox bacteria. Nevertheless, it is possible to draw conclusions with respect to the potential influence of anammox on the overall N isotope effect between total fixed N and N2 gas. For the anammox N isotope effects to be fully expressed in the environment, both ammonium and nitrite have to be in excess, conditions that hardly persist in the ocean. For example, water-column ammonium concentrations in the interior of OMZs are generally at or below detection, indicating that ammonium supply from the degradation of organic matter is limited (32), whereas nitrite can be replete. On the other hand, anammox bacteria living in sediments and associated with sinking aggregates (30–32) may experience limitation with respect to nitrate/nitrite, resulting in a suppressed N isotope effect for benthic NOx conversion to N2 (41, 42). In sediments and aggregates, ammonium is rarely limiting, allowing the N isotope effect of ammonium oxidation to N2 (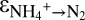 ) by anammox to be expressed.
) by anammox to be expressed.
The anammox NOx isotope effects we report here provide the missing pieces for the interpretation of N isotope signatures of NOx and N2 in the ocean water column. Although anammox bacteria may have the capacity to reduce nitrate, they probably mainly rely on nitrite produced by other co-occurring microorganisms. As a consequence, the N isotope effects of nitrite consumption by anammox could be superimposed on isotope effects by denitrification. Even if nitrite was completely consumed, anammox could still have a significant impact on the net isotope effect for N loss due to the simultaneous preferential return of 15N to the nitrate pool and the corresponding reduction of 15N-depleted nitrite to N2. Hence, we argue that with regard to NOx consumption in the environment, anammox could even act to enhance the net isotope effect of N loss due to canonical denitrification. A similar amplification of the N isotope effect would be expected for nitrite oxidation by nitrite oxidizers; however—and unlike for anammox—this effect is expressed only if nitrite consumption is not quantitative, i.e., only if isotopically altered nitrite is simultaneously converted to N2 by anammox or classical dentrification. These considerations shed unique light on the finding that N isotope effects by denitrification under common environmental conditions in the ocean are likely to be smaller than the 20–30‰ attributed to water-column N loss, namely around 16‰ (39). Anammox and possibly aerobic nitrite oxidation could help to reconcile the apparent discrepancy between these recent N isotope effect estimates and the larger values from field observations in OMZs (43). Moreover, until now, large Δδ15N (i.e., 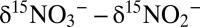 ) of 30–35‰ in the Eastern Tropical North Pacific (44) and up to 39‰ in the Eastern Tropical South Pacific (43) had to be explained by N isotope fractionation caused by denitrification (
) of 30–35‰ in the Eastern Tropical North Pacific (44) and up to 39‰ in the Eastern Tropical South Pacific (43) had to be explained by N isotope fractionation caused by denitrification (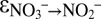 ∼ +25‰), combined with the inverse kinetic isotope effect related to nitrite oxidation (
∼ +25‰), combined with the inverse kinetic isotope effect related to nitrite oxidation (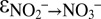 between −9.1‰ and −20.6‰) (35). The large nitrite–nitrate N isotope effects by anammox (
between −9.1‰ and −20.6‰) (35). The large nitrite–nitrate N isotope effects by anammox (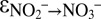 ∼ −31.1‰,
∼ −31.1‰, 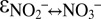 ∼ −60.5‰) provide an alternative explanation for large Δδ15N values, especially for the oxygen-free interior of OMZs (44), where aerobic nitrite oxidation is unlikely to occur. In this context, our observation that anammox catalyzed isotope exchange between nitrate and nitrite when exposed to environmental stress is highly relevant, as it implies that anammox may even—or especially—cause large N isotope offsets between nitrate and nitrite while exhibiting low net activity.
∼ −60.5‰) provide an alternative explanation for large Δδ15N values, especially for the oxygen-free interior of OMZs (44), where aerobic nitrite oxidation is unlikely to occur. In this context, our observation that anammox catalyzed isotope exchange between nitrate and nitrite when exposed to environmental stress is highly relevant, as it implies that anammox may even—or especially—cause large N isotope offsets between nitrate and nitrite while exhibiting low net activity.
Most intriguingly, our results show that N isotope fractionation by a single anammox strain covers the entire variability of N isotope fractionations observed in natural environments. The N isotope effects by anammox play an important, but as yet neglected, role in global N isotope budgets. The observed 15N enrichment of nitrate (by >10‰) in oceanic OMZ waters and concomitant 15N depletion in ambient dissolved N2 (by up to −0.6‰) can also be explained by anammox and not only by N isotope effects related to heterotrophic denitrification in the water column. Moreover, the combination of anammox N isotope fractionation and N isotope effects for the reduction of nitrate to nitrite by co-occurring microorganisms like canonical denitrifiers may amplify the overall N isotope effect for N loss from OMZs.
Methods
Incubation Experiments.
Cultivation of anammox organisms is challenging, and biomass is available only in limited amounts. Therefore, experiments had to be carried out in four campaigns (C1, C2, C3, and C4), which were separated from each other by several months. In C1, C2, and C3 replicate experiments (designated as Ci_a, and Ci_b, i = 1…3) were performed to determine natural abundance N isotope effects. In C3, additional experiments (C3_c, C3_d) were devoted to the determination of N flux rates by 15N labeling. During C4, seven experiments (C4_a to C4_g) were carried out to investigate the impact of different sampling protocols on anammox-mediated nitrogen isotope exchange between nitrate and nitrite.
One-liter cultures of K. stuttgartiensis (∼108 cells⋅mL−1) were harvested by centrifugation at 8,000 × g for 12 min, washed, and resuspended in 1 L medium with a fixed amount of nitrite (∼1.5 mmol⋅L−1). In the case of experiments with 15N isotope labels, the centrifugation step was omitted. The batch cultures were flushed with an Ar:CO2 gas mixture (95:5, vol/vol), incubated at 31 °C, and shaken continuously with a shaking incubator at 125 rpm for the duration of the experiments (up to 2.5 h). The initial ammonium concentrations were between 1.5 mM and 2 mM (Fig. S5). Anammox activity was monitored by nitrite consumption. Subsamples (15–30 mL) were sequentially extracted, filtered (0.22 μm), and stored at −20 °C until analysis. A summary of the sample-handling experiments is given in Table S4.
Analytical.
Dissolved ammonium (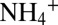 ) concentrations were determined using a colorimetric autoanalyzer (TRAACS 800; Bran & Lubbe; detection limit of 0.3 μmol⋅L−1) (45). NOx (
) concentrations were determined using a colorimetric autoanalyzer (TRAACS 800; Bran & Lubbe; detection limit of 0.3 μmol⋅L−1) (45). NOx (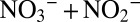 ) and
) and 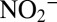 were determined by chemiluminescence after reduction to NO with acidic Vanadium (II) chloride (46). Nitrate was also measured with the same method after
were determined by chemiluminescence after reduction to NO with acidic Vanadium (II) chloride (46). Nitrate was also measured with the same method after 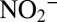 removal with sulfamic acid (9).
removal with sulfamic acid (9). 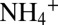 isotope analyses were carried out using the ammonium diffusion method (47, 48) and subsequent analysis of the trapped ammonium sulfate via a continuous-flow isotope ratio mass spectrometer (CF-IRMS), coupled to an elemental analyzer. For nitrite δ15N measurements, sample nitrite was reacted with acetic-buffered sodium azide to form N2O (49). The N2O was then measured using a CF-IRMS with an on-line purge and trap system. The N isotope composition of nitrate plus nitrite (δ15NNOx) was determined by bacterial conversion of NOx to N2O via the denitrifier method (50) and the subsequent N isotope analysis of the N2O as described above. The δ15N of nitrate was determined with the denitrifier method after nitrite removal with sulfamic acid (51). Agreement between directly measured δ15N
isotope analyses were carried out using the ammonium diffusion method (47, 48) and subsequent analysis of the trapped ammonium sulfate via a continuous-flow isotope ratio mass spectrometer (CF-IRMS), coupled to an elemental analyzer. For nitrite δ15N measurements, sample nitrite was reacted with acetic-buffered sodium azide to form N2O (49). The N2O was then measured using a CF-IRMS with an on-line purge and trap system. The N isotope composition of nitrate plus nitrite (δ15NNOx) was determined by bacterial conversion of NOx to N2O via the denitrifier method (50) and the subsequent N isotope analysis of the N2O as described above. The δ15N of nitrate was determined with the denitrifier method after nitrite removal with sulfamic acid (51). Agreement between directly measured δ15N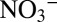 and δ15N
and δ15N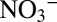 values derived using the mass and N isotope balance approach by ref. 44 was excellent. Analyses were calibrated against the AIR-N2 reference scale, using international and internal nitrate standards (IAEA N3, +4.7‰; USGS S34, −1.8‰; UBN1, +14.15%) and an internal nitrite standard with a calibrated δ15N of −34.9‰. For all NOx isotope measurements, samples were prepared to obtain 100–200 nmol N2O. The SD was generally better than 0.3‰ for measurements with the denitrifier method and better than 0.4‰ for nitrite measurements via the sodium azide method. Determination of
values derived using the mass and N isotope balance approach by ref. 44 was excellent. Analyses were calibrated against the AIR-N2 reference scale, using international and internal nitrate standards (IAEA N3, +4.7‰; USGS S34, −1.8‰; UBN1, +14.15%) and an internal nitrite standard with a calibrated δ15N of −34.9‰. For all NOx isotope measurements, samples were prepared to obtain 100–200 nmol N2O. The SD was generally better than 0.3‰ for measurements with the denitrifier method and better than 0.4‰ for nitrite measurements via the sodium azide method. Determination of  ,
,  in 15N-labeling experiments was carried out by GC-IRMS after conversion to N2 (52, 53).
in 15N-labeling experiments was carried out by GC-IRMS after conversion to N2 (52, 53).
Data Interpretation.
The isotope effects by anammox are likely caused by several enzymatically catalyzed reactions, which may be intrinsically linked. Enzymatically catalyzed reactions are reversible to some degree (e.g., ref. 38). Reversibility during anammox will affect the expression of the biological (i.e., enzyme-level/intrinsic) isotope effects outside the anammox cells (e.g., ref. 20). At the current state of knowledge, the actual anammox reaction network is not fully constrained, nor is the reversibility of the involved individual enzymatic steps. For the assessment and modeling of the anammox N isotope effects, it is therefore necessary to consider a simplified reaction scheme, where N isotope effects are designated to the respective net process, which are attributed to fluxes in isotope mass balance equations (Fig. S1). We used mass balance calculations to investigate the stoichiometry of the anammox reaction (SI Text); iterative, numerical isotope mass balance models to determine isotope label transfer (SI Text); and the evolution of the natural abundance isotope signature of N pools (SI Text), as well as analytical approaches to determine ammonium and nitrite isotope fractionation factors (SI Text).
Supplementary Material
Acknowledgments
We thank G. L. Arnold for critical editing of an earlier version of this manuscript and the editor and reviewers for constructive comments. This work was supported by the Max-Planck-Gesellschaft and the Deutsche Forschungsgemeinschaft.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*For unidirectional processes A→B: 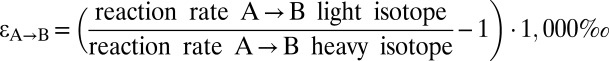 ; for equilibrium processes A↔B:
; for equilibrium processes A↔B: 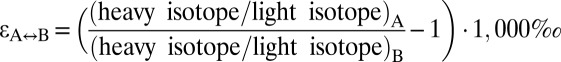 .
.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310488110/-/DCSupplemental.
References
- 1.Cline JD, Kaplan IR. Isotopic fractionation of dissolved nitrate during denitrification in the eastern tropical north pacific ocean. Mar Chem. 1975;3:271–299. [Google Scholar]
- 2.Brandes JA, Devol AH, Yoshinari T, Jayakumar DA, Naqvi SWA. Isotopic composition of nitrate in the Central Arabian Sea and Eastern Tropical North Pacific: A tracer for mixing and nitrogen cycles. Limnol Oceanogr. 1998;43:1680–1689. [Google Scholar]
- 3.Altabet M, Murray D, Prell W. Climatically linked oscillations in Arabian Sea denitrification over the past 1 m.y.: Implications for the marine N cycle. Paleoceanography. 1999;14:732–743. [Google Scholar]
- 4.Brandes J, Devol A. A global marine-fixed nitrogen isotopic budget: Implications for Holocene nitrogen cycling. Global Biogeochem Cycles. 2002;16(4):67-1–67-14. [Google Scholar]
- 5.Deutsch C, Sigman D, Thunell R, Meckler A, Haug G. Isotopic constraints on glacial/interglacial changes in the oceanic nitrogen budget. Global Biogeochem Cycles. 2004 10.1029/2003GB002189. [Google Scholar]
- 6.Wellman RP, Cook FD, Krouse HR. Nitrogen-15: Microbiological alteration of abundance. Science. 1968;161(3838):269–270. doi: 10.1126/science.161.3838.269. [DOI] [PubMed] [Google Scholar]
- 7.Delwiche CC, Steyn PL. Nitrogen isotope fractionation in soils and microbial reactions. Environ Sci Technol. 1970;4:929–935. [Google Scholar]
- 8.Mariotti A, et al. Experimental determination of nitrogen kinetic isotope fractionation: Some principles; illustration for the denitrification and nitrification processes. Plant Soil. 1981;62:413–430. [Google Scholar]
- 9.Granger J, Sigman DM, Lehmann MF, Tortell PD. Nitrogen and oxygen isotope fractionation during dissimilatory nitrate reduction by denitrifying bacteria. Limnol Oceanogr. 2008;53:2533–2545. [Google Scholar]
- 10.Brandes JA, Devol AH, Deutsch C. New developments in the marine nitrogen cycle. Chem Rev. 2007;107(2):577–589. doi: 10.1021/cr050377t. [DOI] [PubMed] [Google Scholar]
- 11.Voss M, Dippner J, Montoya J. Nitrogen isotope patterns in the oxygen-deficient waters of the Eastern Tropical North Pacific Ocean. Deep Sea Res Part I Oceanogr Res Pap. 2001;48:1905–1921. [Google Scholar]
- 12.Kuypers MM, et al. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc Natl Acad Sci USA. 2005;102(18):6478–6483. doi: 10.1073/pnas.0502088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thamdrup B, et al. Anaerobic ammonium oxidation in the oxygen-deficient waters off northern Chile. Limnol Oceanogr. 2006;51:2145–2156. [Google Scholar]
- 14.Devol AH. Nitrogen cycle: Solution to a marine mystery. Nature. 2003;422(6932):575–576. doi: 10.1038/422575a. [DOI] [PubMed] [Google Scholar]
- 15.Arrigo KR. Marine microorganisms and global nutrient cycles. Nature. 2005;437(7057):349–355. doi: 10.1038/nature04159. [DOI] [PubMed] [Google Scholar]
- 16.Devol A. Denitrification including Anammox. In: Capone DG, Bronk DA, Mulholland M, Carpenter J, editors. Nitrogen in the Marine Environment. 2nd Ed. New York: Academic; 2003. pp. 263–301. [Google Scholar]
- 17.Kartal B, Geerts W, Jetten MSM. 2011. Research on Nitrification and Related Processes, Part A, Methods in Enzymology, ed Klotz MG (Academic, New York), pp 89–108. Available at http://www.sciencedirect.com/science/article/pii/B9780123812940000043. [DOI] [PubMed]
- 18.Kartal B, et al. Molecular mechanism of anaerobic ammonium oxidation. Nature. 2011;479(7371):127–130. doi: 10.1038/nature10453. [DOI] [PubMed] [Google Scholar]
- 19.Strous M, et al. Missing lithotroph identified as new planctomycete. Nature. 1999;400(6743):446–449. doi: 10.1038/22749. [DOI] [PubMed] [Google Scholar]
- 20.Tcherkez G, Farquhar GD. Isotopic fractionation by plant nitrate reductase, twenty years later. Funct Plant Biol. 2006;33:531–537. doi: 10.1071/FP05284. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida N. 15N-depleted N2O as a product of nitrification. Nature. 1988;335:528–529. [Google Scholar]
- 22.Casciotti K, Sigman D, Ward B. Linking diversity and stable isotope fractionation in ammonia-oxidizing bacteria. Geomicrobiol J. 2003;20:335–353. [Google Scholar]
- 23.Bigeleisen J, Mayer MG. Calculation of equilibrium constants for isotopic exchange reactions. J Chem Phys. 1947;15:261–267. [Google Scholar]
- 24.Schauble EA. Applying stable isotope fractionation theory to new systems. Rev Mineral Geochem. 2004;55(1):65–111. [Google Scholar]
- 25.Spindel W. The calculation of equilibrium constants for several exchange reactions of Nitrogen-15 between oxy compounds of nitrogen. J Chem Phys. 1954;22:1271–1272. [Google Scholar]
- 26.Begun G, Fletcher W. Partition function ratios for molecules containing nitrogen isotopes. J Chem Phys. 1960;33:1083–1085. [Google Scholar]
- 27.Casciotti KL. Inverse kinetic isotope fractionation during bacterial nitrite oxidation. Geochim Cosmochim Acta. 2009;73:2061–2076. [Google Scholar]
- 28.Strous M, et al. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature. 2006;440(7085):790–794. doi: 10.1038/nature04647. [DOI] [PubMed] [Google Scholar]
- 29.Kartal B, et al. How to make a living from anaerobic ammonium oxidation. FEMS Microbiol Rev. 2013;37(3):428–461. doi: 10.1111/1574-6976.12014. [DOI] [PubMed] [Google Scholar]
- 30.Woebken D, Fuchs BM, Kuypers MMM, Amann R. Potential interactions of particle-associated anammox bacteria with bacterial and archaeal partners in the Namibian upwelling system. Appl Environ Microbiol. 2007;73(14):4648–4657. doi: 10.1128/AEM.02774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocker R. Marine microbes see a sea of gradients. Science. 2012;338(6107):628–633. doi: 10.1126/science.1208929. [DOI] [PubMed] [Google Scholar]
- 32.Kalvelage T, et al. Nitrogen cycling driven by organic matter export in the South Pacific oxygen minimum zone. Nat Geosci. 2013;6:228–234. [Google Scholar]
- 33.Fry B, Ruf W, Gest H, Hayes JM. Sulfur isotope effects associated with oxidation of sulfide by O2 in aqueous solution. Isot Geosci. 1988;73:205–210. doi: 10.1016/0168-9622(88)90001-2. [DOI] [PubMed] [Google Scholar]
- 34.Lücker S, et al. A Nitrospira metagenome illuminates the physiology and evolution of globally important nitrite-oxidizing bacteria. Proc Natl Acad Sci USA. 2010;107(30):13479–13484. doi: 10.1073/pnas.1003860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchwald C, Casciotti KL. Oxygen isotopic fractionation and exchange during bacterial nitrite oxidation. Limnol Oceanogr. 2010;55:1064–1074. [Google Scholar]
- 36.Rees CE. A steady-state model for sulphur isotope fractionation in bacterial reduction processes. Geochim Cosmochim Acta. 1973;37:1141–1162. [Google Scholar]
- 37.Kartal B, et al. Anammox bacteria disguised as denitrifiers: Nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ Microbiol. 2007;9(3):635–642. doi: 10.1111/j.1462-2920.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 38.Holler T, et al. Carbon and sulfur back flux during anaerobic microbial oxidation of methane and coupled sulfate reduction. Proc Natl Acad Sci USA. 2011;108(52):E1484–E1490. doi: 10.1073/pnas.1106032108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kritee K, et al. Reduced isotope fractionation by denitrification under conditions relevant to the ocean. Geochim Cosmochim Acta. 2012;92:243–259. [Google Scholar]
- 40.Bryan BA, Shearer G, Skeeters JL, Kohl DH. Variable expression of the nitrogen isotope effect associated with denitrification of nitrite. J Biol Chem. 1983;258(14):8613–8617. [PubMed] [Google Scholar]
- 41.Lehmann MF, Sigman D, Berelson W. Coupling the N-15/N-14 and O-18/O-16 of nitrate as a constraint on benthic nitrogen cycling. Mar Chem. 2004;88(1):1–20. [Google Scholar]
- 42.Lehmann MF, et al. The distribution of nitrate 15N/14N in marine sediments and the impact of benthic nitrogen loss on the isotopic composition of oceanic nitrate. Geochim Cosmochim Acta. 2007;71:5384–5404. [Google Scholar]
- 43.Casciotti KL, Buchwald C, McIlvin M. Implications of nitrate and nitrite isotopic measurements for the mechanisms of nitrogen cycling in the Peru oxygen deficient zone. Deep Sea Res Part I Oceanogr Res Pap. 2013;80:78–93. [Google Scholar]
- 44.Casciotti KL, McIlvin MR. Isotopic analyses of nitrate and nitrite from reference mixtures and application to Eastern Tropical North Pacific waters. Mar Chem. 2007;107(2):184–201. [Google Scholar]
- 45.Grasshoff K, Kremling K, Ehrhardt M. 1999. Methods of Seawater Analysis (Wiley-VCH, Göttingen, Germany), 3rd Ed.
- 46.Braman RS, Hendrix SA. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Anal Chem. 1989;61(24):2715–2718. doi: 10.1021/ac00199a007. [DOI] [PubMed] [Google Scholar]
- 47.Sigman DM, et al. Natural abundance-level measurement of the nitrogen isotopic composition of oceanic nitrate: An adaptation of the ammonia diffusion method. Mar Chem. 1997;57:227–242. [Google Scholar]
- 48.Holmes RM, McClelland JW, Sigman DM, Fry B, Peterson BJ. Measuring 15N-NH+4 in marine, estuarine and fresh waters: An adaptation of the ammonia diffusion method for samples with low ammonium concentrations. Mar Chem. 1998;60:235–243. [Google Scholar]
- 49.McIlvin MR, Altabet MA. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Anal Chem. 2005;77(17):5589–5595. doi: 10.1021/ac050528s. [DOI] [PubMed] [Google Scholar]
- 50.Sigman DM, et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem. 2001;73(17):4145–4153. doi: 10.1021/ac010088e. [DOI] [PubMed] [Google Scholar]
- 51.Granger J, Sigman DM. Removal of nitrite with sulfamic acid for nitrate N and O isotope analysis with the denitrifier method. Rapid Commun Mass Spectrom. 2009;23(23):3753–3762. doi: 10.1002/rcm.4307. [DOI] [PubMed] [Google Scholar]
- 52.Holtappels M, Lavik G, Jensen MM, Kuypers MMM. 2011. 15N-labeling experiments to dissect the contributions of heterotrophic denitrification and anammox to nitrogen removal in the OMZ waters of the ocean. Research on Nitrification and Related Processes, Part A, Methods in Enzymology, ed Klotz MG (Academic, New York), pp 223–251. [DOI] [PubMed]
- 53.Füssel J, et al. Nitrite oxidation in the Namibian oxygen minimum zone. ISME J. 2012;6(6):1200–1209. doi: 10.1038/ismej.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.